Recent Advances in the Application of Bacteriophages against Common Foodborne Pathogens
Abstract
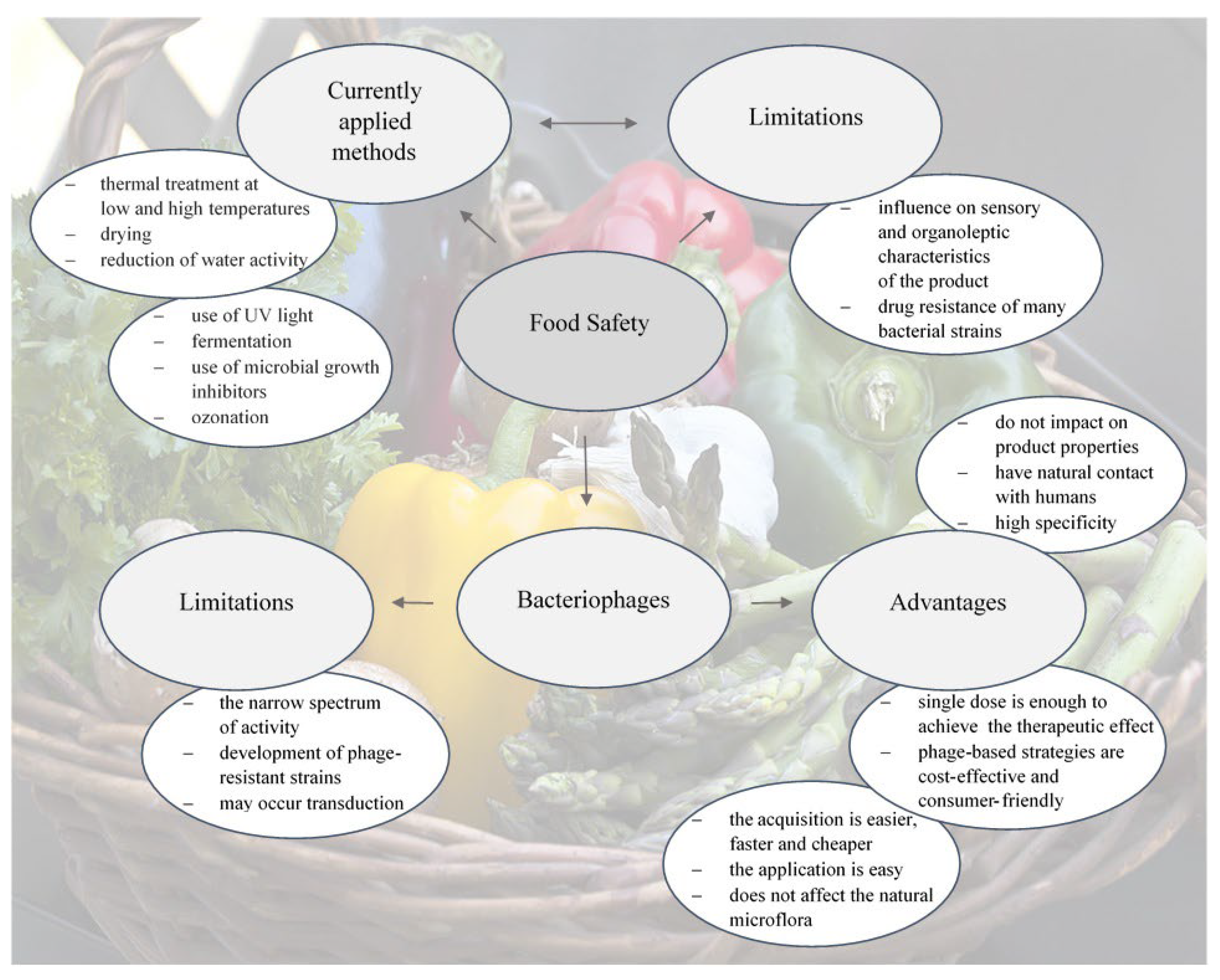
:1. Introduction
1.1. Bacteriophages
1.2. Foodborne Pathogens
1.3. Salmonella
1.4. Escherichia coli
1.5. Listeria
1.6. Campylobacter
1.7. Yersinia
2. Perspectives
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García, P.; Martínez, B.; Obeso, J.M.; Rodríguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and Their Role in Food Safety. Int. J. Microbiol. 2012, 2012, 863945. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, A.; Ebner, P.D.; Syed, Q.A.; Rahman, H.U. Employing list-shield bacteriophage as a bio-control intervention for Listeria monocytogenes from raw beef surface and maintain meat quality during refrigeration storage. LWT 2020, 132, 109784. [Google Scholar] [CrossRef]
- Thung, T.Y.; Lee, E.; Mahyudin, N.A.; Anuradha, K.; Mazlan, N.; Kuan, C.H.; Pui, C.F.; Ghazali, F.M.; Rashid, N.-K.M.A.; Rollon, W.D.; et al. Evaluation of a lytic bacteriophage for bio-control of Salmonella Typhimurium in different food matrices. LWT 2019, 105, 211–214. [Google Scholar] [CrossRef]
- Li, C.; Shi, T.; Sun, Y.; Zhang, Y. A Novel Method to Create Efficient Phage Cocktails via Use of Phage-Resistant Bacteria. Appl. Environ. Microbiol. 2022, 88, e02323-21. [Google Scholar] [CrossRef]
- Ramos-Vivas, J.; Elexpuru-Zabaleta, M.; Samano, M.L.; Barrera, A.P.; Forbes-Hernández, T.Y.; Giampieri, F.; Battino, M. Phages and Enzybiotics in Food Biopreservation. Molecules 2021, 26, 5138. [Google Scholar] [CrossRef]
- Bigot, B.; Lee, W.-J.; McIntyre, L.; Wilson, T.; Hudson, J.A.; Billington, C.; Heinemann, J.A. Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage. Food Microbiol. 2011, 28, 1448–1452. [Google Scholar] [CrossRef]
- Bandara, N.; Jo, J.; Ryu, S.; Kim, K.P. Bacteriophages BCP1-1 and BCP8-2 require divalent cations for efficient control of Bacillus cereus in fermented foods. Food Microbiol. 2012, 31, 9–16. [Google Scholar] [CrossRef]
- Ferguson, S.; Roberts, C.; Handy, E.; Sharma, M. Lytic bacteriophages reduce Escherichia coli O157: H7 on fresh cut lettuce introduced through cross-contamination. Bacteriophage 2013, 3, e24323. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.W.; Kim, J.W.; Jung, T.S.; Woo, G.J. wksl3, a New Biocontrol Agent for Salmonella enterica Serovars Enteritidis and Typhimurium in foods: Characterization, Application, Sequence Analysis, and Oral Acute Toxicity Study. Appl. Environ. Microbiol. 2013, 79, 1956–1968. [Google Scholar] [CrossRef]
- Strange, J.E.; Leekitcharoenphon, P.; Møller, F.D.; Aarestrup, F.M. Metagenomics analysis of bacteriophages and antimicrobial resistance from global urban sewage. Sci. Rep. 2021, 11, 1600. [Google Scholar] [CrossRef]
- Chanishvili, N. Phage therapy—history from Twort and d′Herelle through Soviet experience to current approaches. Adv. Vir. Res. 2012, 83, 3–40. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2017, 62, 17–55. [Google Scholar] [CrossRef]
- Kasman, L.M.; Porter, L.D. Bacteriophages; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kochhar, R. The virus in the rivers: Histories and antibiotic afterlives of the bacteriophage at the sangam in Allahabad. Notes Rec. R Soc. Lond. 2020, 74, 625–651. [Google Scholar] [CrossRef]
- Dowah, A.S.; Clokie, M.R. Review of the nature, diversity and structure of bacteriophage receptor binding proteins that target Gram-positive bacteria. Biophys. Rev. 2018, 10, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Abdelsattar, A.S.; Dawooud, A.; Rezk, N.; Makky, S.; Safwat, A.; Richards, P.J.; El-Shibiny, A. How to Train Your Phage: The Recent Efforts in Phage Training. Biologics 2021, 1, 70–88. [Google Scholar] [CrossRef]
- Abedon, S.T.; García, P.; Mullany, P.; Aminov, R. Phage Therapy: Past, Present and Future. Front. Microbiol. 2017, 8, 981. [Google Scholar] [CrossRef] [Green Version]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a Post-Antibiotic Era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Galtier, M.; De Sordi, L.; Maura, D.; Arachchi, H.; Volant, S.; Dillies, M.A.; Debarbieux, L. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol. 2016, 18, 2237–2245. [Google Scholar] [CrossRef]
- Arumugam, S.N.; Manohar, P.; Sukumaran, S.; Sadagopan, S.; Loh, B.; Leptihn, S.; Nachimuthu, R. Antibacterial efficacy of lytic phages against multidrug-resistant Pseudomonas aeruginosa infections in bacteraemia mice models. BMC Microbiol. 2022, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.M.; Gorman, S.P.; Donnelly, R.F.; Gilmore, B.F. Recent advances in bacteriophage therapy: How delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 2011, 63, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, X. The application and research progress of bacteriophages in food safety. J. Appl. Microbiol. 2022, 133, 2137–2147. [Google Scholar] [CrossRef]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage Biocontrol Applications in Food Production and Processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef]
- Örmälä, A.M.; Jalasvuori, M. Phage therapy: Should bacterial resistance to phages be a concern, even in the long run? Bacteriophage 2013, 3, e24219. [Google Scholar] [CrossRef] [Green Version]
- Molina, F.; Menor-Flores, M.; Fernández, L.; Vega-Rodríguez, M.A.; García, P. Systematic analysis of putative phage-phage interactions on minimum-sized phage cocktails. Sci. Rep. 2022, 12, 2458. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, I, 529–563. [Google Scholar] [CrossRef]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial properties of food nanopackaging: A new focus on foodborne pathogens. Front. Microbiol. 2021, 12, 690706. [Google Scholar] [CrossRef]
- Khare, S.; Tonk, A.; Rawat, A. Foodborne diseases outbreak in India: A Review. Int. J. Food Sci. Nutr. 2018, 3, 9–10. [Google Scholar]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Foodborne Pathogens: Hygiene and Safety. Front. Microbiol. 2019, 10, 1974. [Google Scholar] [CrossRef] [Green Version]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef] [PubMed]
- Mizan, M.F.R.; Cho, H.R.; Ashrafudoulla, M.; Cho, J.; Hossain, M.I.; Lee, D.U.; Ha, S.D. The effect of physico-chemical treatment in reducing Listeria monocytogenes biofilms on lettuce leaf surfaces. Biofouling 2020, 36, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, e04634. [Google Scholar] [CrossRef]
- Oliver, S.P. Foodborne Pathogens and Disease Special Issue on the National and International PulseNet Network. Foodborne Pathog. Dis. 2019, 16, 439–440. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Fátima Barroso, M.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef]
- Cho, I.H.; Ku, S. Current Technical Approaches for the Early Detection of Foodborne Pathogens: Challenges and Opportunities. Int. J. Mol. Sci. 2017, 18, 2078. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.J.; Hungate, B.A.; Price, L.B. Food-animal production and the spread of antibiotic resistance: The role of ecology. Front. Ecol. Environ. 2017, 15, 309–318. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Lindqvist, R.; et al. Evaluation of public and animal health risks in case of a delayed post-mortem inspection in ungulates. EFSA J. 2020, 18, 12. [Google Scholar] [CrossRef]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.S.; Rahman, S.R. Use of Phages to Treat Antimicrobial-Resistant Salmonella Infections in Poultry. Vet. Sci. 2022, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Pelyuntha, W.; Vongkamjan, K. Combined effects of Salmonella phage cocktail and organic acid for controlling Salmonella Enteritidis in chicken meat. Food Control 2022, 133, 108653. [Google Scholar] [CrossRef]
- Yan, T.; Liang, L.; Yin, P.; Zhou, Y.; Mahdy Sharoba, A.; Lu, Q.; Dong, X.; Liu, K.; Connerton, I.; Li, J. Application of a Novel Phage LPSEYT for Biological Control of Salmonella in Foods. Microorganisms 2020, 8, 400. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Zhou, Y.; Liang, L.; Nime, I.; Yan, T.; Wang, X.; Li, J. Application of a Phage Cocktail for Control of Salmonella in Foods and Reducing Biofilms. Viruses 2019, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Thung, T.Y.; Premarathne, J.M.K.J.K.; San Chang, W.; Loo, Y.Y.; Chin, Y.Z.; Kuan, C.H.; Tan, C.W.; Basri, D.F.; Radzi, C.W.J.W.M.; Radu, S. Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food. LWT 2017, 78, 222–225. [Google Scholar] [CrossRef]
- Available online: http://www.intralytix.com/files/prod/02SP/02SP-Desc.pdf (accessed on 1 October 2022).
- Zhang, X.; Niu, Y.D.; Nan, Y.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Gil, J.; Brown, M.; Cesar Tondo, E.; Soraya Martins de Aquino, N.; Eisenberg, M.; Erickson, S. Accurate and sensitive detection of Salmonella in foods by engineered bacteriophages. Sci. Rep. 2020, 10, 17463. [Google Scholar] [CrossRef]
- Alonso, C.A.; González-Barrio, D.; Tenorio, C.; Ruiz-Fons, F.; Torres, C. Antimicrobial resistance in faecal Escherichia coli isolates from farmed red deer and wild small mammals. Detection of a multiresistant E. coli producing extended-spectrum beta-lactamase. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 34–39. [Google Scholar] [CrossRef]
- Riley, L.W. Extraintestinal Foodborne Pathogens. Annu. Rev. Food Sci. Technol. 2020, 11, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Vengarai Jagannathan, B.; Kitchens, S.; Priyesh Vijayakumar, P.; Price, S.; Morgan, M. Efficacy of Bacteriophage Cocktail to Control E. coli O157: H7 Contamination on Baby Spinach Leaves in the Presence or Absence of Organic Load. Microorganisms 2021, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, N.; Picozzi, C.; Cocuzzi, R.; Foschino, R. Evaluation of a Potential Bacteriophage Cocktail for the Control of Shiga-Toxin Producing Escherichia coli in Food. Front. Microbiol. 2020, 11, 1801. [Google Scholar] [CrossRef] [PubMed]
- Dewanggana, M.N.; Evangeline, C.; Ketty, M.D.; Waturangi, D.E.; Magdalena, S. Isolation, characterization, molecular analysis and application of bacteriophage DW-EC to control Enterotoxigenic Escherichia coli on various foods. Sci. Rep. 2022, 12, 495. [Google Scholar] [CrossRef]
- Wang, C.; Hang, H.; Zhou, S.; Niu, Y.D.; Du, H.; Stanford, K.; McAllister, T.A. Bacteriophage biocontrol of Shiga toxigenic Escherichia coli (STEC) O145 biofilms on stainless steel reduces the contamination of beef. Food Microbiol. 2020, 92, 103572. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Chang, Y.; Kim, S.Y.; Han, J. Polycaprolactone film functionalized with bacteriophage T4 promotes antibacterial activity of food packaging toward Escherichia coli. Food Chem. 2021, 346, 128883. [Google Scholar] [CrossRef]
- Kaptchouang Tchatchouang, C.D.; Fri, J.; De Santi, M.; Brandi, G.; Schiavano, G.F.; Amagliani, G.; Ateba, C.N. Listeriosis Outbreak in South Africa: A Comparative Analysis with Previously Reported Cases Worldwide. Microorganisms 2020, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstep. J. Vet. Res. 2020, 87, 1–20. [Google Scholar] [CrossRef]
- Strydom, A. Control of Listeria Monocytogenes in an Avocado Processing Facility. Doctoral dissertation, University of the Free State, Bleomfontei, South Africa, 2015. [Google Scholar]
- Dhama, K.; Karthik, K.; Tiwari, R.; Shabbir, Z.; Barbuddhe, S.; Malik, S.V.S.; Singh, R.K. Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: A comprehensive review. Vet. Q. 2015, 35, 211–235. [Google Scholar] [CrossRef]
- Jakobsen, R.R.; Trinh, J.T.; Bomholtz, L.; Brok-Lauridsen, S.K.; Sulakvelidze, A.; Nielsen, D.S. A Bacteriophage Cocktail Significantly Reduces Listeria monocytogenes without Deleterious Impact on the Commensal Gut Microbiota under Simulated Gastrointestinal Conditions. Viruses 2022, 14, 190. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes-How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 1, 66462. [Google Scholar] [CrossRef] [PubMed]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectives of Phage- Based Inhibiotion of Listria monocytogenes in Food Products and Food Processing Environments. Microorganism 2020, 8, 1764. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.N.; Abuladze, T.; Li, M.; Woolston, J.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiol. 2015, 52, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Bowman, J.P.; Fox, E.M. Novel Biocontrol Methods for Listeria monocytogenes Biofilms in Food Production Facilities. Front. Microbiol. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogovski, P.; Cadamuro, R.D.; da Silva, R.; de Souza, E.B.; Bonatto, C.; Viancelli, A.; Michelon, W.; Elmahdy, E.M.; Treichel, H.; Rodríguez-Lázaro, D.; et al. Uses of Bacteriophages as Bacterial Control Tools and Environmental Safety Indicators. Front. Microbiol. 2021, 12, 3793135. [Google Scholar] [CrossRef]
- Wójcicki, M.; Błażejak, S.; Gientka, I.; Brzezicka, K. The concept of using bacteriophages to improve the microbiological quality of minimally processed foods. Acta Sci. Pol. Technol. Aliment. 2019, 18, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Harrer, A.; Bücker, R.; Boehm, M.; Zarzecka, U.; Tegtmeyer, N.; Sticht, H.; Schulzke, J.D.; Backert, S. Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog. 2019, 11, 4. [Google Scholar] [CrossRef]
- Thung, T.Y.; Lee, E.; Mahyudin, N.A.; Wan Mohamed Radzi, C.W.J.; Mazlan, N.; Tan, C.W.; Radu, S. Partial characterization and in vitro evaluation of a lytic bacteriophage for biocontrol of Campylobacter jejuni in mutton and chicken meat. J. Food Saf. 2020, 40, e12770. [Google Scholar] [CrossRef]
- D’angelantonio, D.; Scattolini, S.; Boni, A.; Neri, D.; Di Serafino, G.; Connerton, P.; Connerton, I.; Pomilio, F.; Di Giannatale, G.; Migliorati, G.; et al. Bacteriophage Therapy to Reduce Colonization of Campylobacter jejuni in Broiler Chickens before Slaughter. Viruses 2021, 13, 1428. [Google Scholar] [CrossRef]
- Richards, P.J.; Connerton, P.L.; Connerton, I.F. Phage Biocontrol of Campylobacter jejuni in Chickens Does Not produce Collateral Effects on the Gut Microbiota. Front. Microbiol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Lv, P.; Huang, J.; Tai, R.; Jin, X.; Wang, J.; Wang, X. Salmonella Phages Affect the Intestinal Barrier in Chicks by Altering the Composition of Early Intestinal Flora: Association With Time of Phage Use. Front. Microbiol. 2022, 13, 947640. [Google Scholar] [CrossRef] [PubMed]
- Sarrami, Z.; Sedghi, M.; Mohammadi, I.; Kim, W.K.; Mahdavi, A.H. Effects of bacteriophage supplement on the growth performance, microbial population, and PGC-1α and TLR4 gene expressions of broiler chickens. Sci. Rep. 2022, 12, 14391. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Karimi Torshizi, M.A.; Rahimi, S.; Dennehy, J.J. Prophylactic Bacteriophage Administration More Effective than Post-infection Administration in Reducing Salmonella enterica serovar Enteritidis Shedding in Quail. Front. Microbiol. 2016, 7, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhaya, S.D.; Ahn, J.M.; Cho, J.H.; Kim, J.Y.; Kang, D.K.; Kim, S.W.; Kim, H.B.; Kim, I.H. Bacteriophage cocktail supplementation improves growth performance, gut microbiome and production traits in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 49. [Google Scholar] [CrossRef]
- Zampara, A.; Sørensen, M.C.H.; Elsser-Gravesen, A.; Brøndsted, L. Significance of phage-host interactions for biocontrol of Campylobacter jejuni in food. Food Control 2017, 73, 1169–1175. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Raza, H.; Niazi, S.; Khan, I.M.; Akhtar, W.; Safdar, W.; Wang, Z. A comprehensive review on the prevalence pathogenesis and detection of Yersinia enterocolitica. RSC 2019, 9, 41010–41021. [Google Scholar] [CrossRef] [Green Version]
- Terentjeva, M.; Ķibilds, J.; Mesitere, I.; Gradovska, S.; Alksne, L.; Streikiša, M.; Ošmjana, J.; Valciņa, O. Virulence Determinants and Genetic Diversity of Yersinia Species Isolated from Retail Meat. Pathogens 2022, 11, 37. [Google Scholar] [CrossRef]
- Available online: https://www.efsa.europa.eu/en/news/campylobacter-and-salmonella-cases-stable-eu (accessed on 25 February 2021).
- Leon-Velarde, C.G.; Jun, J.W.; Skurnik, M. Yersinia Phages and Food Safety. Viruses 2019, 11, 1105. [Google Scholar] [CrossRef] [Green Version]
- Gwak, K.M.; Choi, I.Y.; Lee, J.; Oh, J.H.; Park, M.K. Isolation and Characterization of a Lytic and Highly Specific Phage against Yersinia enterocolitica as a Novel Biocontrol Agent. J. Microbiol. Biotechnol. 2018, 28, 1946–1954. [Google Scholar] [CrossRef] [Green Version]
- Pyra, A.; Filik, K.; Szemer-Olearnik, B.; Czarny, A.; Brzozowska, E. New Insights on the Feature and Function of Tail Tubular Protein B and Tail Fiber Protein of the Lytic Bacteriophage φYeO3-12 Specific for Yersinia enterocolitica Serotype O:3. Molecules 2020, 25, 4392. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.W.; Park, S.C.; Wicklund, A.; Skurnik, M. Bacteriophages reduce Yersinia enterocolitica contamination of food and kitchenware. Int. J. Food Microbiol. 2018, 271, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alomari, M.M.M.; Dec, M.; Urban-Chmiel, R. Bacteriophage as an Alternative Method for Control of Zoonotic and Foodborne Pathogens. Viruses 2021, 13, 2348. [Google Scholar] [CrossRef] [PubMed]
- Soffer, N.; Woolston, J.; Li, M.; Das, C.; Sulakvelidze, A. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS ONE 2017, 12, e0175256. [Google Scholar] [CrossRef] [Green Version]
- Kolenda, C.; Josse, J.; Medina, M.; Fevre, C.; Lustig, S.; Ferry, T.; Laurent, F. Evaluation of the activity of a Combination of Three Bacteriophages Alone or in Association with Antibiotics on Saphylococcus aureus Embedded in Biofilm or Internalized in Osteoblasts. Antimicrob. Agents Chemother. 2020, 64, e02231-19. [Google Scholar] [CrossRef]
- Bueno, E.; Garciía, P.; Martiínez, B.; Rodriíguez, A. Phage inactivation of Staphylococcus aureus in fresh and hard-type cheeses. Int J. Food Microbiol. 2012, 158, 23–27. [Google Scholar] [CrossRef]
- Heir, E.; Moen, B.; Åsli, A.W.; Sunde, M.; Langsrud, S. Antibiotic Resistance and Phylogeny of Pseudomonas spp. Isolated over Three Decades from Chicken Meat in the Norwegian Food Chain. Microorganism 2021, 9, 207. [Google Scholar] [CrossRef]
- Hungaro, H.M.; Vidigal, P.M.P.; do Nascimento, E.C.; da Costa Oliveira, G.F.; Gontijo, M.T.P.; Lopez, M.E.S. Genomic Characterisation of UFJF_PfDIW6: A Novel Lytic Pseudomonas fluorescens-Phage with Potential for Biocontrol in the Dairy Industry. Viruses 2022, 14, 629. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- FEEDAP; Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Durjava, M.F.; Lopez-Alonso, M.; Puente, S.L.; et al. Safety and efficacy of a feed additive consisting on the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 infecting Salmonella Gallinarum B/00111 (Bafasal®) for all avian species (Proteon Pharmaceuticals S.A.). EFSA J. 2021, 19, 6534. [Google Scholar] [CrossRef]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family–A Review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef] [Green Version]
- Putra, R.D.; Lyrawati, D. Interactions between Bacteriophages and Eukaryotic Cells. Scientifica 2020, 2020, 3589316. [Google Scholar] [CrossRef]
- Żaczek, M.; Górski, A.; Skaradzińska, A.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B. Phage penetration of eukaryotic cells: Practical implications. Future Med. 2020, 14, 745–760. [Google Scholar] [CrossRef]
- Podlacha, M.; Grabkowski, Ł.; Kosznik-Kawśnicka, K.; Zdrojewska, K.; Stasiłojć, M.; Węgrzyn, G.; Węgrzyn, A. Interactions of Bacteriophages with Animal and Human Organisms-Safety Issues in the Ligth of Phage Therapy. Int. J. Mol. Sci. 2021, 22, 8937. [Google Scholar] [CrossRef] [PubMed]
- Goode, D.; Allen, V.M.; Barrow, P.A. Reduction of Experimental Salmonella and Campylobacter Contamination of Chicken Skin by Application of Lytic Bacteriophages. Appl. Env. Microbiol. 2003, 69, 5032–5036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, J.A.; Billington, C.; Wilson, T.; On, S.L. Effect of phage and host concentration on the inactivation of Escherichia coli O157:H7 on cooked and raw beef. Food Sci. Technol. Int. 2013, 21, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Env. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Do Nascimento, E.C.; Sabino, M.C.; da Roza Corguinha, L.; Targino, B.N.; Lange, C.C.; de Oliveira Pinto, C.L.; de Faria Pinto, P.; Vidigal, P.M.P.; Sant’Ana, A.S.; Hungaro, H.M. Lytic bacteriophages UFJF_PfDIW6 and UFJF_PfSW6 prevent Pseudomonas fluorescens growth in vitro and the proteolytic-caused spoilage of raw milk during chilled storage. Food Microbiol. 2022, 101, 103892. [Google Scholar] [CrossRef]
- Kauppinen, A.; Siponen, S.; Pitkänen, T.; Holmfeldt, K.; Pursiainen, A.; Torvinen, E.; Miettinen, I.T. Phage biocontrol of Pseudomonas aeruginosa in water. Viruses 2021, 13, 928. [Google Scholar] [CrossRef]
- Hong, Y.; Schmidt, K.; Marks, D.; Hatter, S.; Marshall, A.; Albino, L.; Ebner, P. Treatment of Salmonella-contaminated Eggs and Pork with a Broad-Spectrum, Single Bacteriophage: Assessment of Efficacy and Resistance Development. Foodborne Pathog. Dis. 2016, 13, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Jeon, B.; Ryu, S. Effective inhibition of Salmonella Typhimurium in fresh produce by a phage cocktail targeting multiple host receptors. Food Microbiol. 2019, 77, 52–60. [Google Scholar] [CrossRef]
- Lu, H.; Liu, H.; Lu, M.; Wang, J.; Liu, X. Isolation and Characterization of a Novel myovirus Infecting Shigella dysenteriae from Aeration Tank Water. Appl. Biochem. Biotechnol. 2020, 192, 120–131. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Bao, H. Phage inactivation of foodborne Shigella on ready-to-eat spiced chicken. Poult. Sci. 2013, 92, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Gharieb, R.M.A.; Saad, M.F.; Mohamed, A.S.; Tartor, Y.H. Characterization of two novel lytic bacteriophages for reducing biofilms of zoonotic multidrug-resistant Staphylococcus aureus and controlling their growth in milk. LWT 2020, 124, 109145. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.; Xu, Y.; Wang, L.; Li, X. Protective effectiveness of feeding phage cocktails in controlling Vibrio parahamolyticus infection of sea cucumber Apostichopus japonicus. Aquaculture 2019, 503, 322–329. [Google Scholar] [CrossRef]
- Orquera, S.; Hertwig, S.; Alter, T.; Hammerl, J.A.; Jirova, A.; Golz, G. Development of transient phage resistance in Campylobacter coli against the group II phage CP84. Berl. Munch Tierarztl. Wochenschr. 2015, 128, 141–147. [Google Scholar] [CrossRef]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.J. Don’t Shut the Stable Door after the Phage Has Botled–The Imporatace of bacteriophage Inactivaction in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.N.; Figueiredo, A.C.; Miranda, F.A.; de Castro Almeida, R.C. Control of Listeria monocytogenes growth in soft cheeses by bacteriophage P100. Braz. J. Microbiol. 2014, 45, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.; de Moura, F.H.; Van Den Broek, K.; de Mello, A.S. Effect of ultraviolet light, organic acids and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sadekuzzaman, M.; Ha, S.D. Reduction of Listeria monocytogenes on chicken breast by combined treatment with UV-C light and bacteriophage List Shield. LWT 2017, 86, 193–200. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Fernández, L.; Martínez, B.; Rodríguez, A.; García, P. Applicability of commercial phage-based products against Listeria monocytogenes for improvement of food safety in Spanish dry-cured ham and food contact surfaces. Food Cont. 2017, 73, 1474–1482. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Yang, S.; Kim, H.S.; Mizan, M.F.R.; Ha, S.D. Evaluation of a novel atimicrobiological (lauric arginate ester) susbstance against biofilm of Escherichia coli O157:H17, Listeria monocytogenes and Salmonella spp. Int. J. Food Sci. Technol. 2017, 52, 2058–2067. [Google Scholar] [CrossRef]
- Wottlin, L.R.; Edrington, T.S.; Anderson, R.C. Salmonella Carriage in Peripheral Lymph Nodes and Feces of Cattle at Slaughter is Affected by Cattle Type, Region, and Season. Front. Anim. Sci. 2022, 3, 859800. [Google Scholar] [CrossRef]
- Moye, Z.D.; Das, C.R.; Tokman, J.I.; Fanelli, B.; Karathia, H.; Hasan, N.A.; Marek, P.J.; Senecal, A.G.; Sulakvelidze, A. Treatment of fresh produce with a Salmonella- targeted bacteriophage cocktail is compatible with chlorine or peracetic acid and more consistently preserves the microbial community on produce. J. Food Saf. 2020, 40, e12763. [Google Scholar] [CrossRef]
- Sharma, M.; Dashiell, G.; Handy, E.T.; East, C.; Reynnells, R.; White, C.; Nyarko, E.; Micallef, S.; Hashem, F.; Millner, P.D. Survival of Salmonella Newport on whole and fresh-cut cucumbers treated with lytic bacteriophages. J. Food Prot. 2017, 80, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Ukhanova, M.; Reinhard, M.K.; Li, M.; Sulakvelidze, A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 2015, 5, e1088124. [Google Scholar] [CrossRef] [Green Version]
- Magnone, P.J.; Marek, J.P.; Sulakvelidze, A.; Senecal, A. Additive Approach for Inactivation of Escherichia coli O157:H7, Salmonella, and Shigella spp. on Contaminated Fresh Fruits and Vegetables Using Bacteriophage Cocktail and Produce Wash. JFP 2013, 76, 1336–1341. [Google Scholar] [CrossRef]
- Vikram, A.; Tokman, J.; Woolston, J.; Sulakvelidze, A. Phage biocontrol improves food safety by significantly reducing both the concentration and occurrence of Escherichia coli O157:H7 in various foods. J. Food Prot. Press 2020, 83, 668–676. [Google Scholar] [CrossRef]
- Boyacioglu, O.; Sharma, M.; Sulakvelidze, A.; Goktepe, I. Biocontrol of Escherichia coli O157:H7 on fresh-cut leafy greens. Bacteriophage 2013, 3, e24620. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.D.; Parks, A.; Abuladze, T.; Li, M.; Woolston, J.; Magnone, J.; Senecal, A.; Kropinski, A.M.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces Escherichia coli O157:H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2012, 2, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.intralytix.com/index.php?page=prod&id=12 (accessed on 20 September 2022).
- Mayne, J.; Zhang, X.; Butcher, J.; Walker, K.; Ning, Z.; Wójcik, K.; Bastych, J.; Stintzi, A.; Figeys, D. Examining the Effects of an Anti-Salmonella Bacteriophage Preparation BASFAL, on Ex-Vivo Human Gut Microbiome Composton an Fuction Using a Multi- Omics Approoach. Viruses 2021, 13, 1734. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ahfoodchain.com/en/about/news/2020/08/finalyse-sal-release (accessed on 6 September 2022).
| Bacteria | Symptoms | Transmission | Phages | Results | References |
|---|---|---|---|---|---|
| Campylobacter jejuni | Fever, muscle aches, headaches, arthralgia, abdominal pain and cramps, weakness, bloody diarrhea, gastric or intestinal pain, occurrence of Guillain-Barré syndrome | Poultry meat, milk, contaminated water, swimming in contaminated water bodies, contact with animal. | CJ01 | Mutton and chicken meat were stored at 4 °C and injected with 5 mL of C. jejuni with a concentration of 104 CFU/mL. The samples prepared in this way were incubated for 4 h. Then, they were sprayed with 5 mL of bacteriophage with PFU/mL, and the samples were again incubated for 48 h. The final result was 102 CFU/g. | [4] |
| Φ7-izsam Φ16-izsam | The influence of bacteriophages on naturally or artificially contaminated poultry was investigated. Bacteriophages were given to the animals before slaughter and resulted in a reduction of 1 log10 CFU/g and 2 log10 CFU/g for both test groups. | [72] | |||
| CP20 and CP30A | Poultry were infected and bacteriophages were administered 4 days later. Chickens were sacrificed every 24 h and the intestinal pathogen concentration was examined. The most prominent result was obtained on the second day of incubation and caused a decrease of bacteria by 2.4 log CFU/g in relation to the control. | [73] | |||
| 12673, P22, 29C; | The contaminated skin of chickens was examined. The level of the pathogen decreased by 2 log units when using the MOI of the phage 100:1 or 1000:1. | [100] | |||
| Escherichia coli | Vomiting, headaches, stomach pain, low-grade fever or fever, diarrhea, weakness, bloody stools, hemolytic uremic syndrome, neonatal meningitis, pneumonia, sepsis | Pork, poultry, contaminated ruminants such as goats, deer, sheep, elk, water, milk and dairy products, direct contact with animals | FM10, DP16 and DP19 | The phage cocktail was tested on fresh intubated cucumber at two temperatures of 4 °C and 25 °C for 24 h. The number of bacteria was reduced by 1.97–2.01 log CFU/g and 1.16–2.01 log CFU/g at 25 °C and 4 °C. | [54] |
| DW-EC | It was tested on many matrices, such as chicken meat, lettuce meat, fish meat, and tomato. The samples were contaminated with bacteria, then they were subjected to the phage section. A significant result was obtained at 6 days of incubation. The best effect was seen in the chicken feed samples where the pathogen value decreased by 80.93% after the first day and 87.29% after the 6th day. The weakest effect was observed on lettuce leaves. | [55] | |||
| AZO145A | The effect of phage on the biofilm was investigated. Exposure to bacteriophage at a concentration of 1010 PFU/cm2 for 2 h resulted in a 4.0 log 10 PFU/mL reduction in biofilm on stainless steel. However, on the surface of beef, at 48 h incubation, the pathogen decreased by 3.1 log10 CFU/g. | [56] | |||
| T4 | The aim of the study was to design an antimicrobial package by using the immobilization of T4 phage (105 CFU/mL) on the surface of the PCL foil. Contaminated beef was placed in this package. The bacterial concentration applied to the meat was 107 CFU/mL. After 48 h of incubation, the concentration of bacteria was reduced by 3 log CFU/mL. | [84] | |||
| FAHEc1 | Contaminated raw beef as a test matrix; after using phage, the concentration of bacteria decreased by 2 or 4 log units at the appropriate storage temperatures, 24 °C and 37 °C. | [101] | |||
| Listeria monocytogenes | Fever, chills, muscle aches, headaches, nausea and vomiting, confusion, local infections, inflammation of the lymph nodes, inflammation of the lungs, joints, bone marrow, pericarditis and myocarditis, inflammation of the eyeball, gastrointestinal infections. | Raw vegetables and fruit, unpasteurized dairy products (milk, cheese, ices cream), raw, cooked and frozen poultry meat, raw and smoked fish, delicatessen products, semi-finished products, fast-food products, soil, sewage, water, rotting plants, silage, wild and farm animals. | FWLLm1 | Bacterial levels dropped by 2 log units on the surface of the chicken that had become contaminated with Listeria. The samples were stored in a vacuum package at 4 °C and 30 °C. A positive result was observed only for the sample kept at 30 °C. | [7] |
| A511 | Bacterial levels were tested in milk chocolate, mozzarella and brie cheese. The phage were given and incubated at 6 °C. Bacteria concentration dropped by 5 log units. | [102] | |||
| Pseudomonas spp. | Pneumonia, fever, chills, severe shortness of breath, cough, confusion, chronic lower respiratory tract infection, Roth’s spots, i.e., petechiae on the retina, small painless erythematous changes on the hands and feet—Janeway symptom, painful reddish lumps on the fingers—Osler’s nodules, subungual petechiae | Water, soil, human and animal digestive tract. | UFJF_PfDIW6, UFJF_PfSW6 | The lyophilized phage cocktail was incubated with raw milk at 4 °C for 7 days. After the incubation period, the Pseudomonas bacterial population decreased by 3.2 log CFU/mL. | [103] |
| V523, V524, JG003 | Three bacteriophages used separately and together as a cocktail were used to biocontrol bacteria in the water. The effect of the phages was tested against two bacteriophage strains: PAO1 and the environmental strain 17V1507. Of all the bacteriophages, V523 was most effective in reducing the PAO1 strain (>2.4 log10). The other strain was sensitive only to JG003, resulting in its reduction by 1.2 log10. The phage cocktail resulted in higher reductions in PAO1 (>3.4 log10) compared to using them alone. In contrast, the same reduction was observed in 17V1507 as with JG003 alone. | [104] | |||
| Salmonella spp. | Abdominal pain, vomiting, diarrhea, fever, headache, chills, reduced urine output, dry mucous membranes, excessive sleepiness, apathy. | Chicken, turkey, pig, duck, goose meat, eggs, soil, water, cheese, milk, fruit, vegetables, contact with contaminated animals | LPSTLL, LPST94, LPST153 | The use of the bacteriophage cocktail decreased the concentration of bacteria by 3 log units. Chicken breasts were inoculated using an inoculum. The influence on the biofilm created by Salmonella was also examined, the administered cocktail effectively inhibited the growth after 72 h, the microplates decreased by 5.23 log units. | [46] |
| LYPSET | The biocontrol was tested in milk and on lettuce leaves. In milk samples there was a decrease of bacteria by 2.19 log CFU/mL at 4 °C and 4.3 log CFU/mL at 25 °C. However, in the samples containing lettuce, there was a decrease of 2.2 log CFU/mL at 4 °C and 2.34 log CFU/mL at 25 °C at MOI = 10,000. | [45] | |||
| SE07 | The effects of phage on eggs, beef and poultry meat were tested. The best effect for beef was obtained after 48 h of incubation; the bacterial value dropped from 4.23 log CFU/mL to 2.11 log CFU/mL, while for chicken the effect was even better as there was a decrease from 4.16 log CFU/mL to 2.14 log CFU/mL also at 48 h. | [47] | |||
| SJ2 | The use of phage resulted in a significant reduction of bacteria in the soft pork and eggs. Incubation was carried out at 4 °C. | [105] | |||
| BSPM4, BSP101, BSP22A | The phages were presented as a cocktail. The reduction of bacterial colonies was tested on lettuce leaves and fresh cucumber. There was a reduction of 4.7 log for lettuce and 5.8 log for cucumber. | [106] | |||
| Shigella sp. | Vomiting, anorexia, abdominal cramps, bowel urgency, severe watery diarrhea, fever, diarrhea with an admixture of mucus and blood, rapid breathing, heart rate, low blood pressure, dry mouth and skin (dehydration), pain on palpation of the abdomen | Touching skin of contaminated person, oral cavity (fecal–oral route), contaminated water and food, sexual contact, swimming in contaminated water, by insects, such as housefly. | SSE1, SGF3, SGF2, | The influence of the SFE3 phage and its combination as a cocktail with other phages on the reduction of biofilm on polystyrene surfaces was investigated. It was found that the single SGF2 phage (isolated from wastewater) had the greatest impact on the development of biofilm; it caused growth inhibition by 26.6%. The lowest results were obtained for SGF3, while adding it to a phage cocktail increased its effectiveness by 25%. The phage is active against strains such as S. dysenteriae, S. baumannii, and S. flexneri. | [107] |
| SD-11, SF-A2, SS-92 | The number of pathogens was decreased by 4 log in chicken meat, when they applied phage cocktail. It was stored at 4 °C. | [108] | |||
| Staphylococcus sp. | Infections of the skin and subcutaneous tissue, which are characterized by the presence of purulent discharge, impetigo, folliculitis, boils, furunculosis (multiple boils), abscesses, inflammation of the sweat glands and inflammation of the mammary gland, high fever, drop in blood pressure, organ dysfunction | Transmission mainly by direct contact. Patients after surgery are most at risk | MDR, ME18, ME126 | Reducing biofilm in UHT milk at 25 °C using ME18 (MOI = 10) and MDR. They reduce biofilm in milk. However ME126 (MOI = 10) at 37 °C reduces CFU/mL by 87.2% compared to control sample. | [109] |
| Vibrio parahaemolyticus | Watery diarrhea, abdominal cramps, nausea, vomiting, fever or chills, abscess formation, otitis media, otitis media and conjunctivitis | Contact with contaminated water, fruit, seafood | PVP1 and PVP2 | They treated sea cucumber contaminated with pathogen. MOI = 10 or MOI = 100. Test were performed at in 20 °C and it increased survival of sea cucumber to 80% compared with control sample without phage cocktail treatment, which was only 30%. | [110] |
| Yersinia | Mild or high fever, cramping abdominal pain, loose stools often with mucus or blood, vomiting, right-hand stomach pain, tenderness when examining the abdominal cavity, fast heartbeat, joint pains, mainly in the knee, ankle and wrist, rapid breathing | Pork and pork offal, milk, water, raw vegetables and fruits. | fHe-Yen3-01 fHe-Yen9-01, fHe-Yen9-02 and fHe-Yen9-03 | Infected raw pork and cooking tools with the Rukola/71 strain. The kitchen tools were immersed in an inoculum at a concentration of 104 CFU/mL. The best effect was obtained for the phage fHe-Yen9-01, which reduced the number of bacteria by 1/3. | [86] |
| PY100 | The given phage reduced the amount of bacteria in the meat MOI = 102 by 3 log10 units after 24 h incubation and at a MOI = 104 by 5 log10 units after 1.5 h incubation at 37 °C. However, when incubated at 4 °C, the bacteria count decreased by 2 log units after 24 h. | [111] |
| Company | Product | Target | Reference | Regulatory & Certifications |
|---|---|---|---|---|
| Micreos Food Safety (The Netherlands) | PhageGuard Listex | Listeria sp. | [65,112,113] | Halal, OMRI, Kosher, Skal, FSSC 2200; FDA, GRN 198/21, EFSA; Swiss BAG; Israel Ministry of Health; Health Canada |
| PhageGuard S | Salmonella enterica | [114] | Halal, FSSC 22000; FDA, GRN 468; USDA, FSIS Directive 7120.1, Swiss BAG | |
| PhageGuard E | Escherichia coli O157:H7 | - | FSSC 22000 | |
| Intralytix (USA) | ListShield | Listeria monocytogenes | [3,66,115,116,117] | Kosher; Halal; OMRI; FDA, 21 CFR 172.785; FDA, GRN 528; |
| SalmoFresh | Salmonella enterica | [5,49,118,119,120] | Kosher; Halal; OMRI FDA, GRN 435; USDA, FSIS Directive 7120.1 | |
| ShigaShield | Shigella sp. | [88,121,122] | FDA, GRN 672 | |
| EcoShield PX | Escherichia coli | [9,123,124,125] | FDA, GRN 834; USDA, FSIS Directive 7120.1 | |
| CampyloShield | Campylobacter spp. | [126] | GRAS | |
| Proteon Pharmaceuticals SA (Poland) | Bafasal | Salmonella enterica | [96,127] | - |
| Bafador | Pseudomonas sp., Aeromonas sp. | - | - | |
| Passport Food Safety Solutions | Finalyse | E. coli O157:H7 | - | USDA, FSIS Directive 7120.1 |
| Phagelux | SalmoPro | Salmonella spp. | - | FDA, GRN 603; USDA |
| FINK TEC GmbH (Hamm, Germany) | Secure Shield E1 | E. coli | [93] | FDA,GRN 724 |
| Arm and Hammer Animal & Food Production (USA) | Finalyse SAL | Salmonella | [128] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyla, K.; Dusza, I.; Skaradzińska, A. Recent Advances in the Application of Bacteriophages against Common Foodborne Pathogens. Antibiotics 2022, 11, 1536. https://doi.org/10.3390/antibiotics11111536
Hyla K, Dusza I, Skaradzińska A. Recent Advances in the Application of Bacteriophages against Common Foodborne Pathogens. Antibiotics. 2022; 11(11):1536. https://doi.org/10.3390/antibiotics11111536
Chicago/Turabian StyleHyla, Kinga, Izabela Dusza, and Aneta Skaradzińska. 2022. "Recent Advances in the Application of Bacteriophages against Common Foodborne Pathogens" Antibiotics 11, no. 11: 1536. https://doi.org/10.3390/antibiotics11111536
APA StyleHyla, K., Dusza, I., & Skaradzińska, A. (2022). Recent Advances in the Application of Bacteriophages against Common Foodborne Pathogens. Antibiotics, 11(11), 1536. https://doi.org/10.3390/antibiotics11111536







