Antifungal Carvacrol Loaded Chitosan Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. NPs Preparation
2.2. Bioassay Guided Nanoparticles Optimization
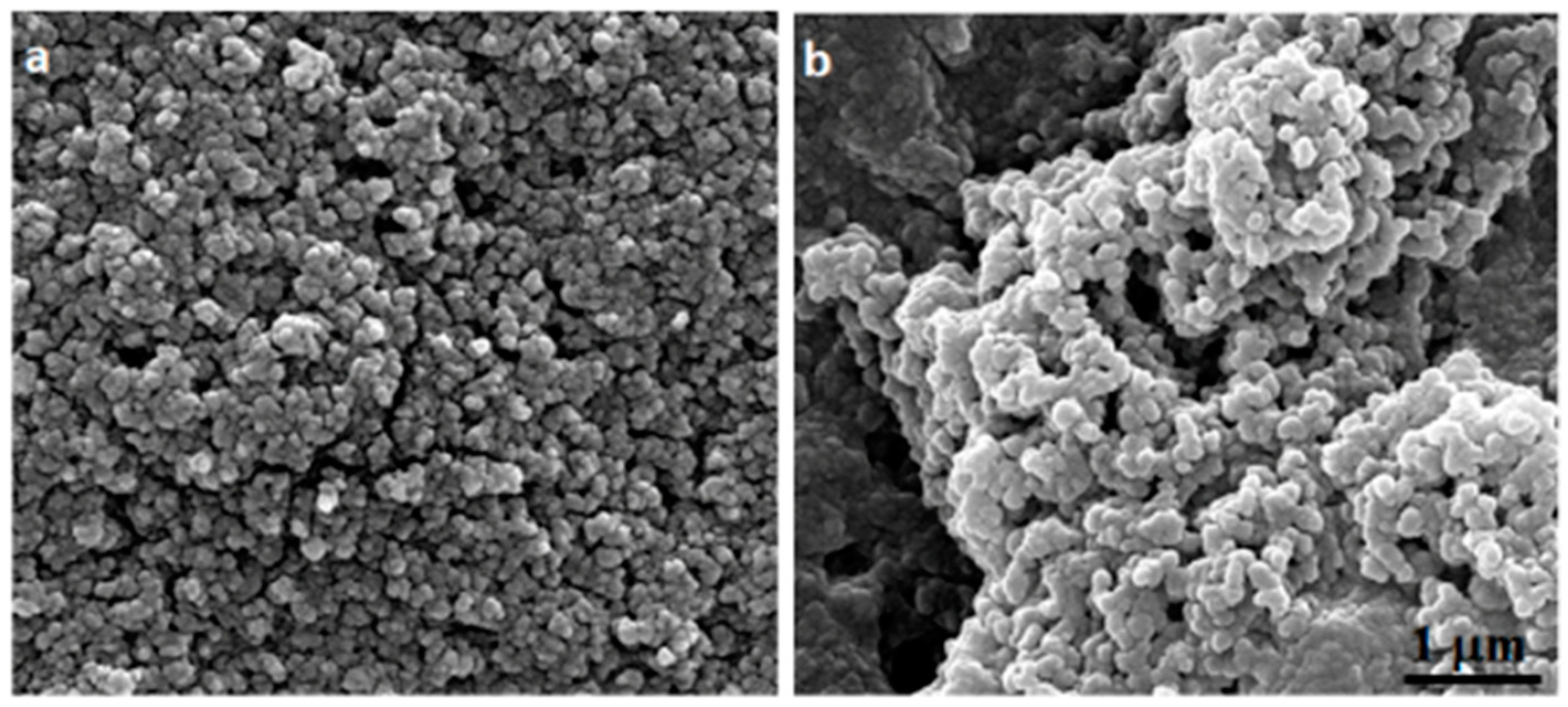
2.3. Cs–Cv-NPs Characterization
2.4. Evaluation of Antimicrobial Activity
2.5. Inhibition on Pre-Formed and Forming Biofilm by Cv–Cs-NPs in Candida spp.
3. Materials and Methods
3.1. Chemicals
3.2. Nanoparticles Preparation
3.3. Nanoparticles Characterization
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
3.3.3. Dynamic Light Scattering
3.3.4. Evaluation of Encapsulation Efficiency and Loading Capacity
3.4. Release Kinetics
3.5. Organisms and Growth Conditions
3.6. Evaluation of Antimicrobial Activity
3.7. In Vitro Time–Kill Kinetics
3.8. Biofilm Formation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm inhibition by biocompatible poly(ε-caprolactone) nanocapsules loaded with essential oils and their cyto/genotoxicity to human keratinocyte cell line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef]
- Beena, S.; Kumar, D.; Rawat, D.S. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg. Med. Chem. Lett. 2013, 23, 641–645. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of Essential Oils Obtained from Abruzzo Autochthonous Plants: Antioxidant and Antimicrobial Activities Assessment for Food Application. Foods 2018, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.S.; Yadegari, M.H.; Rajabibazl, M.; Ghaemi, E.A. Inhibitory effects of carvacrol on the expression of secreted aspartyl proteinases 1-3 in fluconazole-resistant Candida albicans isolates. Iran. J. Microbiol. 2016, 8, 401–409. [Google Scholar]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Fahimirad, S. Efficacy of medicinal essential oils against pathogenic Malassezia sp. isolates. J. Mycol. Med. 2016, 26, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, V.; Rojas, F.; Tedesco, V.; Giusiano, G.; Angiolella, L. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Nat. Prod. Res. 2019, 33, 3273–3277. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol induces Candida albicans apoptosis associated with Ca2+/calcineurin pathway. Front. Cell. Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Bermúdez, J.M.; Aucejo, S.; Cameán, A.M. Cytotoxicity and morphological effects induced by carvacrol and thymol on the human cell line Caco-2. Food Chem. Toxicol. 2014, 64, 281–290. [Google Scholar] [CrossRef]
- Tavares, A.G.; Andrade, J.; Resende, R.; Silva, A.; Suprani Marques, C.; Ramos da Silva, J.O.; Dantas Vanetti, M.C.; Ramos de Melo, N.; de Fátima Ferreira Soar, N. Carvacrol-loaded liposome suspension: Optimization, characterization and incorporation into poly(vinyl alcohol) films. Food Funct. 2021, 12, 6549–6557. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Shakeri, S.; Hojjatoleslami, M. Preparation and characterization of carvacrol loaded polyhydroxybutyrate nanoparticles by nanoprecipitation and dialysis methods. J. Food Sci. 2014, 79, N697–N705. [Google Scholar] [CrossRef] [PubMed]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.; Grande, R.; Di Stefano, A.; Di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Webb, J.P.; Green, J.; Smith, L.J.; Laird, K. From formulation to in vivo model: A comprehensive study of a synergistic relationship between vancomycin, carvacrol, and cuminaldehyde against Enterococcus faecium. Phytother. Res. 2020, 34, 1638–1649. [Google Scholar] [CrossRef]
- Tripathy, A.; Pahal, S.; Mudakavi, R.J.; Raichur, A.M.; Varma, M.M.; Sen, P. Impact of Bioinspired Nanotopography on the Antibacterial and Antibiofilm Efficacy of Chitosan. Biomacromolecules 2018, 19, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Je, H.J.; Kim, E.S.; Lee, J.S.; Lee, H.G. Release Properties and cellular uptake in Caco-2 cells of size-controlled chitosan nanoparticles. J. Agric. Food Chem. 2017, 65, 10899–10906. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Wang, T.M.; Di, L. Natural Products with Activity against Lung Cancer: A Review Focusing on the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10827. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Blagodatskikh, I.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of Candida albicans: Molecular weight-activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Barbos, M.; Vale, N.; Costa, F.M.T.A.; Martins, M.C.L.; Gomes, P. Tethering antimicrobial peptides onto chitosan: Optimization of azide-alkyne “click” reaction conditions. Carbohydr. Polym. 2017, 165, 384–393. [Google Scholar] [CrossRef]
- Layek, B.; Lipp, L.; Singh, J. Cell Penetrating Peptide Conjugated Chitosan for Enhanced Delivery of Nucleic Acid. Int. J. Mol. Sci. 2015, 16, 28912–28930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, N.S.; Shirazi, A.N.; El-Meligy, M.G.; El-Ziaty, A.K.; Nagieb, Z.A.; Parang, K.; Tiwari, R.K. Design, synthesis, and evaluation of chitosan conjugated GGRGDSK peptides as a cancer cell-targeting molecular transporter. Int. J. Biol. Macromol. 2016, 87, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida spp. in the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Zhu, Y.; Thangaraj, B.; Abdel-Samie, M.A.S.; Cui, H. Improving the stability of thyme essential oil solid liposome by using β-cyclodextrin as a cryoprotectant. Carbohydr. Polym. 2018, 188, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Ives, J.A.; Jonas, W.B. Nonlinear effects of nanoparticles: Biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose Response 2013, 12, 202–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keawchaoon, L.; Rangrong, Y. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Kashanian, S.; Askari, M. The effect of ultrasound waves on levothyroxine release from chitosan nanoparticles. Adv. Mater. Res. 2014, 829, 284–288. [Google Scholar] [CrossRef]
- Alarfaj, J. Preparation, Characterization and Antibacterial Effect of Chitosan Nanoparticles against Food Spoilage Bacteria. Pure Appl. Microbiol. 2019, 13, 1273–1278. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Kim, D.Y.; Lee, J.S.; Lee, H.G. Quercetin delivery characteristics of chitosan nanoparticles prepared with different molecular weight polyanion cross-linkers. Carbohydr. Polym. 2021, 267, 118157. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef]
- Nallamuthua, I.; Devib, A.; Khanuma, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Sobhani, Z.; Mohammadi Samani, S.; Montaseri, H.; Khezri, E. Nanoparticles of chitosan loaded ciprofloxacin: Fabrication and antimicrobial activity. Adv. Pharm. Bull. 2017, 7, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Mussin, J.; Robles-Botero, V.; Casañas-Pimentel, R.; Rojas, F.; Angiolella, L.; San Martín-Martínez, E.; Giusiano, G. Antimicrobial and cytotoxic activity of green synthesis silver nanoparticles targeting skin and soft tissue infectious agents. Sci. Rep. 2021, 11, 14566. [Google Scholar] [CrossRef]
- Walker, L.A.; Munro, C.A. Spongin induced cell wall changes of Candida species influences macrophage interactions. Front. Cell. Infect. Microbiol. 2020, 10, 164. [Google Scholar] [CrossRef]
- Angiolella, L.; Maras, B.; Stringaro, A.R.; Arancia, G.; Mondello, F.; Girolamo, A.; Palamara, A.T.; Cassone, A. Glucan-associated protein modulations and ultrastructural changes of the cell wall in Candida albicans treated with micafungin, a water-soluble, lipopeptide antimycotic. J. Chemother. 2005, 4, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Stringaro, A.; Vavala, E.; Colone, M.; Pepi, F.; Mignogna, G.; Garzoli, S.; Cecchetti, S.; Ragno, R.; Angiolella, L. Effects of Mentha suaveolens Essential Oil Alone or in Combination with Other Drugs in Candida albicans. Evid.-Based Complement. Altern. Med. 2014, 2014, 125904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, L.R.; Radu Mihu, M.; Tar, M.; Cordero, R.J.B.; Han, G.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Demonstration of antibiofilm and antifungal efficacy of chitosan against Candidal biofilms, using an in vivo central venous catheter model. J. Infect. Dis. 2010, 201, 1436–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Felipe, V.; Breser, M.L.; Bohl, L.P.; Rodrigues da Silva, E.; Morgante, C.A.; Correa, S.G.; Porporatto, C. Chitosan disrupts biofilm formation and promotes biofilm eradication in Staphylococcus species isolated from bovine mastitis. Int. J. Biol. Macromol. 2019, 126, 60–67. [Google Scholar] [CrossRef]
- Velino, C.; Carella, F.; Adamiano, A.; Sanguinetti, M.; Vitali, A.; Catalucci, D.; Bugli, F.; Iafisco, M. Nanomedicine Approaches for the Pulmonary Treatment of Cystic Fibrosis. Front. Bioeng. Biotechnol. 2019, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Bizerra, F.C.; Nakamura, C.V.; de Poersch, C.; Estivalet Svidzinski, T.I.; Borsato Quesada, R.M.; Goldenberg, S.; Krieger, M.A.; Yamada-Ogatta, S.F. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008, 8, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramage, G.; Vande Walle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Material | Cs/Cv (w/w) | MIC µg/mL |
|---|---|---|
| Cs | - | >2400 |
| Cv | - | 1200 |
| Cs-NPs | - | 1580 |
| Cv–Cs NPs | 1:0.25 | >2400 |
| Cv–Cs-NPs | 1:0.50 | >2400 |
| Cv–Cs-NPs | 1:0.75 | >2400 |
| Cv–Cs-NPs | 1:1 | >2400 |
| Cv–Cs-NPs | 1:1.25 | 1200 |
| Cv–Cs-NPs | 1:1.50 | 24 |
| Cv–Cs-NP | Cs-NP | |
|---|---|---|
| Hydrodynamic diameter (nm) | 281.6 ± 2 | 384.5 ± 3 |
| Polydispersity index (PdI) | 0.235 ± 0.03 | 0.2475 ± 0.02 |
| Strains | Cs | Cv | Cs-NPs | Cv–Cs-NPs 1:1.50 |
|---|---|---|---|---|
| C. albicans AIDS68 | 1560 | 780 | 1560 | 24 |
| C. tropicalis 47829 | >3120 | 780 | 3120 | 1560 |
| C. krusei 45709 | 1560 | 780 | 3120 | 1560 |
| C. glabrata SFY115 | 1560 | 780 | 1560 | 780 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitali, A.; Stringaro, A.; Colone, M.; Muntiu, A.; Angiolella, L. Antifungal Carvacrol Loaded Chitosan Nanoparticles. Antibiotics 2022, 11, 11. https://doi.org/10.3390/antibiotics11010011
Vitali A, Stringaro A, Colone M, Muntiu A, Angiolella L. Antifungal Carvacrol Loaded Chitosan Nanoparticles. Antibiotics. 2022; 11(1):11. https://doi.org/10.3390/antibiotics11010011
Chicago/Turabian StyleVitali, Alberto, Annarita Stringaro, Marisa Colone, Alexandra Muntiu, and Letizia Angiolella. 2022. "Antifungal Carvacrol Loaded Chitosan Nanoparticles" Antibiotics 11, no. 1: 11. https://doi.org/10.3390/antibiotics11010011
APA StyleVitali, A., Stringaro, A., Colone, M., Muntiu, A., & Angiolella, L. (2022). Antifungal Carvacrol Loaded Chitosan Nanoparticles. Antibiotics, 11(1), 11. https://doi.org/10.3390/antibiotics11010011










