Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula angustifolia against Persister-Derived Biofilm of Candida auris
Abstract
:1. Introduction
2. Results
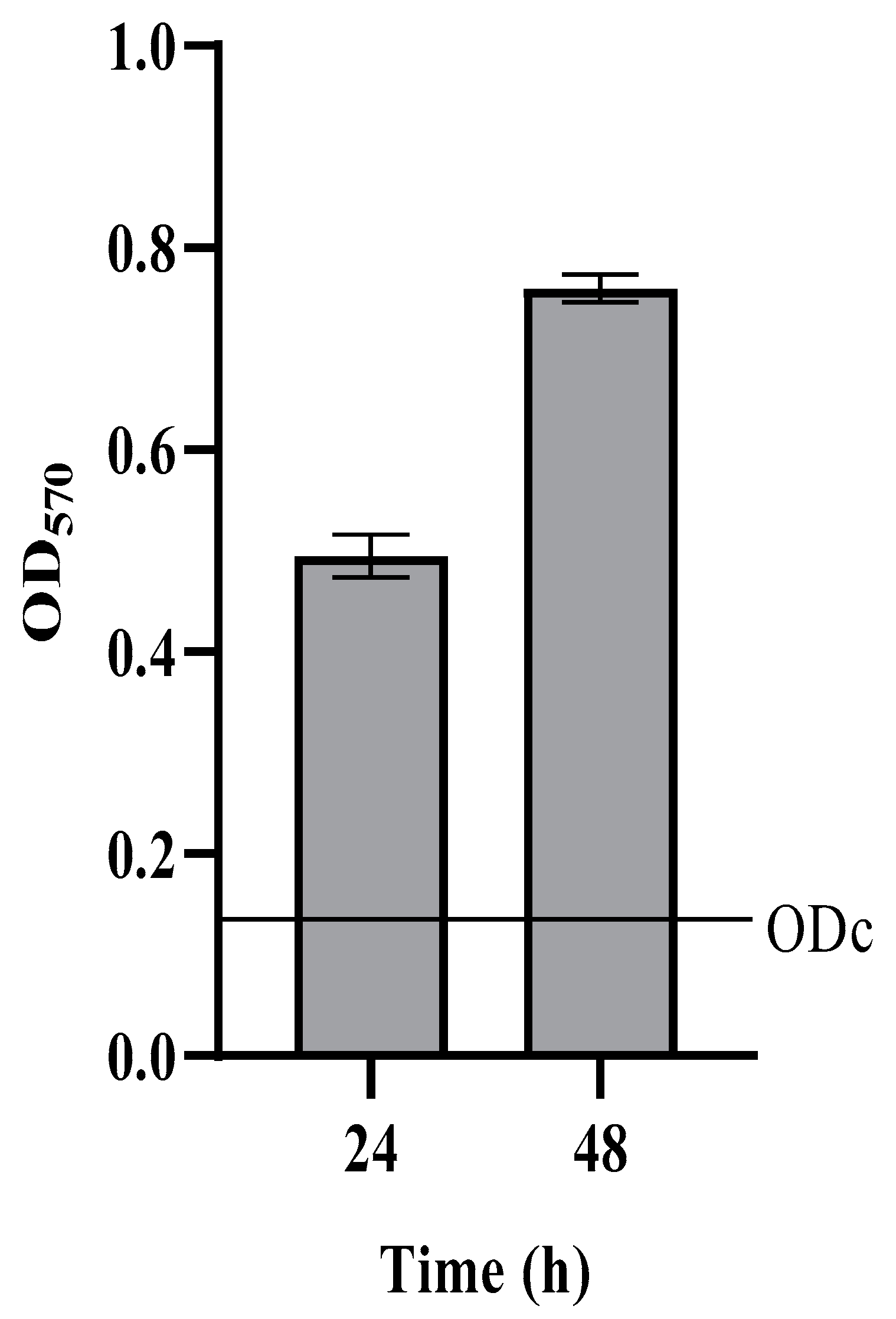
2.1. Detection of Persisters Cells in C. auris Biofilms
2.2. Composition of L. angustifolia Oil
2.3. Characterization of Liposome-Encapsulated Oil of L. angustifolia
2.4. Antifungal Activity of L. angustifolia Free and Encapsulated Oil on Planktonic Cells and Biofilms of C. auris
3. Discussion
4. Materials and Methods
4.1. Strain and Culture Condition
4.2. GC-MS Analysis of L. angustifolia Oil
4.3. Preparation of Liposome-Encapsulated L. angustifolia Oil
4.4. Determination of Caspofungin Minimum Inhibitory Concentration
4.5. Effect of L. aungustifolia Oil and Liposome-Encapsulated Oil on Planktonic C. auris Cells
4.6. Biofilm Formation and Quantification
4.7. Detection of Persisters Cells
4.8. Development of Persister-Derived Biofilm of C. auris
4.9. Eradication of Biofilm with Free Liposome-Encapsulated L. angustifolia Oil
4.10. Determination of iROS in Persisters-Derived Biofilms of C. auris
4.11. Gene Expression in C. auris Persister-Derived Biofilms Treated with L. angustifolia Free and Liposome-Encapsulated Oil
4.12. Confocal Laser Scanning Microscopy
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Chowdhary, A.; Dannaoui, E. Candida auris: An emerging drug resistant yeast—A mini-review. J. Mycol. Méd. 2018, 28, 568–573. [Google Scholar] [CrossRef]
- Chaabane, F.; Graf, A.; Jeqier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.B.; Lorenz, A. What do we know about the biology of the emerging fungal pathogen of humans Candida auris? Microbiol. Res. 2021, 242, 126621. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; Delanaey, C.; Sherry, L.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome Assembly and Profiling of Candida auris Reveals Novel Insights into Biofilm-Mediated Resistance. mSphere 2018, 3, e00334-18. [Google Scholar] [CrossRef] [Green Version]
- Kean, R.; Ramage, G. Combined Antifungal Resistance and Biofilm Tolerance: The Global Threat of Candida auris. mSphere 2019, 4, e00458-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, E.G.; Zarnowski, R.; Choy, H.L.; Zhao, M.; Sanchez, H.; Nett, J.E.; Andes, D.R. Conserved Role for Biofilm Matrix Polysaccharides in Candida auris Drug Resistance. Msphere 2019, 4, e00680-18. [Google Scholar] [CrossRef] [Green Version]
- Short, B.; Borwn, J.; Delanaey, C.; Sherry, L.; Wlliams, C.; Ramage, G.; Kean, R. Candida auris exhibits resilient biofilm characteristics in vitro: Implications for environmental persistence. J. Hosp. Infect. 2019, 103, 92–96. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [Green Version]
- Wuyts, J.P.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef]
- Galdiero, E.; de Alteriis, E.; De Natale, A.; D’Alterio, A.; Siciliano, A.; Guida, M.; Lombardi, L.; Falanga, A.; Galdiero, S. Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Aboualigalehdari, E.; Birgani, M.T.; Fatahinia, M.; Hozzeinzadeh, M. Transcription Factors of CAT1, EFG1, and BCR1 Are Effective in Persister Cells of Candida albicans-Associated HIV-Positive and Chemotherapy Patients. Front. Microbiol. 2021, 12, 651221. [Google Scholar] [CrossRef]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [Green Version]
- Dudiuk, C.; Berrio, I.; Leonardelli, F.; Morales-Lopez, S.; Theill, L.; Macedo, D.; Yesid-Rodriguez, J.; Salcedo, S.; Marin, A.; Gamarra, S.; et al. Antifungal activity and killing kinetics of anidulafungin, caspofungin and amphotericin B against Candida auris. J. Antimicrob. Chemother. 2019, 74, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant Preparations and Compounds with Activities against Biofilms Formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef]
- Butassi, E.; Svetaz, L.; Carpinella, M.C.; Efferth, T.; Zacchino, S. Fungal Biofilms as a Valuable Target for the Discovery of Natural Products That Cope with the Resistance of Medically Important Fungi—Latest Findings. Antibiotics 2021, 10, 1053. [Google Scholar] [CrossRef] [PubMed]
- Tariqa, S.; Wania, S.; Rasoola, W.; Shafia, K.; Bhat, M.A.; Prabhakarb, A.; Shallaa, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Tran, H.N.; Graham, L.; Adukwu, E.C. In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [Green Version]
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol. Immunol. 2003, 47, 681–684. [Google Scholar] [CrossRef]
- Roller, S.; Ernest, N.; Buckle, J. The Antimicrobial Activity of High-Necrodane and Other Lavender Oils on Methicillin-Sensitive and -Resistant Staphylococcus aureus (MSSA and MRSA). J. Altern. Complementary Med. 2009, 15, 275–279. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Gniewosz, M.; Kosakowska, O.; Pobiega, K. Chemical composition and antimicrobial properties of essential oil from lavender (Lavandula angustifolia L.) in commercial available preparation. Postępy Fitoter. 2017, 113–118. [Google Scholar] [CrossRef]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential oils encapsulated in liposomes: A review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by y TiO2–MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef]
- Verma, M.L.; Chaudhary, R.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: Application in cellobiose hydrolysis. Bioresour. Technol. 2013, 135, 2–6. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Liposomes as delivery systems for antibiotics. Int. J. Pharm. 2010, 387, 187–198. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.R.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris during the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef]
- Walraven, C.J.; Bernardo, S.M.; Wiederhold, N.P.; Lee, S.A. Paradoxical antifungal activity and structural observations in biofilms formed by echinocandin-resistant Candida albicans clinical isolates. Med. Mycol. 2013, 52, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1–8. [Google Scholar]
- D’auria, F.D.; Tecca, M.; Strippoli, V.; Salvatore, G.; Battinelli, L.; Mazzanti, G. Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med. Mycol. 2005, 43, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Rapper, S.; Kamatou, G.; Viljoen, A.; van Vuuren, S. The In Vitro Antimicrobial Activity of Lavandula angustifolia Essential Oil in Combination with Other Aroma-Therapeutic Oils. Evid. Based Complement. Altern. Med. 2013, 2013, 852049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, E.L. The effects of sublethal doses of essential oils and their constituents on antimicrobial susceptibility and antibiotic resistance among food-related bacteria: A review. Trends Food Sci. Technol. 2016, 56, 1–12. [Google Scholar] [CrossRef]
- Gupta, P.; Pruthi, V.; Poluri, K.M. Mechanistic insights into Candida biofilm eradication potential of eucalyptol. J. Appl. Microbiol. 2021, 131, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lai, W.L.; Chuang, K.C.; Lee, M.H.; Tsai, Y.C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 2013, 51, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Fothergill, A.W. Antifungal Susceptibility Testing: Clinical Laboratory and Standards Institute (CLSI) Methods. In Interactions of Yeasts, Moulds, and Antifungal Agents: How to Detect Resistance; Hall, G.S., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 65–74. [Google Scholar]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Holowka, T.; Orner, E.P.; Fries, B.C. Gene Duplication Associated with Increased Fluconazole Tolerance in Candida auris cells of Advanced Generational Age. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Wang, L.; Chen, R.; Weng, Q.; Lin, S.; Wang, H.; Li, L.; Fuchs, B.B.; Tan, X.; Mylonakis, E. SPT20 Regulates the Hog1-MAPK Pathway and Is Involved in Candida albicans Response to Hyperosmotic Stress. Front. Microbiol. 2020, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Riesgo, A.; Pérez-Porro, A.R.; Carmona, S.; Leys, S.P.; Giribet, G. Optimization of preservation and storage time of sponge tissues to obtain quality mRNA for next-generation sequencing. Mol. Ecol. Resour. 2012, 12, 312–322. [Google Scholar] [CrossRef] [PubMed]








| Compound | RI | Peak Area (%) |
|---|---|---|
| β-Myrcene | 892 | 0.14 |
| 1,8-Cineole | 932 | 1.20 |
| β-Pinene | 946 | 1.29 |
| trans-Ocimene | 954 | 0.27 |
| Linalool | 1034 | 39.31 |
| Camphor | 1059 | 0.11 |
| Isoborneol | 1078 | 0.95 |
| 4-Terpineol | 1091 | 1.40 |
| Cis-β-Tepineol | 1100 | 0.14 |
| α-Terpineol | 1107 | 0.08 |
| Linalyl acetate | 1180 | 48.45 |
| Lavandulyl acetate | 1207 | 2.17 |
| Geraniol acetate | 1292 | 0.45 |
| Caryophyllene | 1335 | 2.01 |
| β-Farnesene | 1366 | 1.77 |
| β-Cubebene | 1390 | 0.07 |
| Caryophyllene oxide | 1494 | 0.21 |
| Sample | Mean Diameter (nm) | Mean PDI | Mean Zeta Potential (mV) |
|---|---|---|---|
| Liposome-encapsulated Oil | 177.2 ± 11.49 | 0.295 ± 0.053 | −3.73 ± 0.099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Alteriis, E.; Maione, A.; Falanga, A.; Bellavita, R.; Galdiero, S.; Albarano, L.; Salvatore, M.M.; Galdiero, E.; Guida, M. Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula angustifolia against Persister-Derived Biofilm of Candida auris. Antibiotics 2022, 11, 26. https://doi.org/10.3390/antibiotics11010026
de Alteriis E, Maione A, Falanga A, Bellavita R, Galdiero S, Albarano L, Salvatore MM, Galdiero E, Guida M. Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula angustifolia against Persister-Derived Biofilm of Candida auris. Antibiotics. 2022; 11(1):26. https://doi.org/10.3390/antibiotics11010026
Chicago/Turabian Stylede Alteriis, Elisabetta, Angela Maione, Annarita Falanga, Rosa Bellavita, Stefania Galdiero, Luisa Albarano, Maria Michela Salvatore, Emilia Galdiero, and Marco Guida. 2022. "Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula angustifolia against Persister-Derived Biofilm of Candida auris" Antibiotics 11, no. 1: 26. https://doi.org/10.3390/antibiotics11010026
APA Stylede Alteriis, E., Maione, A., Falanga, A., Bellavita, R., Galdiero, S., Albarano, L., Salvatore, M. M., Galdiero, E., & Guida, M. (2022). Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula angustifolia against Persister-Derived Biofilm of Candida auris. Antibiotics, 11(1), 26. https://doi.org/10.3390/antibiotics11010026












