Antimicrobial Photosensitizing Material Based on Conjugated Zn(II) Porphyrins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedures
2.2. Spectroscopic Determinations
2.3. Photooxidation of 9,10-Dimethylanthracene (DMA)
2.4. Photoreduction of Nitro Blue Tetrazolium (NBT)
2.5. Bacterial Strains and Growth Conditions
2.6. Photosensitized Inactivation of Bacterial Suspensions
2.7. Photosensitized Inactivation of Bacteria with PZnTEP Adsorbed on a PLA Surface
2.8. Statistical Analysis
3. Results and Discussion
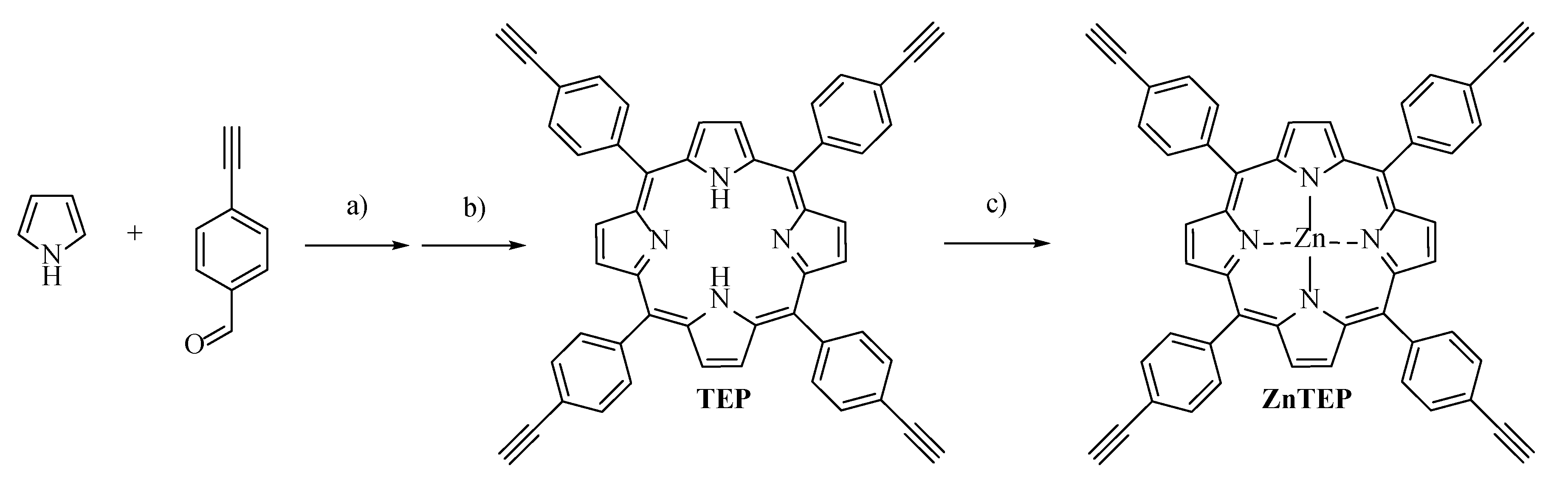
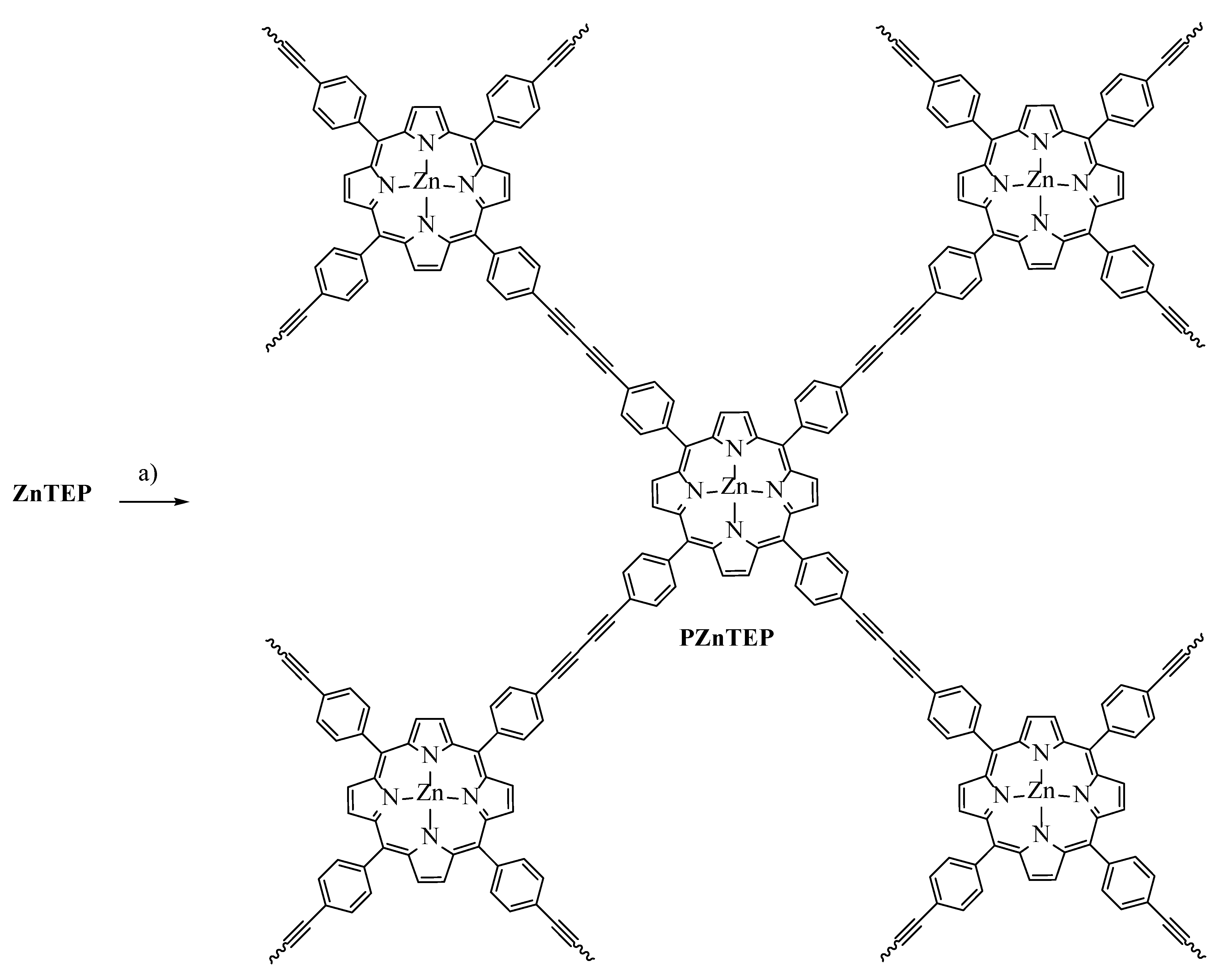
3.1. Synthesis of ZnTEP and PZnTEP
3.2. SEM Images of the Polymer
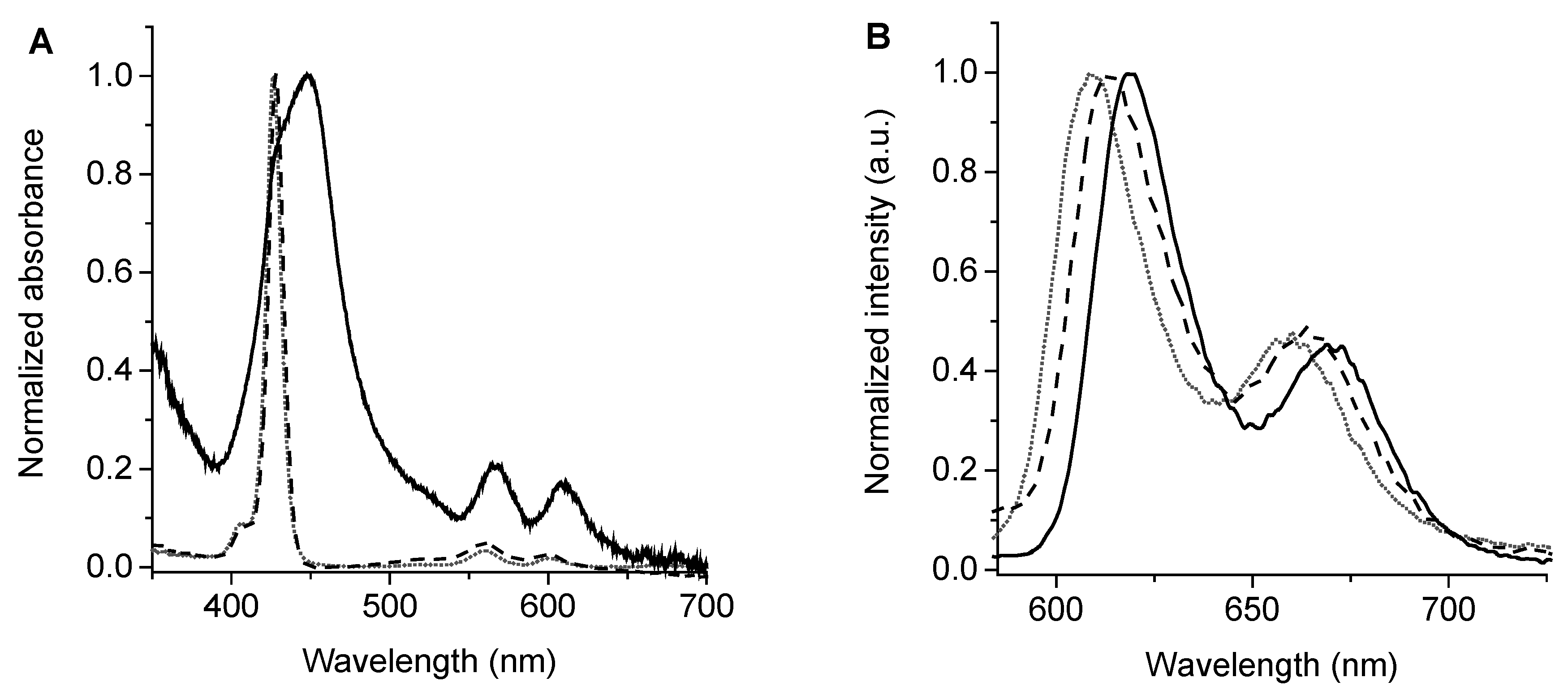
3.3. Absorption and Fluorescence Spectroscopic Characterization
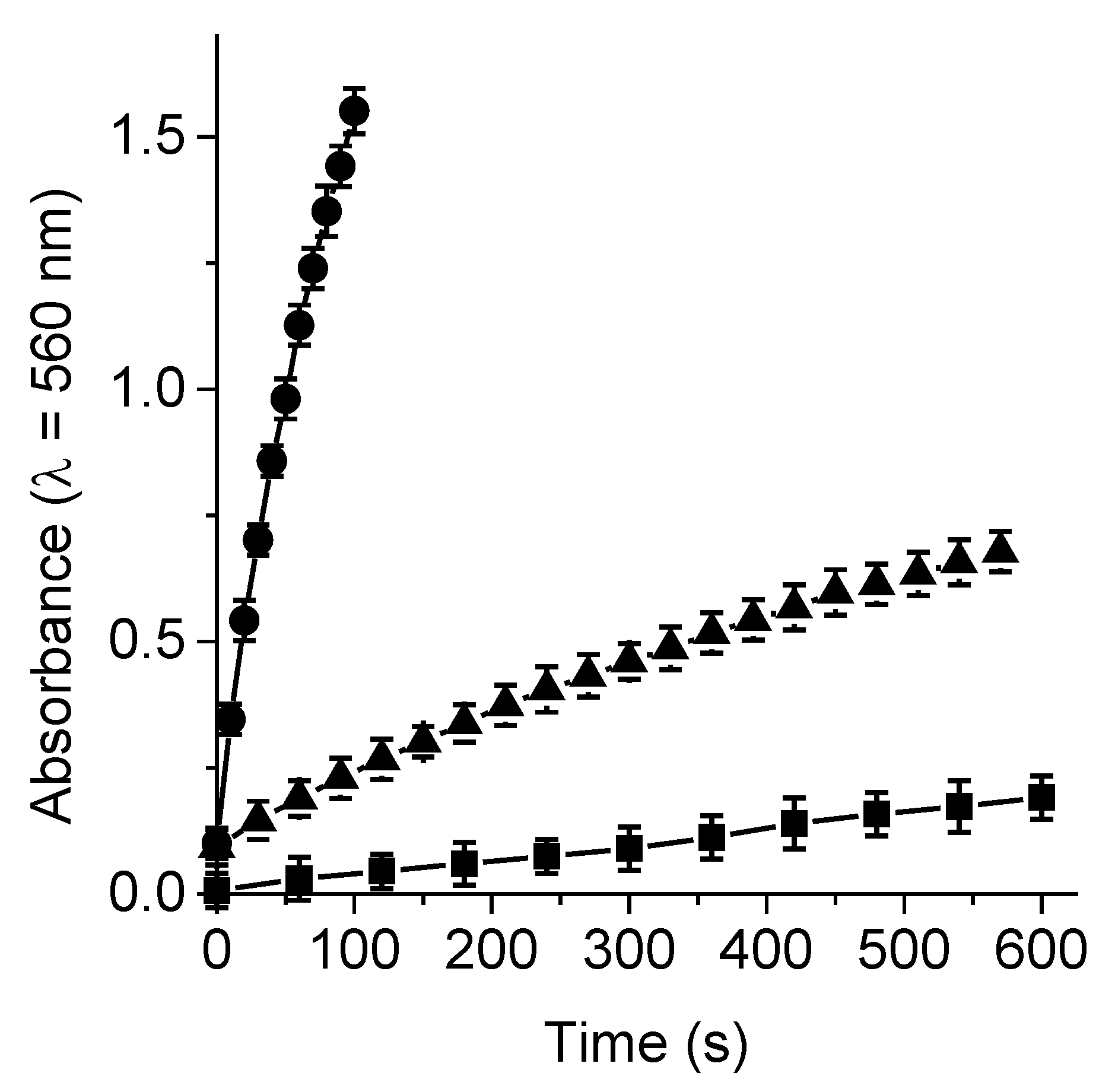
3.4. Production of O2(1Δg)
3.5. Formation of O2•−
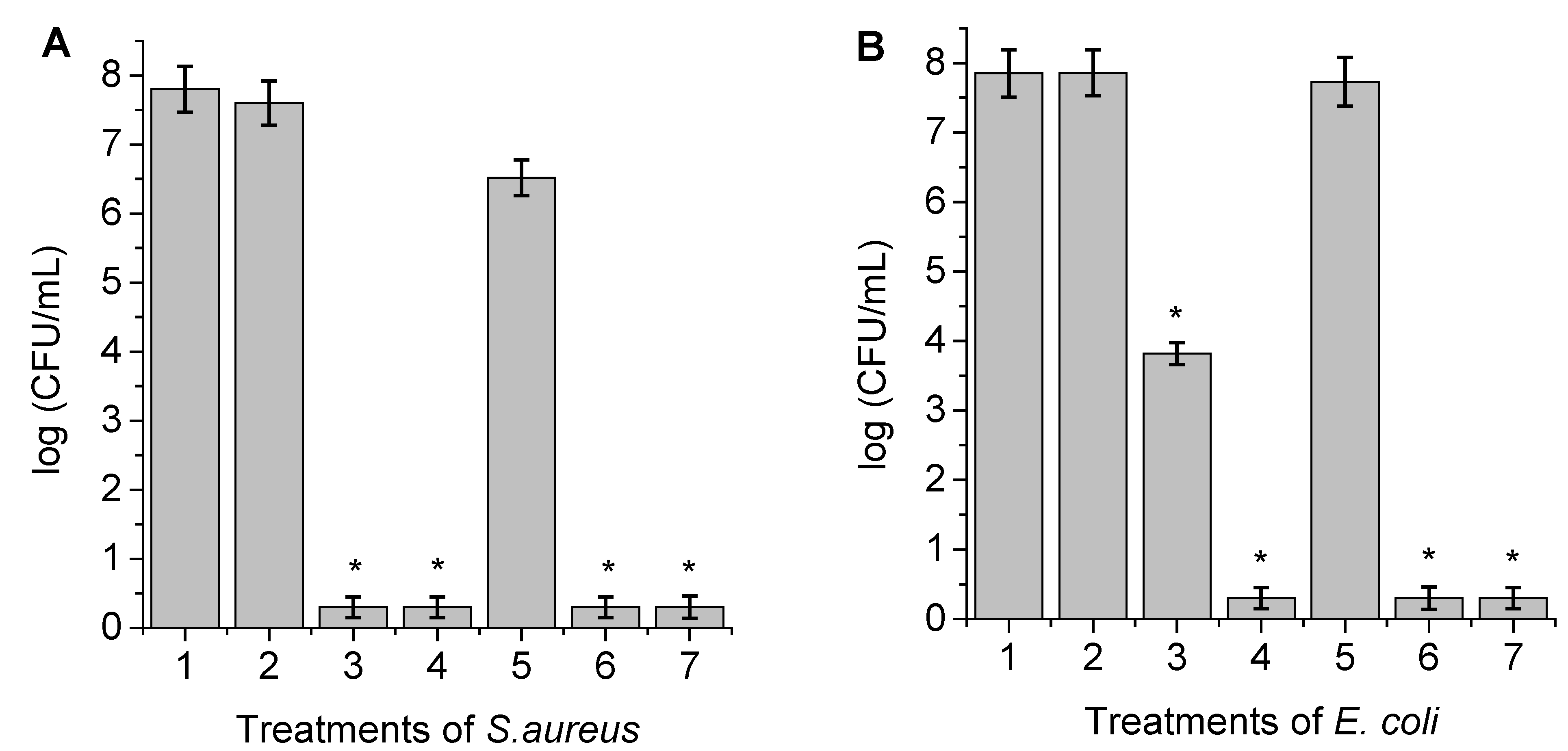
3.6. Photosensitized Inactivation of Bacteria
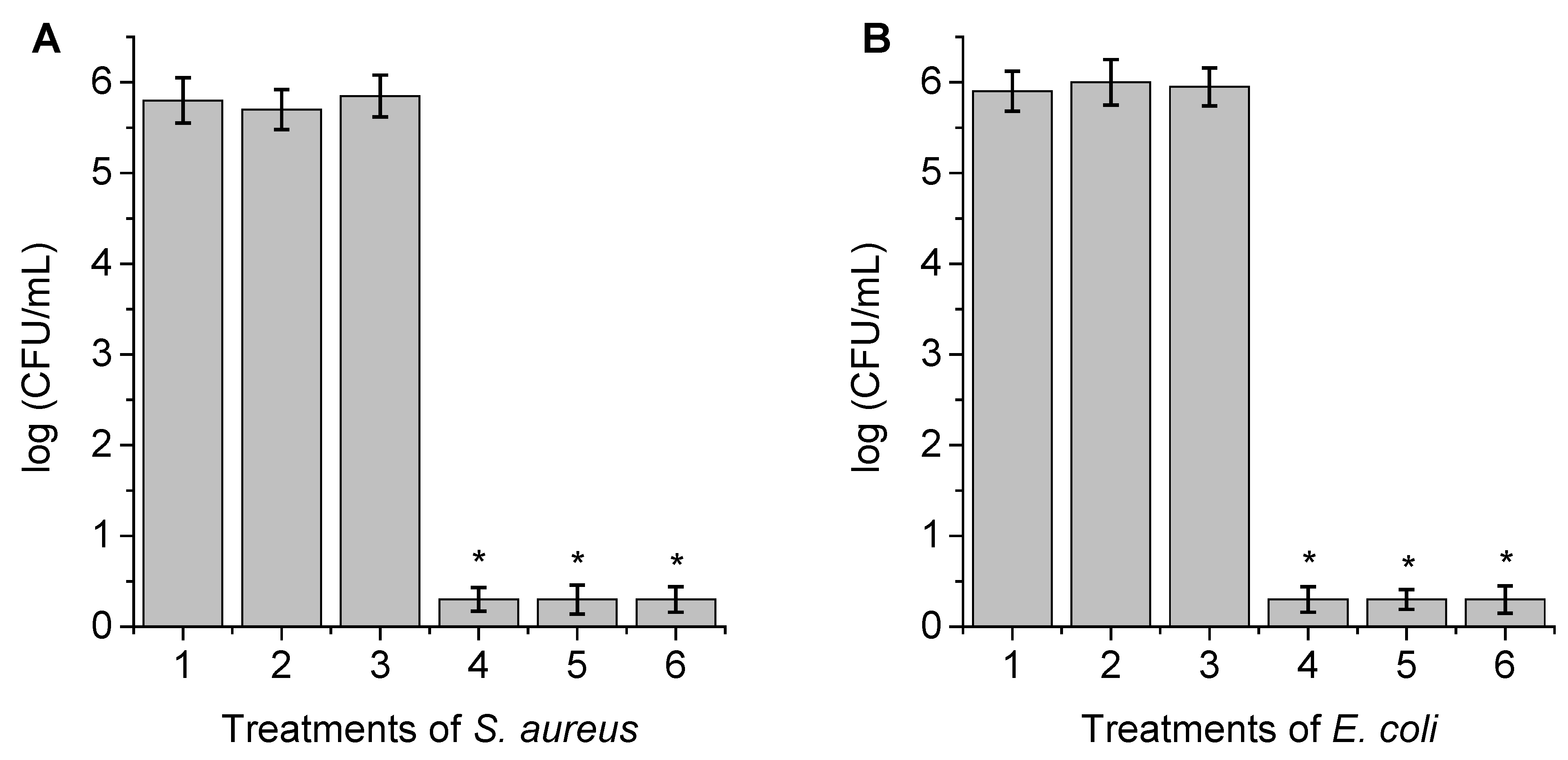
3.7. Photosensitized Inactivation of Bacteria with PZnTEP Coating a Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassoun-Kheir, N.; Stabholz, Y.; Kreft, J.-U.; de la Cruz, R.; Romalde, J.L.; Nesme, J.; Sørensen, S.J.; Smets, B.F.; Gra-ham, D.; Paul, M. Comparison of antibiotic-resistant bacteria and antibiotic resistance genes abundance in hospital and community wastewater: A systematic review. Sci. Total Environ. 2020, 743, 140804. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, M.N.; Thomason, M.K.; Hsu, C.; Nugent, P.; Gage, J.; Samadpour, A.N.; Kariisa, A.; Merrikh, C.N.; Miller, S.I.; Sherman, D.R.; et al. Inhibiting the evolution of antibiotic resistance. Mol. Cell 2019, 73, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.-H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Olofsson, M.; Matussek, A.; Ehricht, R.; Lindgren, P.-E.; Östgren, C.J. Differences in molecular epidemiology of Staphylococcus aureus and Escherichia coli in nursing home residents and people in unassisted living situations. J. Hosp. Infect. 2019, 101, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Shankar, N.; Soe, P.-m.; Tam, C.C. Prevalence and risk of acquisition of methicillin-resistant Staphylococcus aureus among households: A systematic review. Int. J. Infect. Dis. 2020, 92, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Dunn, S.J.; Connor, C.; McNally, A. The evolution and transmission of multi-drug resistant Escherichia coli and Klebsiella pneumoniae: The complexity of clones and plasmids. Curr. Opin. Microbiol. 2019, 51, 51–56. [Google Scholar] [CrossRef]
- Aijuka, M.; Buys, E.M. Persistence of foodborne diarrheagenic Escherichia coli in the agricultural and food production environment: Implications for food safety and public health. Food Microbiol. 2019, 82, 363–370. [Google Scholar] [CrossRef]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.D.C.; Teixeira, J.A.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial photodynamic therapy: Latest developments with a focus on combinatory strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Szaciłowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic photochemistry: Frontiers and mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet oxygen: There is still something new under the sun, and it is better than ever Photochem. Photobiol. Sci. 2010, 9, 1543–1560. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Durantini, A.M.; Heredia, D.A.; Durantini, J.E.; Durantini, E.N. BODIPYs to the rescue: Potential applications in photodynamic inactivation. Eur. J. Med. Chem. 2018, 144, 651–661. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Zhang, W. Synthesis, self-assembly and applications of functional polymers based on porphyrins. Prog. Polym. Sci. 2019, 95, 65–117. [Google Scholar] [CrossRef]
- Ji, W.; Wang, T.-X.; Ding, X.; Lei, S.; Han, B.-H. Porphyrin- and phthalocyanine-based porous organic polymers: From synthesis to application. Coord. Chem. Rev. 2021, 439, 213875. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Gao, S.; Jin, Y.; Zhao, W.; Liu, H.; Yang, X.; Hu, S.; Cheng, K.; Zhang, J. Porous organic polymer-coated band-aids for phototherapy of bacteria-induced wound infection. ACS Appl. Bio Mater. 2019, 2, 613–618. [Google Scholar] [CrossRef]
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Durantini, E.N. Antimicrobial photodynamic polymeric films bearing biscarbazol triphenylamine end-capped dendrimeric Zn(II) porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. [Google Scholar] [CrossRef]
- Milanesio, M.E.; Alvarez, M.G.; Yslas, E.I.; Borsarelli, C.D.; Silber, J.J.; Rivarola, V.; Durantini, E.N. Photodynamic studies of metallo 5,10,15,20-tetrakis(4-methoxyphenyl) porphyrin: Photochemical characterization and biological consequences in a human carcinoma cell line. Photochem. Photobiol. 2001, 74, 14–21. [Google Scholar] [CrossRef]
- Ferreyra, D.D.; Reynoso, E.; Cordero, P.; Spesia, M.B.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Synthesis and properties of 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl] chlorin as potential broad-spectrum antimi-crobial photosensitizers. J. Photochem. Photobiol. B Biol. 2016, 158, 243–251. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Durantini, J.E.; Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. A novel tricationic fullerene C60 as broad-spectrum antimicrobial photosensitizer: Mechanisms of action and potentiation with potassium iodide. Photochem. Photobiol. Sci. 2021, 20, 327–341. [Google Scholar] [CrossRef]
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin-schiff base conjugates bearing basic amino groups as antimicrobial phototherapeutic agents. Molecules 2021, 26, 5877. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.R.; Palacios, Y.B.; Heredia, D.A.; Aiassa, V.; Bartolilla, A.; Durantini, A.M. Self-sterilizing 3D-printed polylactic acid surfaces coated with a BODIPY photosensitizer. ACS Appl. Mater. Interfaces 2021, 13, 11597–11608. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Guo, J.; Wang, C. Gelation of metalloporphyrin-based conjugated microporous polymers by oxidative homocoupling of terminal alkynes. Chem. Mater. 2014, 26, 6241–6250. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, S.; Han, L.; Zhang, Z.; Xue, Z.; Gao, J.; Li, Y.; Huang, C.; Yi, Y.; Liu, H.; et al. High conductive two-dimensional covalent organic framework for lithium storage with large capacity. ACS Appl. Mater. Interfaces 2016, 8, 5366–5375. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Li, C.; Liu, G.; Luo, X.; Wu, F. Oxidase mimetic activity of a metalloporphyrin-containing porous organic polymer and its applications for colorimetric detection of both ascorbic acid and glutathione. ACS Sustain. Chem. Eng. 2021, 9, 5412–5421. [Google Scholar] [CrossRef]
- Meng, S.; Ma, H.; Jiang, L.; Ren, H.; Zhu, G. A facile approach to prepare porphyrinic porous aromatic frameworks for small hydrocarbon separation. J. Mater. Chem. A 2014, 2, 14536–14541. [Google Scholar] [CrossRef]
- Scalise, I.; Durantini, E.N. Photodynamic effect of metallo 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl)porphyrins in biomimetic AOT reverse micelles containing urease. J. Photochem. Photobiol. A Chem. 2004, 162, 105–113. [Google Scholar] [CrossRef]
- Spellane, P.J.; Gouterman, M.; Antipas, A.; Kim, S.; Liu, Y.C. Porphyrins. 40. Electronic spectra and four-orbital energies of free-base, zinc, copper, and palladium tetrakis(perfluorophenyl)-porphyrins. Inorg. Chem. 1980, 19, 386–391. [Google Scholar] [CrossRef]
- Gsponer, N.S.; Agazzi, M.L.; Spesia, M.B.; Durantini, E.N. Approaches to unravel pathways of reactive oxygen species in the photoinactivation of bacteria induced by a dicationic fulleropyrrolidinium derivative. Methods 2016, 109, 167–174. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, D.D.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Synthesis and photodynamic properties of 5,10,15,20-tetrakis[3-(N-ethyl-N-methylcarbazoyl)]chlorin and itsanalogous porphyrin in solution and in human red blood cells. J. Photochem. Photobiol. A Chem. 2014, 282, 16–24. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Mechanistic insight into the photodynamic effect mediated by porphyrin-fullerene C60 dyads in solution and in Staphylococcus aureus cells. RSC Adv. 2018, 8, 22876–22886. [Google Scholar] [CrossRef] [Green Version]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [Green Version]
- Reynoso, E.; Quiroga, E.D.; Agazzi, M.L.; Ballatore, M.B.; Bertolotti, S.G.; Durantini, E.N. Photodynamic inactivation of microorganisms sensitized by cationic BODIPY derivatives potentiated by potassium iodide. Photochem. Photobiol. Sci. 2017, 16, 1524–1536. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; El-Hussein, A.; Xuan, W.; Hamblin, M.R. Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J. Photochem. Photobiol. B Biol. 2018, 178, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Baigorria, E.; Durantini, J.E.; Martínez, S.R.; Milanesio, M.E.; Palacios, Y.B.; Durantini, A.M. Potentiation effect of iodine species on the antimicrobial capability of surfaces coated with electroactive phthalocyanines. ACS Appl. Bio. Mater. 2021, 4, 8559–8570. [Google Scholar] [CrossRef]
- Mosinger, J.; Janoškova, M.; Lang, K.; Kubat, P. Lightinduced aggregation of cationic porphyrins. J. Photochem. Photobiol. A Chem. 2006, 181, 283–289. [Google Scholar] [CrossRef]
- Vieira, C.; Gome, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An insight into the potentiation effect of potassium iodide on aPDT efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballatore, M.B.; Durantini, J.; Gsponer, N.S.; Suarez, M.B.; Gervaldo, M.; Otero, L.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Photodynamic inactivation of bacteria using novel electrogenerated porphyrin-fullerene C60 polymeric films. Environ. Sci. Technol. 2015, 49, 7456–7463. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Photodynamic properties and photoinactivation of microorganisms mediated by 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin covalently linked to silica-coated magnetite nanoparticles. Photochem. Photobiol. A Chem. 2017, 346, 452–461. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Milanesio, M.E.; Fujita, H.; Lindsey, J.S.; Durantini, E.N. Bacteriochlorinbis(spermine) conjugate affords an effective photodynamic action to eradicate microorganisms. J. Biophotonics 2020, 13, e201960061. [Google Scholar] [CrossRef]


| PS | λSoret (nm) | εSoret a | λem (nm) | ΦF b | kobsDMA (s−1) c | ΦΔ d |
|---|---|---|---|---|---|---|
| ZnTMP | 426 | 4.07 × 105 | 608 | 0.049 ± 0.004 | (2.02 ± 0.02) × 10−2 | 0.73 ± 0.03 |
| ZnTEP | 428 | 5.56 × 105 | 612 | 0.030 ± 0.003 | (1.60 ± 0.02) × 10−2 | 0.57 ± 0.03 |
| PZnTEP | 446 | - | 620 | 0.008 ± 0.002 | (5.30 ± 0.08) × 10−4 | 0.019 ± 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamarina, S.C.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Antimicrobial Photosensitizing Material Based on Conjugated Zn(II) Porphyrins. Antibiotics 2022, 11, 91. https://doi.org/10.3390/antibiotics11010091
Santamarina SC, Heredia DA, Durantini AM, Durantini EN. Antimicrobial Photosensitizing Material Based on Conjugated Zn(II) Porphyrins. Antibiotics. 2022; 11(1):91. https://doi.org/10.3390/antibiotics11010091
Chicago/Turabian StyleSantamarina, Sofía C., Daniel A. Heredia, Andrés M. Durantini, and Edgardo N. Durantini. 2022. "Antimicrobial Photosensitizing Material Based on Conjugated Zn(II) Porphyrins" Antibiotics 11, no. 1: 91. https://doi.org/10.3390/antibiotics11010091
APA StyleSantamarina, S. C., Heredia, D. A., Durantini, A. M., & Durantini, E. N. (2022). Antimicrobial Photosensitizing Material Based on Conjugated Zn(II) Porphyrins. Antibiotics, 11(1), 91. https://doi.org/10.3390/antibiotics11010091







