Empiric “Three-in-One” Bismuth Quadruple Therapy for Second-Line Helicobacter pylori Eradication: An Intervention Study in Southern Italy
Abstract
:1. Introduction
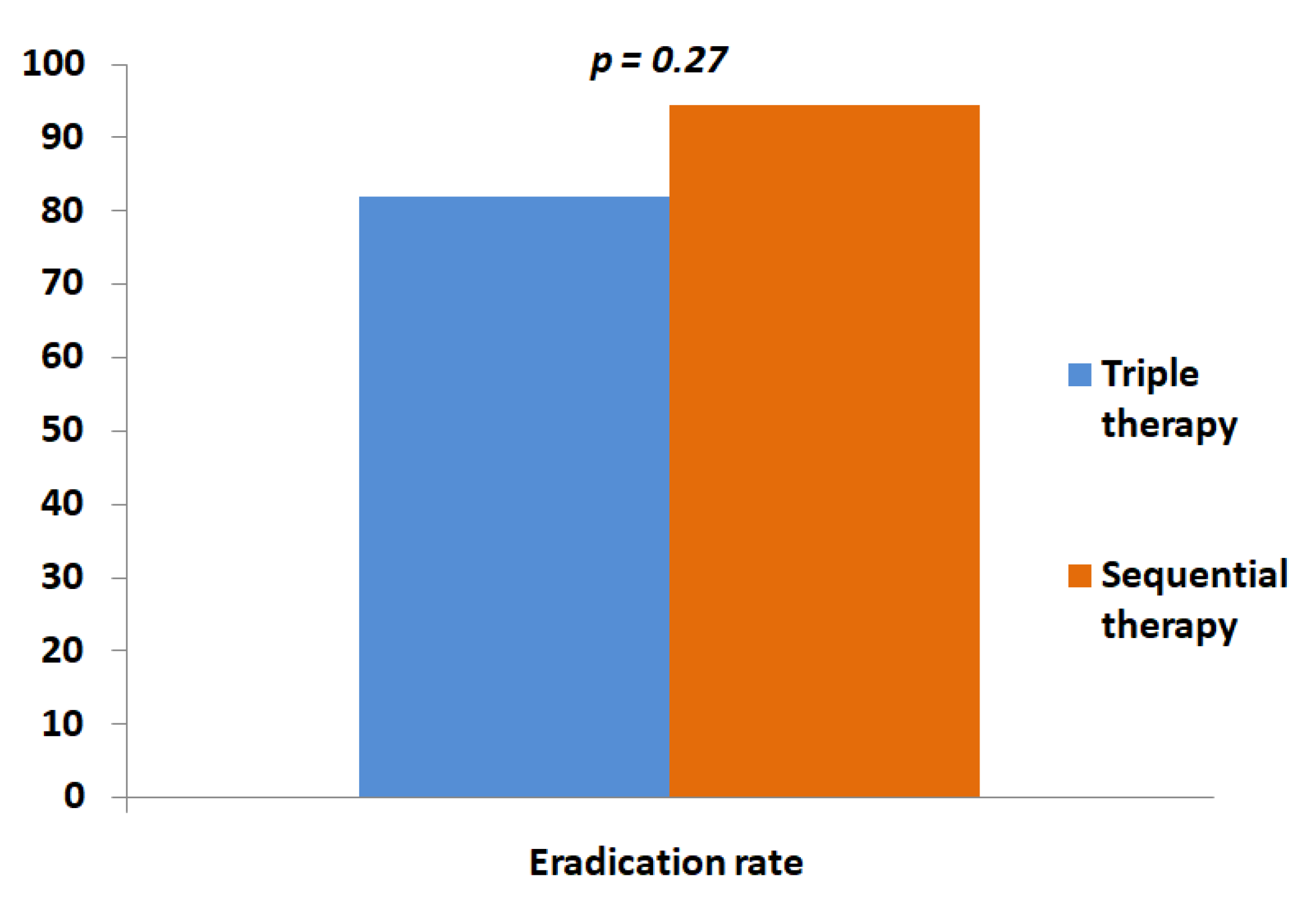
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. H. pylori Investigation
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodwin, C.S.; Mendall, M.M.; Northfield, T.C. Helicobacter pylori infection. Lancet 1997, 349, 265–269. [Google Scholar] [CrossRef]
- Sugano, K.; Tack, J.; Kuipers, E.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef]
- Ierardi, E.; Goni, E.; Losurdo, G.; Di Mario, F. Helicobacter pylori and nonmalignant diseases. Helicobacter 2014, 19 (Suppl. 1), 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Giorgio, F.; Losurdo, G.; Di Leo, A.; Principi, M. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World J. Gastroenterol. 2013, 19, 8168–8180. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Pellicano, R.; Ribaldone, D.G.; Fagoonee, S.; Astegiano, M.; Saracco, G.M.; Mégraud, F. A 2016 panorama of Helicobacter pylori infection: Key messages for clinicians. Panminerva Med. 2016, 58, 304–317. [Google Scholar]
- Ierardi, E.; Losurdo, G.; Mileti, A.; Paolillo, R.; Giorgio, F.; Principi, M.; Di Leo, A. The Puzzle of Coccoid Forms of Helicobacter pylori: Beyond Basic Science. Antibiotics 2020, 9, 293. [Google Scholar] [CrossRef]
- Shah, S.C.; Iyer, P.G.; Moss, S.F. AGA Clinical practice update on the management of refractory Helicobacter pylori infection: Expert review. Gastroenterology 2021, 160, 1831–1841. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Astegiano, M.; Pellicano, R. Helicobacter pylori eradication: Poor medical compliance from East to West of the world. Scand. J. Gastroenterol. 2018, 53, 265. [Google Scholar] [CrossRef]
- Fiorini, G.; Saracino, I.M.; Zullo, A.; Gatta, L.; Pavoni, M.; Vaira, D. Rescue therapy with bismuth quadruple regimen in patients withHelicobacter pylori-resistant strains. Helicobacter 2017, 22, e12448. [Google Scholar] [CrossRef]
- Zagari, R.M.; Romiti, A.; Ierardi, E.; Gravina, A.G.; Panarese, A.; Grande, G.; Savarino, E.; Maconi, G.; Stasi, E.; Eusebi, L.H.; et al. The “three-in-one” formulation of bismuth quadruple therapy for Helicobacter pylori eradication with or without probiotics supplementation: Efficacy and safety in daily clinical practice. Helicobacter 2018, 23, e12502. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Perez-Aisa, A.; Castro-Fernandez, M.; Pellicano, R.; Huguet, J.M.; Rodrigo, L.; Ortuño, J.; Gomez-Rodriguez, B.J.; Pinto, R.M.; Areia, M.; et al. European Registry on Helicobacter pylori management: Single-capsule bismuth quadruple therapy is effective in real-world clinical practice. United Eur. Gastroenterol. J. 2021, 9, 38–46. [Google Scholar] [CrossRef]
- Shin, K.; Cho, M.J.; Oh, J.H.; Lim, C.H. Second-Line Bismuth-Containing Quadruple Therapy for Helicobacter pylori Infection: A 12-Year Study of Annual Eradication Rates. J. Clin. Med. 2021, 10, 3273. [Google Scholar] [CrossRef]
- Yao, X.; Xiao, S.; Zhou, L. Integrative proteomic and metabolomic analyses reveal the mechanism by which bismuth enables Helicobacter pylori eradication. Helicobacter 2021, 26, e12846. [Google Scholar] [CrossRef]
- Ierardi, E.; Giangaspero, A.; Losurdo, G.; Giorgio, F.; Amoruso, A.; De Francesco, V.; Di Leo, A.; Principi, M. Quadruple rescue therapy after first and second line failure for Helicobacter pylori treatment: Comparison between two tetracycline-based regimens. J. Gastrointestin. Liver Dis. 2014, 23, 367–370. [Google Scholar] [CrossRef]
- Dore, M.P.; Lu, H.; Graham, D.Y. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016, 65, 870–878. [Google Scholar] [CrossRef]
- Graham, D.Y.; Dore, M.P.; Lu, H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Heli-cobacter pylori eradication therapies. Expert Rev. Anti-Infect. Ther. 2018, 16, 679–687. [Google Scholar] [CrossRef]
- Ko, S.W.; Kim, Y.-J.; Chung, W.C.; Lee, S.J. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: Systemic review and meta-analysis. Helicobacter 2019, 24, e12565. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Tung, Y.-C.; Tu, Y.-K.; Yeh, H.-Z.; Yang, J.-C.; Hsu, P.-I.; Kim, S.-E.; Wu, M.-F.; Liou, W.-S.; Shiu, S.-I. Efficacy of second-line regimens for Helicobacter pylori eradication treatment: A systemic review and network meta-analysis. BMJ Open Gastroenterol. 2020, 7, e000472. [Google Scholar] [CrossRef]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef]
- Romano, M.; Gravina, A.G.; Nardone, G.; Federico, A.; Dallio, M.; Martorano, M.; Mucherino, C.; Romiti, A.; Avallone, L.; Granata, L.; et al. Non-bismuth and bismuth quadruple therapies based on previous clarithromycin exposure are as effective and safe in an area of high clarithromycin resistance: A real-life study. Helicobacter 2020, 25, e12694. [Google Scholar] [CrossRef] [PubMed]
- Caldas, M.; Pérez-Aisa, Á.; Castro-Fernández, M.; Bujanda, L.; Lucendo, A.J.; Rodrigo, L.; Huguet, J.M.; Pérez-Lasala, J.; Molina-Infante, J.; Barrio, J.; et al. European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain. Antibiotics 2020, 10, 13. [Google Scholar] [CrossRef]
- Lee, M.; Kemp, J.A.; Canning, A.; Egan, C.; Tataronis, G.; Farraye, F.A. A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch. Intern. Med. 1999, 159, 2312–2316. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Perez-Aisa, A.; Tepes, B.; Castro-Fernandez, M.; Kupcinskas, J.; Jonaitis, L.; Bujanda, L.; Lucendo, A.; Jurecic, N.B.; Perez-Lasala, J.; et al. Adverse Event Profile during the Treatment of Helicobacter pylori: A Real-World Experience of 22,000 Patients from the European Registry on H. pylori Management (Hp-EuReg). Am. J. Gastroenterol. 2021, 116, 1220–1229. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; La Fortezza, R.F.; Principi, M.; Barone, M.; Di Leo, A. Optimizing proton pump inhibitors in Helicobacter pylori treatment: Old and new tricks to improve effectiveness. World J. Gastroenterol. 2019, 25, 5097–5104. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Cubisino, R.; Barone, M.; Principi, M.; Leandro, G.; Ierardi, E.; Di Leo, A. Probiotic monotherapy and Helicobacter pylori eradication: A systematic review with pooled-data analysis. World J. Gastroenterol. 2018, 24, 139–149. [Google Scholar] [CrossRef]
- Francavilla, R.; Polimeno, L.; Demichina, A.; Maurogiovanni, G.; Principi, B.; Scaccianoce, G.; Ierardi, E.; Russo, F.; Riezzo, G.; Di Leo, A.; et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: A randomized, double-blind, placebo-controlled study. J. Clin. Gastroenterol. 2014, 48, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Moreno Márquez, C.; Fernández Álvarez, P.; Valdés Delgado, T.; Castro Laria, L.; Argüelles Arias, F.; Caunedo Álvarez, A.; Gómez Rodríguez, B.J. Randomized, double-blind, placebo-controlled clinical trial on the usefulness of probiotic Lactobacillus reuteri in bismuth-containing quadruple eradication therapy for infection with Helicobacter pylori. Rev. Esp. Enferm. Dig. 2021. [Google Scholar] [CrossRef]
- Nyssen, O.P.; McNicholl, A.G.; Gisbert, J.P. Meta-analysis of three-in-one single capsule bismuth-containing quadruple therapy for the eradication of Helicobacter pylori. Helicobacter 2019, 24, e12570. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Giorgio, F.; Pricci, M.; Girardi, B.; Russo, F.; Riezzo, G.; Martulli, M.; Piazzolla, M.; Cocomazzi, F.; Abbruzzi, F.; et al. Helicobacter pylori Primary and Secondary Genotypic Resistance to Clarithromycin and Levofloxacin Detection in Stools: A 4-Year Scenario in Southern Italy. Antibiotics 2020, 9, 723. [Google Scholar] [CrossRef]
- Losurdo, G.; Ierardi, E.; Di Leo, A. Helicobacter pylori Antibiotic Resistance: Stewardship, Tailored Therapies, and Future Perspectives. Gastroenterology 2021, 161, 1071–1072. [Google Scholar] [CrossRef]
- Sukri, A.; Lopes, B.S.; Hanafiah, A. The Emergence of Multidrug-Resistant Helicobacter pylori in Southeast Asia: A Systematic Review on the Trends and Intervention Strategies Using Antimicrobial Peptides. Antibiotics 2021, 10, 1061. [Google Scholar] [CrossRef]
| Variable (n = 73) | Mean ± SD, or n (%) |
|---|---|
| Female/male ratio | 41/32 |
| Age (years) | 53.0 ± 13.1 |
Symptoms
| 45 (61.6%) 26 (35.6%) 39 (53.4%) 12 (16.4%) 17 (23.3%) 14 (19.2%) |
Endoscopic and histological picture (n = 54)
| 3 (5.5%) 36 (66.7%) 27 (50%) 16 (29.6%) 8 (14.8%) |
Previous failed regimen
| 55 (75.3%) 18 (24.7%) |
| Variable | Success (n = 62) | Failure (n = 11) | p |
|---|---|---|---|
| Age | 51.6 ± 13.3 | 59.5 ± 9.4 | 0.07 |
| Male sex | 25 (40.3%) | 7 (63.6%) | 0.19 |
PPI
| 17 (27.4%) 10 (16.1%) 20 (32.2%) 10 (16.1%) 5 (8.2%) | 1 (9.2%) 5 (45.4%) 5 (45.4%) 0 (0%) 0 (0%) | 0.08 |
First line
| 45 (72.6%) 17 (27.4%) | 10 (90.9%) 1 (9.1%) | 0.27 |
| Adverse events | 11 (17.7%) | 3 (37.3%) | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losurdo, G.; Lacavalla, I.; Russo, F.; Riezzo, G.; Brescia, I.V.; Rendina, M.; Ierardi, E.; Di Leo, A. Empiric “Three-in-One” Bismuth Quadruple Therapy for Second-Line Helicobacter pylori Eradication: An Intervention Study in Southern Italy. Antibiotics 2022, 11, 78. https://doi.org/10.3390/antibiotics11010078
Losurdo G, Lacavalla I, Russo F, Riezzo G, Brescia IV, Rendina M, Ierardi E, Di Leo A. Empiric “Three-in-One” Bismuth Quadruple Therapy for Second-Line Helicobacter pylori Eradication: An Intervention Study in Southern Italy. Antibiotics. 2022; 11(1):78. https://doi.org/10.3390/antibiotics11010078
Chicago/Turabian StyleLosurdo, Giuseppe, Ilaria Lacavalla, Francesco Russo, Giuseppe Riezzo, Irene Vita Brescia, Maria Rendina, Enzo Ierardi, and Alfredo Di Leo. 2022. "Empiric “Three-in-One” Bismuth Quadruple Therapy for Second-Line Helicobacter pylori Eradication: An Intervention Study in Southern Italy" Antibiotics 11, no. 1: 78. https://doi.org/10.3390/antibiotics11010078
APA StyleLosurdo, G., Lacavalla, I., Russo, F., Riezzo, G., Brescia, I. V., Rendina, M., Ierardi, E., & Di Leo, A. (2022). Empiric “Three-in-One” Bismuth Quadruple Therapy for Second-Line Helicobacter pylori Eradication: An Intervention Study in Southern Italy. Antibiotics, 11(1), 78. https://doi.org/10.3390/antibiotics11010078








