Diagnostic Testing for Sepsis: A Systematic Review of Economic Evaluations
Abstract
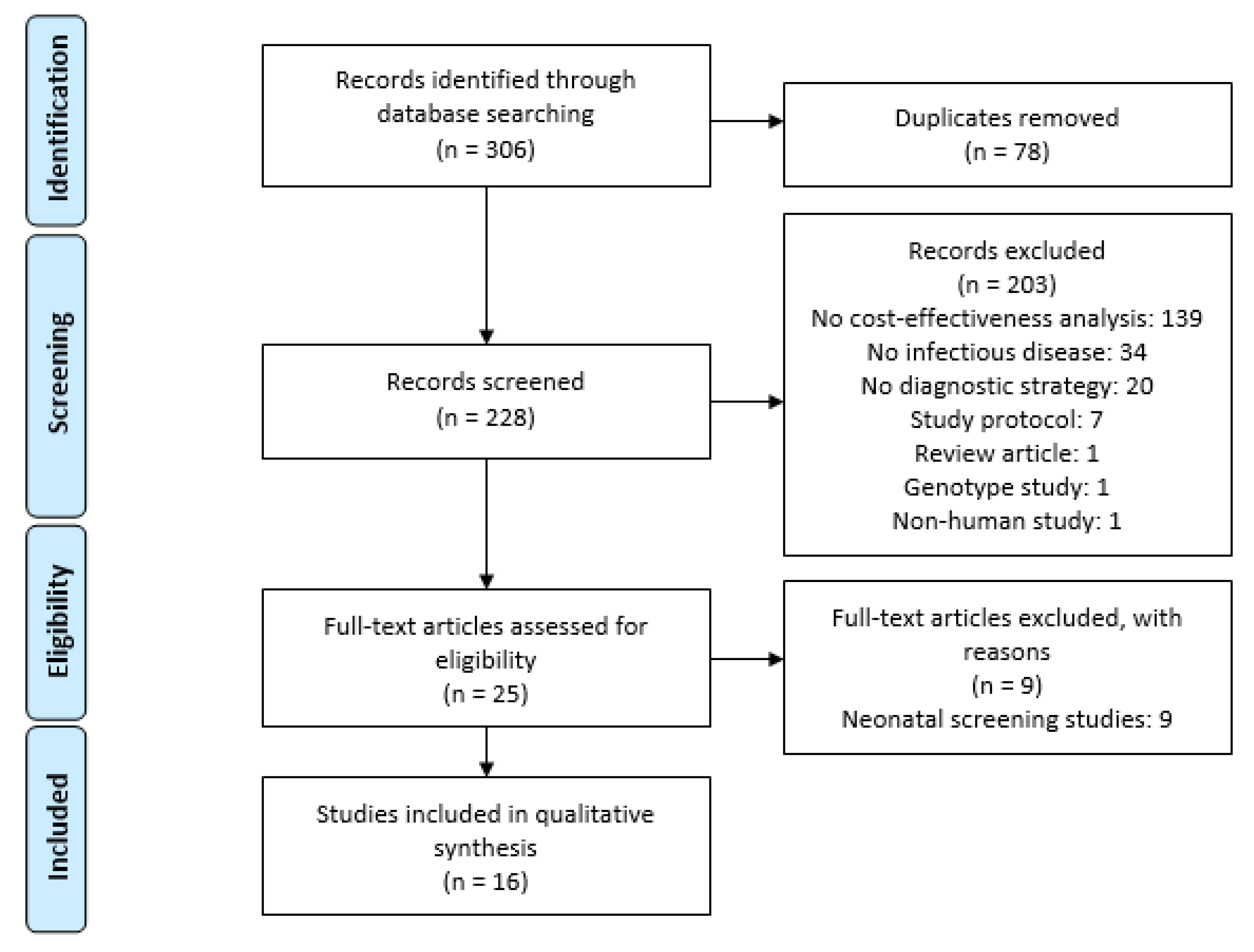
:1. Introduction
2. Materials and Methods
2.1. Type of Studies
2.2. Search Strategy and Selection Criteria
2.3. Data Extraction and Analysis
3. Results
3.1. Country and Setting of the Articles
3.2. Perspective, Time Horizon, and Population
3.3. Type of Model and Assessed Interventions
3.4. Cost-Effectiveness Results
3.5. Antimicrobial Resistance in the Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Teggert, A.; Datta, H.; Ali, Z. Biomarkers for Point-of-Care Diagnosis of Sepsis. Micromachines 2020, 11, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017, analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, C.; Bouadma, L.; Ruckly, S.; Perozziello, A.; Van-Gysel, D.; Mageau, A.; Mourvillier, B.; de Montmollin, E.; Bailly, S.; Papin, G.; et al. Sepsis and septic shock in France: Incidences, outcomes and costs of care. Ann. Intensive Care 2020, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Torio, C.M.; Moore, B.J. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013, Statistical Brief #204; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. Available online: http://europepmc.org/abstract/MED/27359025 (accessed on 24 November 2021).
- Hadžić, D.; Skokić, F.; Brkić, S.; Saračević, A.; Softić, D.; Softić, D. Epidemiology of neonatal sepsis caused by multidrug resistant pathogens in a neonatal intensive care unit level 3. Med. Glas. (Zenica) 2020, 17, 375–382. [Google Scholar] [CrossRef]
- Riedel, S. Procalcitonin and the role of biomarkers in the diagnosis and management of sepsis. Diagn. Microbiol. Infect. Dis. USA 2012, 73, 221–227. [Google Scholar] [CrossRef]
- World Health Organization. World Health Assembly 70, Resolution 70.7, Improving the Prevention, Diagnosis and Clinical Management of Sepsis. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/275646/A70_R7-en.pdf?sequence=1&isAllowed=y (accessed on 1 February 2021).
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. USA 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.; Townsend, S.; Dellinger, R.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Sterling, S.A.; Miller, W.R.; Pryor, J.; Puskarich, M.A.; Jones, A.E. The Impact of Timing of Antibiotics on Outcomes in Severe Sepsis and Septic Shock: A Systematic Review and Meta-Analysis*. Crit. Care Med. 2015, 43, 1907–1915. [Google Scholar] [CrossRef] [Green Version]
- Nauclér, P.; Huttner, A.; van Werkhoven, C.H.; Singer, M.; Tattevin, P.; Einav, S.; Tängdén, T. Impact of time to antibiotic therapy on clinical outcome in patients with bacterial infections in the emergency department: Implications for antimicrobial stewardship. Clin. Microbiol. Infect. 2021, 27, 175–181. [Google Scholar] [CrossRef]
- Weiss, A.; Dym, H. Review of antibiotics and indications for prophylaxis. Dent. Clin. N. Am. USA 2012, 56, 235–244. [Google Scholar] [CrossRef]
- Ferorelli, D.; Zotti, F.; Tafuri, S.; Pezzolla, A.; Dalfino, L.; Brienza, N.; Dell’Erba, A. Good medical practices in the use of antibiotic prophylaxis in a surgery ward: Results of a 2013 Apulian study. Am. J. Infect. Control. USA 2015, 43, e79–e81. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.L.; Patel, M.; Claypool, S. Sepsis as a model for improving diagnosis. Diagnosis 2018, 5, 3–10. [Google Scholar] [CrossRef]
- D’Onofrio, V.; Salimans, L.; Bedenić, B.; Cartuyvels, R.; Barišić, I.; Gyssens, I.C. The Clinical Impact of Rapid Molecular Microbiological Diagnostics for Pathogen and Resistance Gene Identification in Patients With Sepsis: A Systematic Review. Open Forum Infect. Dis. 2020, 7, ofaa352. [Google Scholar] [CrossRef]
- Hincu, M.-A.; Zonda, G.-I.; Stanciu, G.D.; Nemescu, D.; Paduraru, L. Relevance of Biomarkers Currently in Use or Research for Practical Diagnosis Approach of Neonatal Early-Onset Sepsis. Children 2020, 7, 309. [Google Scholar] [CrossRef]
- Bressan, S.; Gomez, B.; Mintegi, S.; Da Dalt, L.; Blazquez, D.; Olaciregui, I.; de la Torre, M.; Palacios, M.; Berlese, P.; Ruano, A. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr. Infect. Dis. J. USA 2012, 31, 1239–1244. [Google Scholar]
- Goldstein, E.; MacFadden, D.R.; Karaca, Z.; Steiner, C.A.; Viboud, C.; Lipsitch, M. Antimicrobial resistance prevalence, rates of hospitalization with septicemia and rates of mortality with sepsis in adults in different US states. Int. J. Antimicrob. Agents 2019, 54, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Folgori, L.; Ellis, S.J.; Bielicki, J.A.; Heath, P.T.; Sharland, M.; Balasegaram, M. Tackling antimicrobial resistance in neonatal sepsis. Lancet Glob. Health 2017, 5, e1066–e1068. [Google Scholar] [CrossRef] [Green Version]
- Le Doare, K.; Bielicki, J.; Heath, P.T.; Sharland, M. Systematic Review of Antibiotic Resistance Rates Among Gram-Negative Bacteria in Children With Sepsis in Resource-Limited Countries. J. Pediatric Infect. Dis. Soc. 2015, 4, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Laxminarayan, R. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 8. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, R.; Logan, S.; Moyer, V.A.; Elliott, E.J. Assessing diagnostic and screening tests: Part 1. Concepts. West J. Med. 2001, 174, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Rojas García, P.; van der Pol, S.; van Asselt, A.D.I.; Postma, M.; Rodríguez-Ibeas, R.; Juárez-Castelló, C.A.; González, M.; Antoñanzas, F. Efficiency of Diagnostic Testing for Helicobacter pylori Infections—A Systematic Review. Antibiotics 2021, 10, 55. Available online: https://www.mdpi.com/2079-6382/10/1/55 (accessed on 10 November 2021). [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013, 346, f1049. Available online: https://www.bmj.com/content/346/bmj.f1049 (accessed on 13 November 2021). [CrossRef] [Green Version]
- Microsoft Excel. Microsoft 365. 2019. Available online: https://www.microsoft.com/zh-cn/microsoft-365/previous-versions/microsoft-office-2019 (accessed on 31 May 2021).
- Zotero. Center for History and New Media de la Universidad George Mason. 2020. Available online: https://chnm.gmu.edu/zotero/ (accessed on 31 May 2021).
- Brown, J.; Paladino, J.A. Impact of Rapid Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Testing on Mortality and Cost Effectiveness in Hospitalized Patients with Bacteraemia: A Decision Model. Pharmacoeconomics 2010, 28, 567–575. [Google Scholar] [CrossRef]
- Alvarez, J.; Mar, J.; Varela-Ledo, E.; Garea, M.; Matinez-Lamas, L.; Rodriguez, J.; Regueiro, B. Cost Analysis of Real-Time Polymerase Chain Reaction Microbiological Diagnosis in Patients with Septic Shock. Anaesth. Intensive Care 2012, 40, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Buendía, J.A. Costo-Efectividad de la Proteína C Reactiva, Procalcitonina y Escala de Rochester: Tres Estrategias Diagnosticas para la Identificación de Infección Bacteriana Severa en Lactantes Febriles sin Foco. Value Health Reg. Issues 2013, 2, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, N.; Sambri, V.; Corti, C.; Ghidoli, N.; Paolucci, M.; Clerici, D.; Carletti, S.; Greco, R.; Tassara, M.; Pizzorno, B. Cost-effectiveness of blood culture and a multiplex real-time PCR in hematological patients with suspected sepsis: An observational propensity score-matched study. Expert Rev. Mol. Diagn. 2014, 14, 623–632. [Google Scholar] [CrossRef]
- Harrison, M.; Collins, C.D. Is Procalcitonin-Guided Antimicrobial Use Cost-Effective in Adult Patients with Suspected Bacterial Infection and Sepsis? Infect. Control. Hosp. Epidemiol. 2015, 36, 265–272. [Google Scholar] [CrossRef]
- Penno, E.C.; Crump, J.A.; Baird, S.J. Performance Requirements to Achieve Cost-Effectiveness of Point-of-Care Tests for Sepsis among Patients with Febrile Illness in Low-Resource Settings. Am. J. Trop. Med. Hyg. 2015, 93, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westwood, M.; Ramaekers, B.; Whiting, P.; Tomini, F.; Joore, M.; Armstrong, N.; Ryder, S.; Stirk, L.; Severens, J.; Kleijnen, J. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2015, 19, 1–236. [Google Scholar] [CrossRef] [Green Version]
- Cambau, E.; Durand-Zaleski, I.; Bretagne, S.; Brun-Buisson, C.; Cordonnier, C.; Duval, X.; Herwegh, S.; Pottecher, J.; Courcol, R.; Bastuji-Garin, S.; et al. Performance and economic evaluation of the molecular detection of pathogens for patients with severe infections: The EVAMICA open-label, cluster-randomised, interventional crossover trial. Intensive Care Med. 2017, 43, 1613–1625. [Google Scholar] [CrossRef] [Green Version]
- Kip, M.M.A.; van Oers, J.A.; Shajiei, A.; Beishuizen, A.; Berghuis, A.M.S.; Girbes, A.R.; De Jong, E.; De Lange, D.W.; Nijsten, M.W.N.; Ijzerman, M.J.; et al. Cost-effectiveness of procalcitonin testing to guide antibiotic treatment duration in critically ill patients: Results from a randomised controlled multicentre trial in the Netherlands. Crit. Care 2018, 22, 293. [Google Scholar] [CrossRef] [Green Version]
- Pliakos, E.E.; Andreatos, N.; Shehadeh, F.; Ziakas, P.D.; Mylonakis, E. The Cost-Effectiveness of Rapid Diagnostic Testing for the Diagnosis of Bloodstream Infections with or without Antimicrobial Stewardship. Clin. Microbiol. Rev. 2018, 31, e00095-17. [Google Scholar] [CrossRef] [Green Version]
- Steuten, L.; Mewes, J.; Lepage-Nefkens, I.; Vrijhoef, H. Is Procalcitonin Biomarker-Guided Antibiotic Therapy a Cost-Effective Approach to Reduce Antibiotic Resistant and Clostridium difficile Infections in Hospitalized Patients? A J. Integr. Biol. 2018, 22, 616–625. [Google Scholar] [CrossRef]
- Collins, C.D.; Brockhaus, K.; Sim, T.; Suneja, A.; Malani, A.N. Analysis to determine cost-effectiveness of procalcitonin-guided antibiotic use in adult patients with suspected bacterial infection and sepsis. Am. J. Health-Syst. Pharm. 2019, 76, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Geisler, B.P. Model to evaluate the impact of hospital-based interventions targeting false-positive blood cultures on economic and clinical outcomes. J. Hosp. Infect. 2019, 102, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mewes, J.C.; Pulia, M.S.; Mansour, M.K.; Broyles, M.R.; Nguyen, H.B.; Steuten, L.M. The cost impact of PCT-guided antibiotic stewardship versus usual care for hospitalised patients with suspected sepsis or lower respiratory tract infections in the US: A health economic model analysis. PLoS ONE 2019, 14, e0214222. [Google Scholar] [CrossRef] [Green Version]
- Shehadeh, F.; Zacharioudakis, I.M.; Zervou, F.N.; Mylonakis, E. Cost-effectiveness of rapid diagnostic assays that perform directly on blood samples for the diagnosis of septic shock. Diagn. Microbiol. Infect. Dis. USA 2019, 94, 378–384. [Google Scholar] [CrossRef]
- Zacharioudakis, I.M.; Zervou, F.N.; Shehadeh, F.; Mylonakis, E. Cost-effectiveness of molecular diagnostic assays for the therapy of severe sepsis and septic shock in the emergency department. PLoS ONE 2019, 14, e0217508. [Google Scholar] [CrossRef] [PubMed]
- Antonio Buendia, J.; Patricia Sanchez-Villamil, J.; Urman, G. Cost-effectiveness of diagnostic strategies of severe bacterial infection in infants with fever without a source. Biomedica 2016, 36, 406–414. [Google Scholar]
- Singh, N.; Rogers, P.; Atwood, C.W.; Wagener, M.M.; Yu, V.L. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am. J. Respir. Crit. Care Med. USA 2000, 162, 505–511. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.-Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs 15 Days of Antibiotic Therapy for Ventilator-Associated Pneumonia in AdultsA Randomized Trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- van der Maas, M.; Kip, M.; Mantjes, G.; Steuten, L. A procalcitonin algorithm used in adult ICU patients with sepsis saves costs by reducing antibiotic resistance and C. difficile infections. In Proceedings of the ISPOR 18th Annual European Congress, Milan, Italy, 7–11 November 2015. [Google Scholar]
- Winters, B.D.; Eberlein, M.; Leung, J.; Needham, D.M.; Pronovost, P.J.; Sevransky, J.E. Long-term mortality and quality of life in sepsis: A systematic review*. Crit. Care Med. 2010, 38, 1276–1283. [Google Scholar] [CrossRef]
- Martínez, H.O.R.; Lago, G.S. Sepsis, causas directas de muerte y resistencia bacteriana en una unidad de cuidados intensivos. Rev. De Cienc. Médicas De Pinar Del Río 2019, 23, 6. [Google Scholar]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [Green Version]
- Mastrigt GAPG van Hiligsmann, M.; Arts, J.J.C.; Broos, P.H.; Kleijnen, J.; Evers, S.M.A.A.; Majoie, M.H.J.M. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: A five-step approach (part 1/3). Expert Rev. Pharm. Outcomes Res. 2016, 16, 689–704. [Google Scholar]
- Tattoli, L.; Dell’Erba, A.; Ferorelli, D.; Gasbarro, A.; Solarino, B. Sepsis and Nosocomial Infections: The Role of Medico-Legal Experts in Italy. Antibiotics 2019, 8, 199. Available online: https://www.mdpi.com/2079-6382/8/4/199 (accessed on 15 November 2021). [CrossRef] [Green Version]
- Rorat, M.; Jurek, T. Sepsis: Medical errors in Poland. Med. Sci. Law Engl. 2016, 56, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Umscheid, C.A.; Margolis, D.J.; Grossman, C.E. Key Concepts of Clinical Trials: A Narrative Review. Postgrad. Med. 2011, 123, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, E.R.; Venuto, C.; Venkataraman, V.; Harris, D.A.; Kieburtz, K. Novel Methods and Technologies for 21st-Century Clinical Trials: A Review. JAMA Neurol. 2015, 72, 582. [Google Scholar] [CrossRef] [PubMed]
- Huskins, W.C.; Fowler, V.G.; Evans, S. Adaptive Designs for Clinical Trials: Application to Healthcare Epidemiology Research. Clin. Infect. Dis. 2018, 66, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.A.; Sommer, H.; de Velde, F.; Gravestock, I.; Weiss, E.; McAleenan, A.; Nikolakopoulos, S.; Amit, O.; Ashton, T.; Beyersmann, J.; et al. Optimizing the Design and Analysis of Clinical Trials for Antibacterials Against Multidrug-Resistant Organisms: A White Paper From COMBACTE’s STAT-Net. Clinical Infectious Diseases. 2018. Available online: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciy516/5043522 (accessed on 19 April 2021).
- Hirschfeld, S.; Lagler, F.B.; Kindblom, J.M. Prerequisites to support high-quality clinical trials in children and young people. Arch. Dis. Child. 2020, 106, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Lagler, F.B.; Hirschfeld, S.; Kindblom, J.M. Challenges in clinical trials for children and young people. Arch. Dis. Child. 2021, 106, 321–325. [Google Scholar] [CrossRef] [PubMed]
| First Author (Year) | Country | Setting | Perspective, Time Horizon, and Population | Type of Model | Strategies Compared (*) | Cost-Effectiveness Results (*) | Turn-Around | Treatment | AMR Included | Uncertainty Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Brown (2010) [28] | Europe and USA | Hospital | Healthcare center’s
| Decision tree | (1) Empiric vancomycin; (2) semi-synthetic penicillin; (3) PCR that distinguishes MRSA and MSSA | In EU (1) EUR 695 per life-year saved; (2) EUR 687 per life-year saved; (3) EUR 636 per life-year saved. In USA (1) USD 898 per life-year saved; (2) NA; (3) USD 820 per life-year saved | (3) In less than 1 h | Semi-synthetic penicillin if MSSA and vancomycin if MRSA | Test can detect and differentiate between MSSA and MRSA (treatment is guided) | DSA, sensitivity analysis graph |
| Alvarez (2012) [29] | Spain | Hospital and ICU |
| Individual sampling model | (1) PCR; (2) standard care (broad-spectrum antibiotic) | (1) EUR 32,228 per patient; (2) EUR 42,198 per patient | (1) In a few hours | Antibiotic treatment | Test can narrow the spectrum of antibiotics and lower rate of ICU patients | DSA |
| Buendía (2013) [30] | Argentina | Hospital |
| Decision tree | (1) PCT; (2) PCR; (3) Rochester criteria | (1) USD 943 per correctly diagnosed case; (2) USD 937 per correctly diagnosed case; (3) USD 1241 per correctly diagnosed case | NA | Antibiotic treatment | No | DSA, tornado diagram |
| Mancini (2014) [31] | Italy | Hospital |
| Observational, propensity score-matched analysis | (1) PCR; (2) standard diagnostic assays | (1) EUR 1579 per patient; (2) EUR 2010 per patient | NA | Antibiotic treatment | Mentioned as a limitation | PSA |
| Harrison (2015) [32] | USA | Hospital and ICU |
| Decision tree | (1) PCT; (2) standard care (broad-spectrum antibiotic) | (1) vs. (2) +0.0002 QALYs gained and − USD 65 per patient USD 245,501 (ICER) | NA | Vancomycin and cefepime | Test can detect and differentiate between MSSA and MRSA (treatment is guided) | DSA, PSA |
| Penno (2015) [33] | Ethiopia, Gambia, Papua New Guinea, and the Philippines | Hospital |
| Decision tree | (1) POCT; (2) clinical assessment | (1) vs. (2) + USD 147 per life saved (lowest prevalence) (1) vs. (2) + USD 4988 per life saved (highest prevalence) | (1) Results available in a timeframe that can inform initial patient management. | Ampicillin, gentamicin, and ceftriaxone | Mentioned as a limitation | DSA, sensitivity analysis graph |
| Westwood (2015) [34] | United Kingdom | ED and ICU |
| Decision tree | (1) PCT; (2) standard care (broad-spectrum antibiotic) | (1) vs. (2) +0.005 QALYs gained | NA | Antibiotic treatment | Mentioned as a limitation | DSA, PSA, CE plane, CE acceptability curve |
| Cambau (2017) [35] | France | Hospital |
| Decision tree | (1) Blood cultures; (2) LSF | (2) vs. (1) − EUR 535 per patient | (1) 2–3 days (2) a shorter time to results | Beta-lactams, cephalosporins, and other antibiotics | Test can detect resistant infection (treatment is guided) | PSA, CE plane |
| Kip (2018) [36] | The Netherlands | Hospital and ICU |
| Decision tree | (1) PCT; (2) standard care (broad-spectrum antibiotic) | (1) EUR 46,081 and +0.47 QALY per patient gained (2) EUR 46,146 per patient | (1) Result available in the first 24 h | Antibiotic treatment | Mentioned as a limitation | CE plane, CE acceptability curve |
| Pliakos (2018) [37] | USA | Hospital |
| Decision tree | 12 strategies: MALDI-TOF analysis with an ASP; conventional laboratory methods without an ASP; others | Rapid diagnostic tests results in less than 24 h. MALDI-TOF resulted in + USD 29,205 per quality-adjusted life year compared to conventional laboratory methods | Conventional laboratory methods up to 5 days for Results. | Antibiotic treatment | Mentioned as a limitation | CE plane, CE acceptability curve, PSA |
| Steuten (2018) [38] | United Kingdom, Germany, and the Netherlands | Hospital |
| Decision tree | (1) PCT; (2) standard care (broad-spectrum antibiotic) | (1) vs. (2) − EUR 1071 (Germany), – EUR 1124 (the Netherlands), and −EUR 1163 (UK) hospital costs. Societal cost savings of + EUR 1309; + EUR 1371, and + EUR 1321 per patient, respectively. | NA | Antibiotic treatment based on the concentration of PCT | The incidence of AMR was included in the model | DSA, tornado diagram, sensitivity analysis graph, PSA |
| Collins (2019) [39] | USA | ICU |
| Decision tree | (1) PCT; (2) standard care (broad-spectrum antibiotic) | (1) vs. (2) +0.0001 QALYs gained and -USD 45 per patient | (1) Result available in the first 24 h | Antibiotic treatment | Mentioned as a limitation | PSA |
| Geisler (2019) [40] | USA | Hospital |
| Decision tree | (1) Blood cultures; (2) ISDD; (3) phlebotomists | (2) annual savings in a hospital of USD 1.9 million and prevent 34 hospital-acquired conditions | NA | Antibiotic treatment | Antibiotic use and adverse clinical consequences as outcomes of the model | DSA, tornado diagram |
| Mewes (2019) [41] | USA | Hospital and ICU |
| Decision tree | (1) PCT; (2) standard care (broad-spectrum antibiotic) | (1) vs. (2) saved USD 11,311 per patient | (1) Result available in the first 24 h | Antibiotic treatment | Patients with antibiotic resistant infections and antibiotics as outcomes of the model | DSA, tornado diagram of DSA |
| Shehadeh (2019) [42] | USA | ICU |
| Decision tree | (1) Only blood culture; (2) molecular testing and blood culture | (2) vs. (1) USD 3000 per death averted | (2) In 2–7 h | Antibiotic treatment | Test can detect resistant infection (treatment is guided) | DSA |
| Zacharioudakis (2019) [43] | USA | ED |
| Decision tree | (1) PCT; (2) standard care | (1) vs. (2) − USD 20,000 per death averted | NA | Antibiotic treatment | Test can detect resistant infection (treatment is guided) | DSA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Garcia, P.; van der Pol, S.; van Asselt, A.D.I.; Postma, M.J.; Rodríguez-Ibeas, R.; Juárez-Castelló, C.A.; González, M.; Antoñanzas, F. Diagnostic Testing for Sepsis: A Systematic Review of Economic Evaluations. Antibiotics 2022, 11, 27. https://doi.org/10.3390/antibiotics11010027
Rojas-Garcia P, van der Pol S, van Asselt ADI, Postma MJ, Rodríguez-Ibeas R, Juárez-Castelló CA, González M, Antoñanzas F. Diagnostic Testing for Sepsis: A Systematic Review of Economic Evaluations. Antibiotics. 2022; 11(1):27. https://doi.org/10.3390/antibiotics11010027
Chicago/Turabian StyleRojas-Garcia, Paula, Simon van der Pol, Antoinette D. I. van Asselt, Maarten J. Postma, Roberto Rodríguez-Ibeas, Carmelo A. Juárez-Castelló, Marino González, and Fernando Antoñanzas. 2022. "Diagnostic Testing for Sepsis: A Systematic Review of Economic Evaluations" Antibiotics 11, no. 1: 27. https://doi.org/10.3390/antibiotics11010027
APA StyleRojas-Garcia, P., van der Pol, S., van Asselt, A. D. I., Postma, M. J., Rodríguez-Ibeas, R., Juárez-Castelló, C. A., González, M., & Antoñanzas, F. (2022). Diagnostic Testing for Sepsis: A Systematic Review of Economic Evaluations. Antibiotics, 11(1), 27. https://doi.org/10.3390/antibiotics11010027









