Local Treatment of Driveline Infection with Bacteriophages
Abstract
1. Introduction
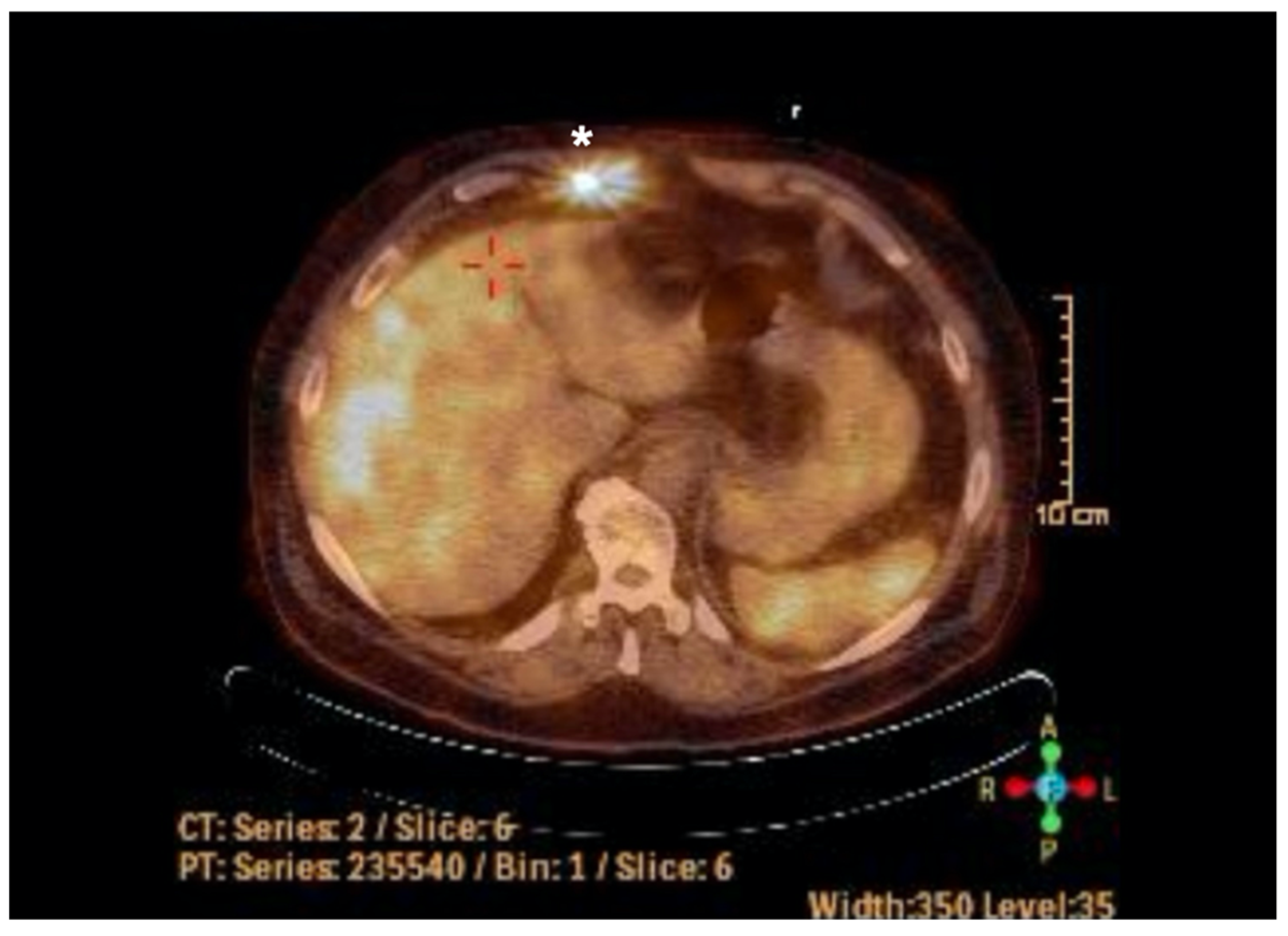
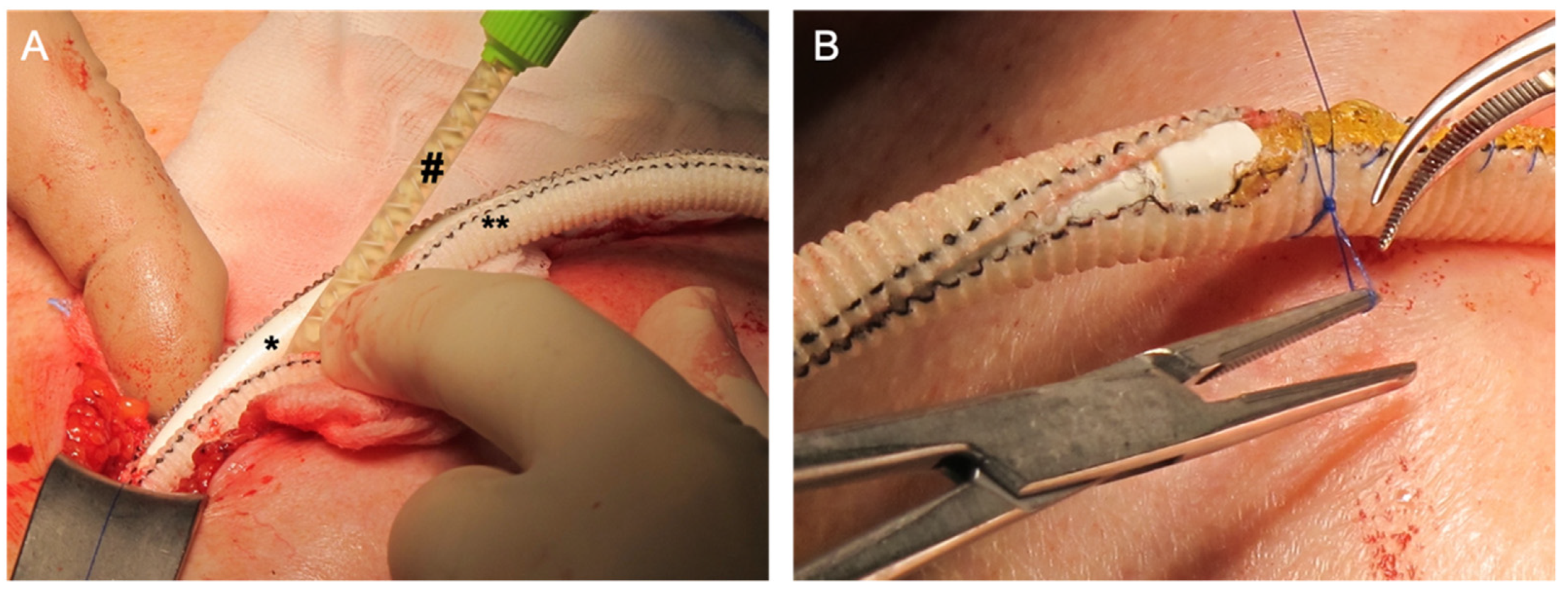
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gordon, R.J.; Quagliarello, B.; Lowy, F.D. Ventricular assist device-related infections. Lancet Infect. Dis. 2006, 6, 426–437. [Google Scholar] [CrossRef]
- Pereda, D.; Conte, J.V. Left Ventricular Assist Device Driveline Infections. Cardiol. Clin. 2011, 29, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Leuck, A.-M. Left ventricular assist device driveline infections: Recent advances and future goals. J. Thorac. Dis. 2015, 7, 2151–2157. [Google Scholar] [CrossRef]
- Nienaber, J.J.C.; Kusne, S.; Riaz, T.; Walker, R.C.; Baddour, L.M.; Wright, A.J.; Park, S.J.; Vikram, H.R.; Keating, M.R.; Arabia, F.A.; et al. Clinical Manifestations and Management of Left Ventricular Assist Device-Associated Infections. Clin. Infect. Dis. 2013, 57, 1438–1448. [Google Scholar] [CrossRef]
- Macia, M.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef]
- Levy, D.; Guo, Y.; Simkins, J.; Puius, Y.; Muggia, V.; Goldstein, D.; D’Alessandro, D.; Minamoto, G. Left ventricular assist device exchange for persistent infection: A case series and review of the literature. Transpl. Infect. Dis. 2014, 16, 453–460. [Google Scholar] [CrossRef]
- Comeau, A.M.; Hatfull, G.F.; Krisch, H.M.; Lindell, D.; Mann, N.H.; Prangishvili, D. Exploring the prokaryotic virosphere. Res. Microbiol. 2008, 159, 306–313. [Google Scholar] [CrossRef]
- Hesse, S.; Adhya, S. Phage Therapy in the Twenty-First Century: Facing the Decline of the Antibiotic Era; Is It Finally Time for the Age of the Phage? Annu. Rev. Microbiol. 2019, 73, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef]
- Greene, W.; Chan, B.; Bromage, E.; Grose, J.H.; Walsh, C.; Kortright, K.; Forrest, S.; Perry, G.; Byrd, L.; Stamper, M.A. The Use of Bacteriophages and Immunological Monitoring for the Treatment of a Case of Chronic Septicemic Cutaneous Ulcerative Disease in a Loggerhead Sea Turtle Caretta caretta. J. Aquat. Anim. Health 2021, 33, 139–154. [Google Scholar] [CrossRef]
- Herten, M.; Idelevich, E.A.; Sielker, S.; Becker, K.; Scherzinger, A.S.; Osada, N.; Torsello, G.B.; Bisdas, T. Vascular Graft Impregnation with Antibiotics: The Influence of High Concentrations of Rifampin, Vancomycin, Daptomycin, and Bacteriophage Endolysin HY-133 on Viability of Vascular Cells. Med Sci. Monit. Basic Res. 2017, 23, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage Therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Alisky, J.; Iczkowski, K.; Rapoport, A.; Troitsky, N. Bacteriophages show promise as antimicrobial agents. J. Infect. 1998, 36, 5–15. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.-C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Aslam, S.; Pretorius, V.; Lehman, S.M.; Morales, S.; Schooley, R.T. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J. Hear. Lung Transplant. 2019, 38, 475–476. [Google Scholar] [CrossRef]
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.-P.; Costechareyre, D.; Molle, V. Bacteriophage Therapy for Staphylococcus Aureus Infections: A Review of Animal Models, Treatments, and Clinical Trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314. [Google Scholar] [CrossRef]
- Hughes, K.A.; Sutherland, I.W.; Jones, M.V. Biofilm susceptibility to bacteriophage attack: The role of phage-borne polysaccharide depolymerase. Microbiology 1998, 144, 3039–3047. [Google Scholar] [CrossRef]
- Spellberg, B.; Gilbert, D.N. The Future of Antibiotics and Resistance: A Tribute to a Career of Leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. 2), S71–S75. [Google Scholar] [CrossRef]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef]
- Seed, K.D. Battling Phages: How Bacteria Defend against Viral Attack. PLOS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef]
- Yilmaz, C.; Colak, M.; Yilmaz, B.C.; Ersoz, G.; Kutateladze, M.; Gozlugol, M. Bacteriophage Therapy in Implant-Related Infections. J. Bone Jt. Surg. 2013, 95, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, W.N.; Concepción-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed]
| Antibiotics | Proteus mirabilis | Staphylococcus aureus |
|---|---|---|
| Oxacillin | R | S |
| Ampicillin | S | S |
| Ampicillin/Sulbactam | S | S |
| Piperacillin/Tazobactam | S | S |
| Cefuroxime | I | S |
| Cefotaxime | S | S |
| Ceftazidime | S | S |
| Imipenem | I | S |
| Meropenem | S | S |
| Gentamicin | S | S |
| Tetracycline | R | S |
| Cotimoxazole | S | S |
| Erythromycin | R | S |
| Clindamycin | R | S |
| Vancomycin | R | S |
| Fosfomycin | S | S |
| Fusidic acid | R | S |
| Rifampin | R | S |
| Linezolid | R | S |
| Daptomycin | R | S |
| Tigecycline | R | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Püschel, A.; Skusa, R.; Bollensdorf, A.; Gross, J. Local Treatment of Driveline Infection with Bacteriophages. Antibiotics 2022, 11, 1310. https://doi.org/10.3390/antibiotics11101310
Püschel A, Skusa R, Bollensdorf A, Gross J. Local Treatment of Driveline Infection with Bacteriophages. Antibiotics. 2022; 11(10):1310. https://doi.org/10.3390/antibiotics11101310
Chicago/Turabian StylePüschel, Anja, Romy Skusa, Antonia Bollensdorf, and Justus Gross. 2022. "Local Treatment of Driveline Infection with Bacteriophages" Antibiotics 11, no. 10: 1310. https://doi.org/10.3390/antibiotics11101310
APA StylePüschel, A., Skusa, R., Bollensdorf, A., & Gross, J. (2022). Local Treatment of Driveline Infection with Bacteriophages. Antibiotics, 11(10), 1310. https://doi.org/10.3390/antibiotics11101310






