Comparison of Consumption Data and Phenotypical Antimicrobial Resistance in E. coli Isolates of Human Urinary Samples and of Weaning and Fattening Pigs from Surveillance and Monitoring Systems in Germany
Abstract
:1. Introduction
2. Material and Methods
2.1. Data Collection and Processing
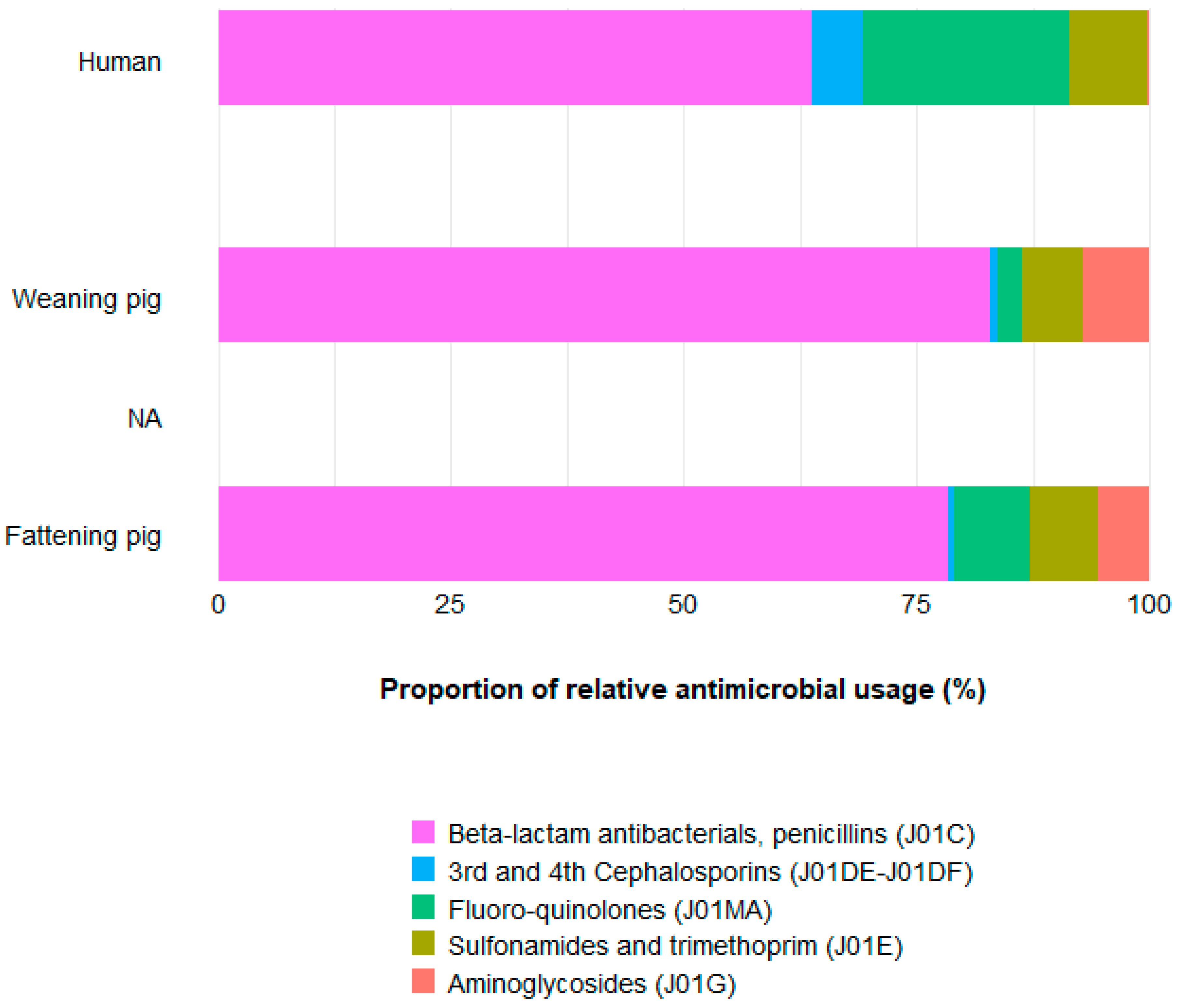
2.2. Approach to Compare AMU between Population Types
2.3. Statistical Analysis
- First hypotheses:
- (a)
- The resistance level in E. coli differs between urban and rural isolates of humans.
- (b)
- There are differences on the resistance level of E. coli between age groups in pigs.
- (c)
- The level of E. coli resistance is higher in clinical isolates in comparison to nonclinical isolates from pigs.
- Second hypotheses:
- (d)
- There are differences between the resistance levels of clinical and nonclinical isolates from weaning and fattening pigs and those from the human areas in the community.
- (e)
- There are similar AMR patterns between pigs and humans.
- (f)
- There is an association between AMU and AMR in humans and pigs.
- (g)
- E. coli resistance levels are reduced due to measures taken to control AMR.
- (a)
- Isolates from urban and rural human populations across years.
- (b)
- Clinical isolates of weaning pigs and fattening pigs across years.
- (c)
- Nonclinical isolates of weaning pigs and fattening pigs in 2015.
- (d)
- Clinical isolates and nonclinical isolates of weaning pigs in 2015.
- (e)
- Clinical isolates and nonclinical isolates of fattening pigs in 2015 and 2017.
3. Results
3.1. Comparison of Subgroups within the Population
3.2. Comparison between Human and Pig Isolates
3.3. Association of AMU and Year with Resistance within Populations
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://www.who.int/antimicrobial-resistance/global-action-plan/en/ (accessed on 11 August 2020).
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations—The Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 23 August 2021).
- EFSA/EMA/ECDC. Analysis of Antimicrobial Consumption and Resistance (Report I.JIACRA 2011–2012). 2015. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/analysis-antimicrobial-consumption-resistance-jiacra-reports#report-on-2011-12-(jiacra-i)-section (accessed on 14 July 2021).
- EFSA/EMA/ECDC. Analysis of Antimicrobial Consumption and Resistance (Report II.JIACRA 2013–2015). 2017. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/analysis-antimicrobial-consumption-resistance-jiacra-reports#report-on-2013–15-(jiacra-ii)-section (accessed on 14 July 2021).
- EFSA/EMA/ECDC. Analysis of Antimicrobial Consumption and Resistance (Report III.JIACRA 2016–2018). 2021. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/analysis-antimicrobial-consumption-resistance-jiacra-reports#report-on-2016%E2%80%9318-(jiacra-iii)-(new)-section (accessed on 14 July 2021).
- Ceccarelli, D.; Hesp, A.; Van Der Goot, J.; Joosten, P.; Sarrazin, S.; Wagenaar, J.A.; Dewulf, J.; Mevius, D.J.; The Effort Consortium. Antimicrobial resistance prevalence in commensal Escherichia coli from broilers, fattening turkeys, fattening pigs and veal calves in European countries and association with antimicrobial usage at country level. J. Med. Microbiol. 2020, 69, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y.; on behalf of the Study Group participants. Helicobacter pyloriresistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.P.; Horton, R.A.; Lemma, F.; Martelli, F.; Duggett, N.A.; Smith, R.P.; Kirchner, M.J.; Ellis, R.J.; Rogers, J.P.; Williamson, S.M.; et al. Longitudinal study on the occurrence in pigs of colistin-resistant Escherichia coli carrying mcr-1 following the cessation of use of colistin. J. Appl. Microbiol. 2018, 125, 596–608. [Google Scholar] [CrossRef]
- Murphy, C.P.; Carson, C.; Smith, B.; Chapman, B.; Marrotte, J.; McCann, M.; Primeau, C.; Sharma, P.; Parmley, E.J. Factors potentially linked with the occurrence of antimicrobial resistance in selected bacteria from cattle, chickens and pigs: A scoping review of publications for use in modelling of antimicrobial resistance (IAM.AMR Project). Zoonoses Public Health 2018, 65, 957–971. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). The European Surveillance of Antimicrobial Consumption Network (ESAC-Net). Available online: https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/esac-net (accessed on 3 December 2021).
- European Medicines Agency (EMA). European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). 2019. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2017_en.pdf (accessed on 3 December 2021).
- World Health Organization (WHO). Global Action Plan (GAP). 2015. Available online: http://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html (accessed on 21 June 2021).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, e05598. [Google Scholar] [CrossRef]
- Rojas-Lopez, M.; Monteiro, R.; Pizza, M.; Desvaux, M.; Rosini, R. Intestinal Pathogenic Escherichia coli: Insights for Vaccine Development. Front. Microbiol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Djordjevic, S.P.; Stokes, H.W.; Chowdhury, P.R. Mobile elements, zoonotic pathogens and commensal bacteria: Conduits for the delivery of resistance genes into humans, production animals and soil microbiota. Front. Microbiol. 2013, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, e06007. [Google Scholar] [CrossRef] [Green Version]
- Köck, R.; Becker, K.; Cookson, B.; E Van Gemert-Pijnen, J.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J.; et al. Systematic literature analysis and review of targeted preventive measures to limit healthcare-associated infections by meticillin-resistant Staphylococcus aureus. Eurosurveillance 2014, 19, 20860. [Google Scholar] [CrossRef] [Green Version]
- Kinross, P.; Petersen, A.; Skov, R.; Van Hauwermeiren, E.; Pantosti, A.; Laurent, F.; Voss, A.; Kluytmans, J.; Struelens, M.J.; Heuer, O.; et al. Livestock-associated meticillin-resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area countries, 2013. Eurosurveillance 2017, 22, 00696. [Google Scholar] [CrossRef] [Green Version]
- Varona, O.M.; Chaintarli, K.; Muller-Pebody, B.; Anjum, M.F.; Eckmanns, T.; Norström, M.; Boone, I.; Tenhagen, B.-A. Monitoring Antimicrobial Resistance and Drug Usage in the Human and Livestock Sector and Foodborne Antimicrobial Resistance in Six European Countries. Infect. Drug Resist. 2020, 13, 957–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flor, M.; Käsbohrer, A.; Kaspar, H.; Tenhagen, B.-A.; Wallmann, J. Arbeitsgruppe Antibiotikaresistenz des Bundesinstituts für Risikobewertung und des Bundesamtes für Verbraucherschutz und Lebensmittelsicherheit. Themenkomplex 1: Entwicklung der Antibiotikaabgabe-und-verbrauchsmengen sowie der Therapiehäufigkeit. 2019. Available online: https://www.bmel.de/SharedDocs/Downloads/DE/_Tiere/Tiergesundheit/Tierarzneimittel/16-AMG-Novelle-Anlage2.pdf?__blob=publicationFile&v=2 (accessed on 3 December 2021).
- World Health Organization (WHO). Critically Important Antibacterial Agents for Human Medicine for Risk Management Strategies of Non-Human Use: Report of a WHO Working Group Consultation, 15–18 February 2005, Canberra, Australia. 2005. Available online: https://apps.who.int/iris/handle/10665/43330 (accessed on 3 December 2021).
- FAO/WHO/OIE. Joint FAO/WHO/OIE Expert Meeting on Critically Important Antimicrobials; FAO, Food and Agriculture Organization of the United Nations, Ed.; FAO/WHO/OIE, 2007; Available online: https://www.fao.org/3/i0204e/i0204e.pdf (accessed on 3 December 2021).
- Bennani, H.; Mateus, A.; Mays, N.; Eastmure, E.; Stärk, K.D.C.; Häsler, B. Overview of Evidence of Antimicrobial Use and Antimicrobial Resistance in the Food Chain. Antibiotics 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.; Cork, S.C.; Ronksley, P.E.; Barkema, H.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Bundesinstitut für Bau-Stadt-und Raumforschung (BBSR). INKAR—Indikatoren und Karten zur Raum-und Stadtentwicklung. 2019. Available online: https://www.inkar.de/documents/Erlaeuterungen%20Raumbezuege19.pdf (accessed on 3 June 2021).
- Bundesamt für Verbarucherschutz und Lebensmittelsicherheit (BVL). Zoonosen-Monitoring. Available online: https://www.bvl.bund.de/DE/Arbeitsbereiche/01_Lebensmittel/01_Aufgaben/02_AmtlicheLebensmittelueberwachung/06_ZoonosenMonitoring/lm_zoonosen_monitoring_node.html (accessed on 27 May 2021).
- Bundesamt für Verbraucherschutz und Lebensmittelsicherkeit (BVL). Bericht zum GERM-Vet Monitoring programm 2016. Available online: https://www.bvl.bund.de/SharedDocs/Fachmeldungen/07_untersuchungen/2018/2018_10_09_Fa_GERM-Vet-2016.html (accessed on 3 December 2021).
- Irrgang, A.; Tenhagen, B.-A.; Pauly, N.; Schmoger, S.; Kaesbohrer, A.; Hammerl, J.A. Characterization of VIM-1-Producing E. coli Isolated From a German Fattening Pig Farm by an Improved Isolation Procedure. Front. Microbiol. 2019, 10, 2256. [Google Scholar] [CrossRef]
- Noll, I.; Schweickert, B.; Abu Sin, M.; Feig, M.; Claus, H.; Eckmanns, T. Daten zur Antibiotikaresistenzlage in Deutschland. Bundesgesundheitsblatt Gesundh. Gesundh. 2012, 55, 1370–1376. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Global Antimicrobial Resistance Surveillance System (GLASS). The Detectionand Reporting of Colistin Resistance. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/277175/WHO-WSI-AMR-2018.4-eng.pdf (accessed on 1 June 2021).
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Consumption Database (ESAC-Net). Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database (accessed on 3 December 2021).
- EUCAST. New S, I and R Definitions. Available online: https://www.eucast.org/newsiandr/ (accessed on 3 December 2021).
- Bundesamt für Verbraucherschutz und Lebensmittelsicherkeit (BVL). Veterinärmedizinischer Informationsdienst für Arzneimittelanwendung, Toxikologie und Arzneimittelrecht (VETIDATA). Available online: www.vetidata.de (accessed on 29 October 2021).
- Sundqvist, M. Reversibility of antibiotic resistance. Upsala J. Med. Sci. 2014, 119, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Varona, O.; Mader, R.; Velasova, M.; Madec, J.-Y.; Granier, S.; Perrin-Guyomard, A.; Norstrom, M.; Kaspar, H.; Grobbel, M.; Jouy, E.; et al. Comparison of Phenotypical Antimicrobial Resistance between Clinical and Nonclinical E. coli Isolates from Broilers, Turkeys and Calves in Four European Countries. Microorganisms 2021, 9, 678. [Google Scholar] [CrossRef]
- Aasmäe, B.; Häkkinen, L.; Kaart, T.; Kalmus, P. Antimicrobial resistance of Escherichia coli and Enterococcus spp. isolated from Estonian cattle and swine from 2010 to 2015. Acta Veter Scand. 2019, 61, 5. [Google Scholar] [CrossRef]
- Mesa-Varona, O.; Kaspar, H.; Grobbel, M.; Tenhagen, B.-A. Phenotypical antimicrobial resistance data of clinical and nonclinical Escherichia coli from poultry in Germany between 2014 and 2017. PLoS ONE 2020, 15, e0243772. [Google Scholar] [CrossRef]
- Burow, E.; Rostalski, A.; Harlizius, J.; Gangl, A.; Simoneit, C.; Grobbel, M.; Kollas, C.; Tenhagen, B.-A.; Käsbohrer, A. Antibiotic resistance in Escherichia coli from pigs from birth to slaughter and its association with antibiotic treatment. Prev. Veter Med. 2019, 165, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Netherlands Veterinary Medicines Insitute (SDa). SDa-Report ‘Usage of Antibiotics in Agricultural Livestock in the Netherlands in 2019’. Available online: https://www.autoriteitdiergeneesmiddelen.nl/en/publications/general-reports (accessed on 26 August 2021).
- Hemme, M.; Ruddat, I.; Hartmann, M.; Werner, N.; Van Rennings, L.; Käsbohrer, A.; Kreienbrock, L. Antibiotic use on German pig farms—A longitudinal analysis for 2011, 2013 and 2014. PLoS ONE 2018, 13, e0199592. [Google Scholar] [CrossRef] [PubMed]
- Dorado-García, A.; Smid, J.H.; Van Pelt, W.; Bonten, M.J.M.; Fluit, A.C.; Bunt, G.V.D.; Wagenaar, J.A.; Hordijk, J.; Dierikx, C.M.; Veldman, K.T.; et al. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: A pooled analysis. J. Antimicrob. Chemother. 2018, 73, 339–347. [Google Scholar] [CrossRef]
- Osbiston, K.; Oxbrough, A.; Fernández-Martínez, L.T. Antibiotic resistance levels in soils from urban and rural land uses in Great Britain. Access Microbiol. 2021, 3, 000181. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Community-Based Surveillance of Antimicrobial Use and Resistance in Resource-Constrained Settings. 2009. Available online: https://www.who.int/medicines/publications/community_based_may09.pdf (accessed on 18 June 2021).
- Mathai, E.; Chandy, S.; Thomas, K.; Antoniswamy, B.; Joseph, I.; Mathai, M.; Sorensen, T.L.; Holloway, K. Antimicrobial resistance surveillance among commensal Escherichia coli in rural and urban areas in Southern India. Trop. Med. Int. Health 2008, 13, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Bisdorff, B.; Scholhölter, J.L.; Claußen, K.; Pulz, M.; Nowak, D.; Radon, K. MRSA-ST398 in livestock farmers and neighbouring residents in a rural area in Germany. Epidemiol. Infect. 2012, 140, 1800–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moennighoff, C.; Thomas, N.; Nienhaus, F.; Hartmann, M.; Menrath, A.; Merkel, J.; Detlefsen, H.; Kreienbrock, L.; Hennig-Pauka, I. Phenotypic antimicrobial resistance in Escherichia coli strains isolated from swine husbandries in North Western Germany—temporal patterns in samples from laboratory practice from 2006 to 2017. BMC Veter. Res. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Wageningen University. MARAN Report. 2019. Available online: https://www.wur.nl/en/Research-Results/Research-Institutes/Bioveterinary-Research/In-the-spotlight/Antibiotic-resistance/MARAN-reports.htm (accessed on 22 October 2021).
- Mader, R.; Bourély, C.; Amat, J.-P.; Broens, E.M.; Busani, L.; Callens, B.; Crespo, P.; Damborg, P.; Filippitzi, M.-E.; Fitzgerald, W.; et al. Defining the scope of the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet): A bottom-up and One Health approach. bioRxiv 2021, 1–33. [Google Scholar] [CrossRef]
- Teale, C.; Borriello, P. A proposed scheme for the monitoring of antibiotic resistance in veterinary pathogens of food animals in the UK. Veter Rec. 2021, 189, e201. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Liu, C.; Kudinha, T.; Liu, X.; Luo, Y.; Yang, Q.; Sun, H.; Hu, J.; Xu, Y.-C. Comparison of five commonly used automated susceptibility testing methods for accuracy in the China Antimicrobial Resistance Surveillance System (CARSS) hospitals. Infect. Drug Resist. 2018, 11, 1347–1358. [Google Scholar] [CrossRef] [Green Version]
- Bobenchik, A.M.; Deak, E.; Hindler, J.A.; Charlton, C.; Humphries, R.M. Performance of Vitek 2 for Antimicrobial Susceptibility Testing of Enterobacteriaceae with Vitek 2 (2009 FDA) and 2014 CLSI Breakpoints. J. Clin. Microbiol. 2014, 53, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Høg, B.B.; Andersen, V.D.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- Kasabova, S.; Hartmann, M.; Freise, F.; Hommerich, K.; Fischer, S.; Wilms-Schulze-Kump, A.; Rohn, K.; Käsbohrer, A.; Kreienbrock, L. Antibiotic Usage Pattern in Broiler Chicken Flocks in Germany. Front. Veter Sci. 2021, 8, 673809. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health. List of Antimicrobial Agents of Veterinary Importance; OIE International Committee, 2015; Available online: https://www.oie.int/app/uploads/2021/03/oie-list-antimicrobials.pdf (accessed on 3 December 2021).


| 2015 | 2016 | 2017 | |||
|---|---|---|---|---|---|
| Clinical | Nonclinical | Clinical | Clinical | Nonclinical | |
| Human isolates from rural areas | |||||
| ampicillin | 39.5% (37.5–41.52) 915/2317 | NA | 40.3% (38.2–42.43) 851/2112 | 39.8% (37.53–42.04) 734/1846 | NA |
| cefotaxime | 6.8% (6.14–7.58) 333/4877 | NA | 6.8% (6.05–7.66) 263/3862 | 7.1% (6.29–7.92) 274/3880 | NA |
| ciprofloxacin | 16.5% (15.69–17.41) 1207/7302 | NA | 16.0% (15.11–16.98) 965/6024 | 16.4% (15.49–17.44) 928/5645 | NA |
| co-trimoxazole | 22.3% (21.36–23.28) 1627/7295 | NA | 21.5% (20.5–22.59) 1296/6021 | 21.5% (20.47–22.64) 1215/5642 | NA |
| gentamicin | 7.1% (6.57–7.77) 521/7291 | NA | 8.8% (8.08–9.53) 528/6017 | 4.9% (4.33–5.47) 275/5646 | NA |
| trimethoprim | 22.2% (20.38–24.12) 433/1951 | NA | 23.3% (21.38–25.32) 423/1816 | 24.3%(22.08–26.59) 346/1426 | NA |
| Human isolates from urban areas | |||||
| ampicillin | 41.4% (37.84–45.06) 306/739 | NA | 42.2% (38.61–45.84) 313/742 | 39.7% (36.17–43.39) 290/730 | NA |
| cefotaxime | 7.4% (6.65–8.3) 296/3983 | NA | 7.0% (6.2–7.98) 233/3311 | 8.3% (7.4–9.27) 287/3463 | NA |
| ciprofloxacin | 17.0% (15.92–18.06) 814/4799 | NA | 17.0% (15.88–18.2) 696/4092 | 17.7% (16.52–18.91) 707/3999 | NA |
| co-trimoxazole | 22.9% (21.74–24.14) 1099/4796 | NA | 22.2% (20.98–23.55) 909/4088 | 22.0% (20.77–23.36) 881/3998 | NA |
| gentamicin | 5.7% (5.04–6.37) 272/4799 | NA | 5.2% (4.54–5.92) 212/4086 | 5.0% (4.4–5.78) 202/4003 | NA |
| trimethoprim | 23.4% (20.39–26.63) 172/736 | NA | 24.6% (21.54–27.91) 180/732 | 22.5% (18.97–26.38) 115/512 | NA |
| Isolates of fattening pigs | |||||
| ampicillin | 63.2% (54.06–71.51) 79/125 | 33.2% (26.95–40.02) 70/211 | 52.9% (45.19–60.43) 92/174 | 46.8% (39.17–54.54) 80/171 | 31.8% (27.51–36.43) 139/437 |
| cefotaxime | 5.6% (2.48–11.62) 7/125 | 2.4% (0.88–5.75) 5/211 | 5.7% (2.95–10.61) 10/174 | 5.8% (3.0–10.79) 10/171 | 1.6% (0.7–3.42) 7/437 |
| ciprofloxacin | 2.4% (0.62–7.38) 3/125 | 0.9% (0.16–3.75) 2/211 | 4.6% (2.15–9.17) 8/174 | 7.6% (4.28–12.92) 13/171 | 2.5% (1.33–4.59) 11/437 |
| co-trimoxazole | 39.2% (30.71–48.36) 49/125 | NA | 40.2% (32.96–47.94) 70/174 | 34.5% (27.52–42.2) 59/171 | NA |
| gentamicin | 6.4% (3.01–12.62) 8/125 | 3.3% (1.46–7.0) 7/211 | 5.2% (2.55–9.9) 9/174 | 4.7% (2.19–9.33) 8/171 | 2.3% (1.17–4.31) 10/437 |
| trimethoprim | NA | 26.1% (20.39–32.63) 55/211 | NA | NA | 24.0% (20.15–28.37) 105/437 |
| Isolates of weaning pigs | |||||
| ampicillin | 62.6% (53.38–71.03) 77/123 | 50.8% (44.44–57.13) 127/250 | 56.2% (47.72–64.28) 82/146 | 67.9% (59.36–75.35) 95/140 | NA |
| cefotaxime | 10.6% (5.97–17.72) 13/123 | 4.4% (2.33–7.95) 11/250 | 3.4% (1.27–8.22) 5/146 | 6.4% (3.17–12.21) 9/140 | NA |
| ciprofloxacin | 10.6% (5.97–17.72) 13/123 | 4.0% (2.05–7.46) 10/250 | 9.6% (5.54–15.86) 14/146 | 7.2% (3.7–13.18) 10/139 | NA |
| co-trimoxazole | 44.7%(35.83–53.93) 55/123 | NA | 44.5% (36.37–52.96) 65/146 | 49.6% (41.1–58.2) 69/139 | NA |
| gentamicin | 6.5% (3.06–12.82) 8/123 | 3.2% (1.5–6.44) 8/250 | 8.2% (4.51–14.23) 12/146 | 9.4% (5.28–15.77) 13/139 | NA |
| trimethoprim | NA | 36.0% (30.11–42.32) 90/250 | NA | NA | NA |
| Human Area | Antimicrobial | Variable | p-Value | OR (CI) |
|---|---|---|---|---|
| Clinical isolates from humans vs. clinical isolates from fattening pigs | ||||
| Rural | ampicillin | Human/Animal | <0.001 | 1.74 (1.44–2.1) |
| Year | 0.811 | 0.99 (0.94–1.05) | ||
| cefotaxime | Human/Animal | 0.328 | 0.82 (0.54–1.19) | |
| Year | 0.698 | 1.02 (0.94–1.1) | ||
| ciprofloxacin | Human/Animal | <0.001 | 0.27 (0.18–0.41) | |
| Year | 0.815 | 0.99 (0.95–1.04) | ||
| co-trimoxazole | Human/Animal | <0.001 | 2.2 (1.81–2.65) | |
| Year | 0.329 | 0.98 (0.94–1.02) | ||
| gentamicin | Human/Animal | 0.159 | 0.75 (0.48–1.1) | |
| Year | <0.001 | 0.85 (0.79–0.91) | ||
| Urban | ampicillin | Human/Animal | <0.001 | 1.65 (1.35–2.01) |
| Year | 0.123 | 0.93 (0.85–1.02) | ||
| cefotaxime | Human/Animal | 0.137 | 0.74 (0.49–1.08) | |
| Year | 0.199 | 1.06 (0.97–1.15) | ||
| ciprofloxacin | Human/Animal | <0.001 | 0.26 (0.17–0.38) | |
| Year | 0.402 | 1.02 (0.97–1.08) | ||
| co-trimoxazole | Human/Animal | <0.001 | 2.12 (1.75–2.56) | |
| Year | 0.358 | 0.98 (0.93–1.03) | ||
| gentamicin | Human/Animal | 0.989 | 1.0 (0.65–1.47) | |
| Year | 0.161 | 0.94 (0.85–1.03) | ||
| Clinical isolates from humans vs. clinical isolates from weaning pigs | ||||
| Rural | ampicillin | Human/Animal | <0.001 | 2.42 (1.97–2.97) |
| Year | 0.431 | 1.02 (0.96–1.09) | ||
| cefotaxime | Human/Animal | 0.749 | 0.94 (0.62–1.37) | |
| Year | 0.822 | 1.01 (0.93–1.1) | ||
| ciprofloxacin | Human/Animal | <0.001 | 0.5 (0.35–0.69) | |
| Year | 0.736 | 0.99 (0.95–1.04) | ||
| co-trimoxazole | Human/Animal | <0.001 | 3.05 (2.51–3.71) | |
| Year | 0.488 | 0.99 (0.95–1.03) | ||
| gentamicin | Human/Animal | 0.446 | 1.15 (0.79–1.62) | |
| Year | <0.001 | 0.86 (0.8–0.92) | ||
| Urban | ampicillin | Human/Animal | <0.001 | 2.29 (1.85–2.85) |
| Year | 0.975 | 1.0 (0.91–1.1) | ||
| cefotaxime | Human/Animal | 0.407 | 0.85 (0.56–1.23) | |
| Year | 0.262 | 1.05 (0.96–1.14) | ||
| ciprofloxacin | Human/Animal | <0.001 | 0.47 (0.33–0.65) | |
| Year | 0.48 | 1.02 (0.97–1.08) | ||
| co-trimoxazole | Human/Animal | <0.001 | 2.95 (2.42–3.59) | |
| Year | 0.556 | 0.99 (0.94–1.03) | ||
| gentamicin | Human/Animal | 0.021 | 1.54 (1.05–2.18) | |
| Year | 0.304 | 0.95 (0.87–1.04) | ||
| Clinical isolates from humans vs. nonclinical isolates from weaning pigs 1 | ||||
| Rural | ampicillin | Human/Animal | <0.001 | 1.58 (1.22–2.06) |
| cefotaxime | Human/Animal | 0.138 | 0.63 (0.32–1.11) | |
| ciprofloxacin | Human/Animal | <0.001 | 0.21 (0.1–0.38) | |
| gentamicin | Human/Animal | 0.02 | 0.43 (0.19–0.82) | |
| trimethoprim | Human/Animal | <0.001 | 1.97 (1.49–2.6) | |
| Urban | ampicillin | Human/Animal | 0.01 | 1.46 (1.1–1.95) |
| cefotaxime | Human/Animal | 0.077 | 0.57 (0.29–1.01) | |
| ciprofloxacin | Human/Animal | <0.001 | 0.2 (0.1–0.37) | |
| gentamicin | Human/Animal | 0.101 | 0.55 (0.25–1.05) | |
| trimethoprim | Human/Animal | <0.001 | 1.84 (1.35–2.51) | |
| Human Area | Antimicrobial | Variable | p-Value | OR (CI) |
|---|---|---|---|---|
| Clinical isolates from humans vs. nonclinical isolates from fattening pigs 1 | ||||
| Rural | ampicillin | Human/Animal | <0.001 | 0.72 (0.6–0.85) |
| Year | 0.934 | 1.0 (0.95–1.06) | ||
| cefotaxime | Human/Animal | <0.001 | 0.25 (0.13–0.43) | |
| Year | 0.751 | 1.01 (0.93–1.1) | ||
| ciprofloxacin | Human/Animal | <0.001 | 0.1 (0.06–0.17) | |
| Year | 0.928 | 1.0 (0.95–1.05) | ||
| gentamicin | Human/Animal | <0.001 | 0.38 (0.23–0.6) | |
| Year | <0.001 | 0.85 (0.8–0.91) | ||
| trimethoprim | Human/Animal | 0.5 | 1.07 (0.88–1.29) | |
| Year | 0.277 | 1.04 (0.97–1.12) | ||
| Urban | ampicillin | Human/Animal | <0.001 | 0.69 (0.57–0.83) |
| Year | 0.46 | 0.97 (0.88–1.06) | ||
| cefotaxime | Human/Animal | <0.001 | 0.22 (0.12–0.38) | |
| Year | 0.22 | 1.05 (0.97–1.15) | ||
| ciprofloxacin | Human/Animal | <0.001 | 0.1 (0.05–0.16) | |
| Year | 0.323 | 1.03 (0.97–1.09) | ||
| gentamicin | Human/Animal | 0.005 | 0.49 (0.29–0.78) | |
| Year | 0.152 | 0.93 (0.85–1.03) | ||
| trimethoprim | Human/Animal | 0.492 | 1.08 (0.87–1.33) | |
| Year | 0.581 | 0.97 (0.87–1.08) | ||
| AMU | Year | |||
|---|---|---|---|---|
| Antimicrobial | p-Value | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) |
| Clinical isolates from humans | ||||
| ampicillin | 0.71 | 0.97 (0.81–1.16) | 0.788 | 0.99 (0.95–1.04) |
| cefotaxime | 0.311 | 0.52 (0.15–1.84) | 0.346 | 1.03 (0.97–1.08) |
| ciprofloxacin | 0.259 | 0.83 (0.61–1.15) | 0.319 | 1.02 (0.98–1.05) |
| co-trimoxazole | 0.045 | 189.62 (1.12–3.1 × 104) | 0.103 | 0.98 (0.95–1.01) |
| gentamicin | <0.001 | 1.09 × 10185 (3.8 × 1095–3.5 × 10274) | <0.001 | 0.88 (0.84–0.93) |
| trimethoprim | 0.336 | 8.4 × 1020 (1.6 × 10−22–2.6 × 1063) | 0.288 | 1.04 (0.97–1.11) |
| Clinical isolates from fattening pig | ||||
| ampicillin | 0.006 | 2.22 (1.27–3.92) | 0.006 | 0.72 (0.57–0.91) |
| cefotaxime | NA | NA | 0.928 | 1.02 (0.63–1.69) |
| ciprofloxacin | 0.122 | 9.3 × 10−15 (7.9 × 10−36–51.31) | 0.046 | 1.8 (1.03–3.32) |
| co-trimoxazole | 0.502 | 1.89 (0.29–12.16) | 0.37 | 0.9 (0.71–1.14) |
| gentamicin | 0.51 | 16.0 (3.1 × 10−3–5.45 × 104) | 0.524 | 0.85 (0.51–1.42) |
| Clinical isolates from weaning pig | ||||
| ampicillin | 0.898 | 0.99 (0.91–1.09) | 0.344 | 1.13 (0.88–1.45) |
| cefotaxime | 0.137 | 2.06 × 106 (0.01–5.85 × 1014) | 0.206 | 0.73 (0.44–1.19) |
| ciprofloxacin | 0.328 | 2.5 × 10–4 (7 × 10−12–2.2 × 103) | 0.339 | 0.81 (0.53–1.24) |
| co-trimoxazole | 0.38 | 0.73 (0.35–1.48) | 0.414 | 1.11 (0.87–1.41) |
| gentamicin | 0.436 | 0.56 (0.12–2.23) | 0.402 | 1.21 (0.78–1.92) |
| Nonclinical isolates from fattening pig | ||||
| ampicillin | 0.727 | 1.08 (0.7–1.64) | 0.727 | 0.97 (0.81–1.16) |
| cefotaxime | NA | NA | 0.5 | 0.82 (0.46–1.51) |
| ciprofloxacin | 0.199 | 4.3 × 10−15 (3.4 × 10−42–6.78 × 104) | 0.199 | 1.64 (0.85–4.19) |
| gentamicin | 0.445 | 24.12 (4.6 × 10−3–7.9 × 104) | 0.445 | 0.83 (0.51–1.38) |
| trimethoprim | NA | NA | 0.573 | 0.95 (0.79–1.15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesa-Varona, O.; Boone, I.; Flor, M.; Eckmanns, T.; Kaspar, H.; Grobbel, M.; Tenhagen, B.-A. Comparison of Consumption Data and Phenotypical Antimicrobial Resistance in E. coli Isolates of Human Urinary Samples and of Weaning and Fattening Pigs from Surveillance and Monitoring Systems in Germany. Antibiotics 2022, 11, 28. https://doi.org/10.3390/antibiotics11010028
Mesa-Varona O, Boone I, Flor M, Eckmanns T, Kaspar H, Grobbel M, Tenhagen B-A. Comparison of Consumption Data and Phenotypical Antimicrobial Resistance in E. coli Isolates of Human Urinary Samples and of Weaning and Fattening Pigs from Surveillance and Monitoring Systems in Germany. Antibiotics. 2022; 11(1):28. https://doi.org/10.3390/antibiotics11010028
Chicago/Turabian StyleMesa-Varona, Octavio, Ides Boone, Matthias Flor, Tim Eckmanns, Heike Kaspar, Mirjam Grobbel, and Bernd-Alois Tenhagen. 2022. "Comparison of Consumption Data and Phenotypical Antimicrobial Resistance in E. coli Isolates of Human Urinary Samples and of Weaning and Fattening Pigs from Surveillance and Monitoring Systems in Germany" Antibiotics 11, no. 1: 28. https://doi.org/10.3390/antibiotics11010028
APA StyleMesa-Varona, O., Boone, I., Flor, M., Eckmanns, T., Kaspar, H., Grobbel, M., & Tenhagen, B.-A. (2022). Comparison of Consumption Data and Phenotypical Antimicrobial Resistance in E. coli Isolates of Human Urinary Samples and of Weaning and Fattening Pigs from Surveillance and Monitoring Systems in Germany. Antibiotics, 11(1), 28. https://doi.org/10.3390/antibiotics11010028






