Effect of Doxycycline in Decreasing the Severity of Clostridioides difficile Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. difficile Strain
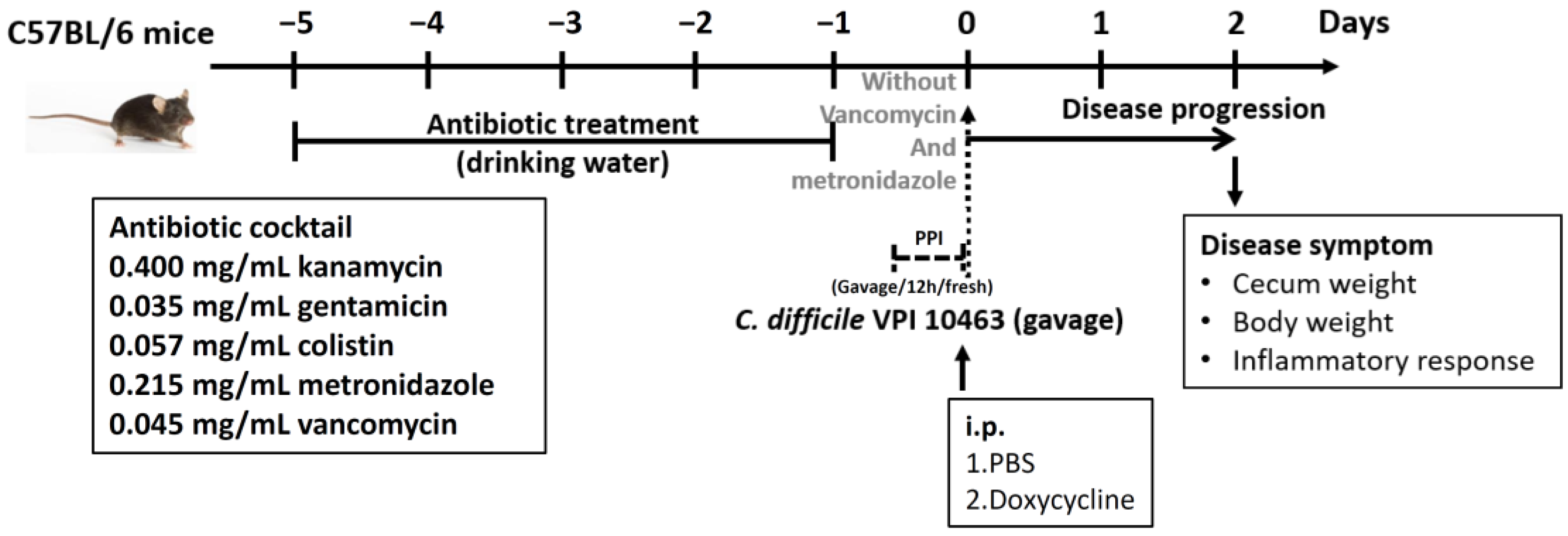
2.2. Concurrent Doxycycline Exposure and C. difficile Challenge in Mice
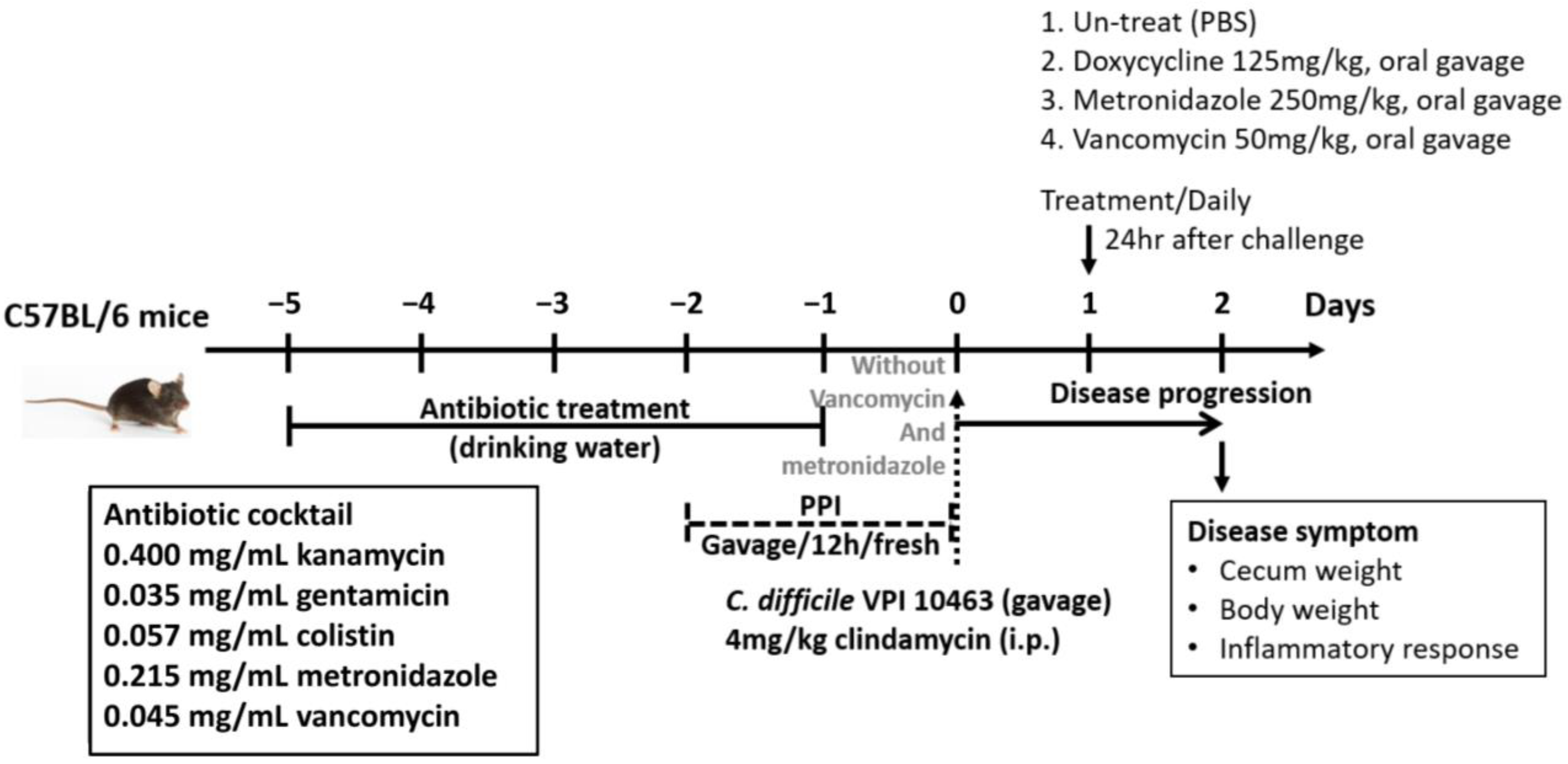
2.3. One Dose of Oral Antibiotic Therapy for CDI in Mice
2.4. Phenotypic Analysis of CDI
2.5. Bacterial Burden
2.6. Expression of Inflammatory Cytokines and Tight Junction Proteins in Colonic Tissues
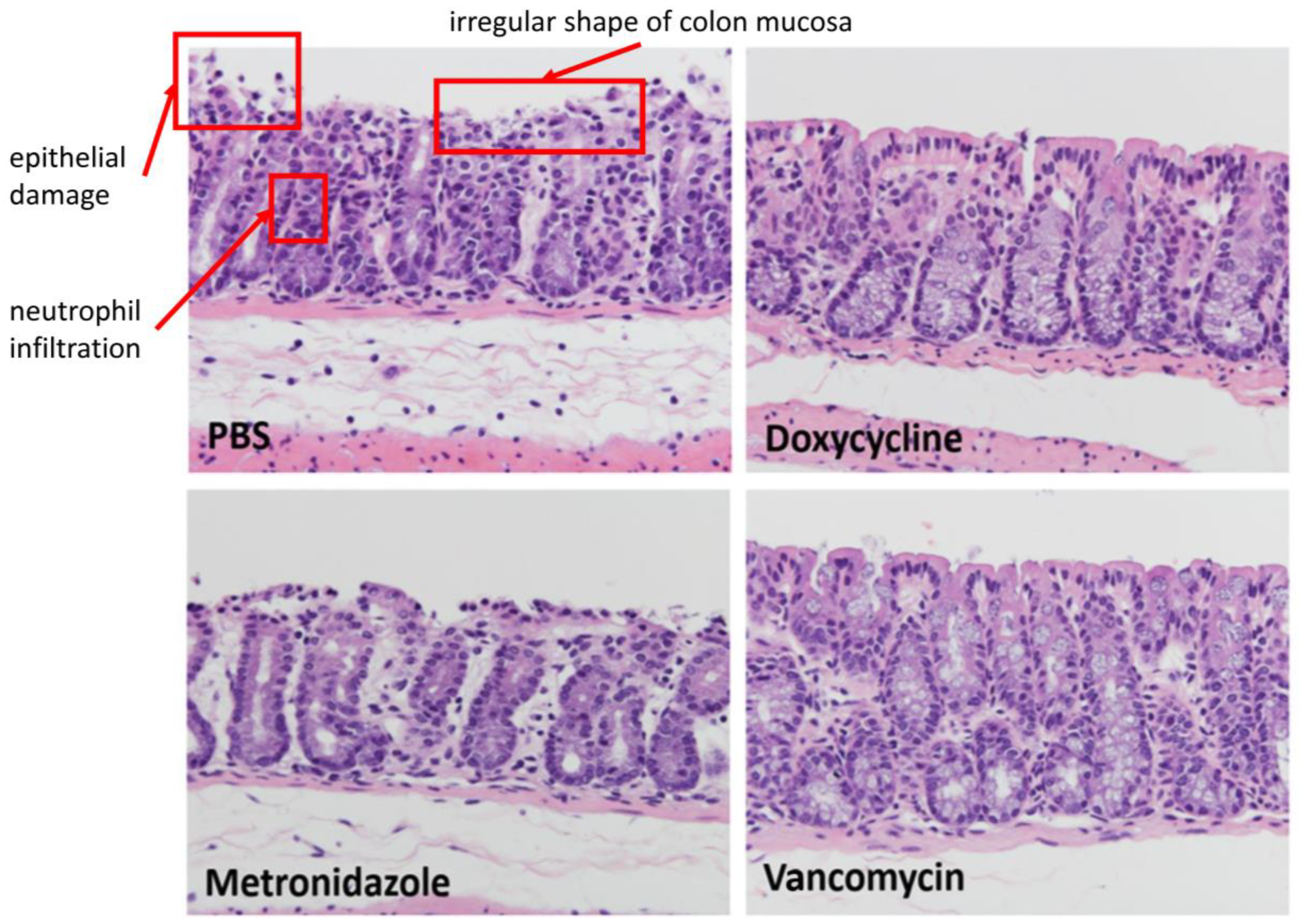
2.7. Histopathologic Examination
2.8. Statistical Method
3. Results
3.1. Doxycycline Exposure before C. difficile Infection
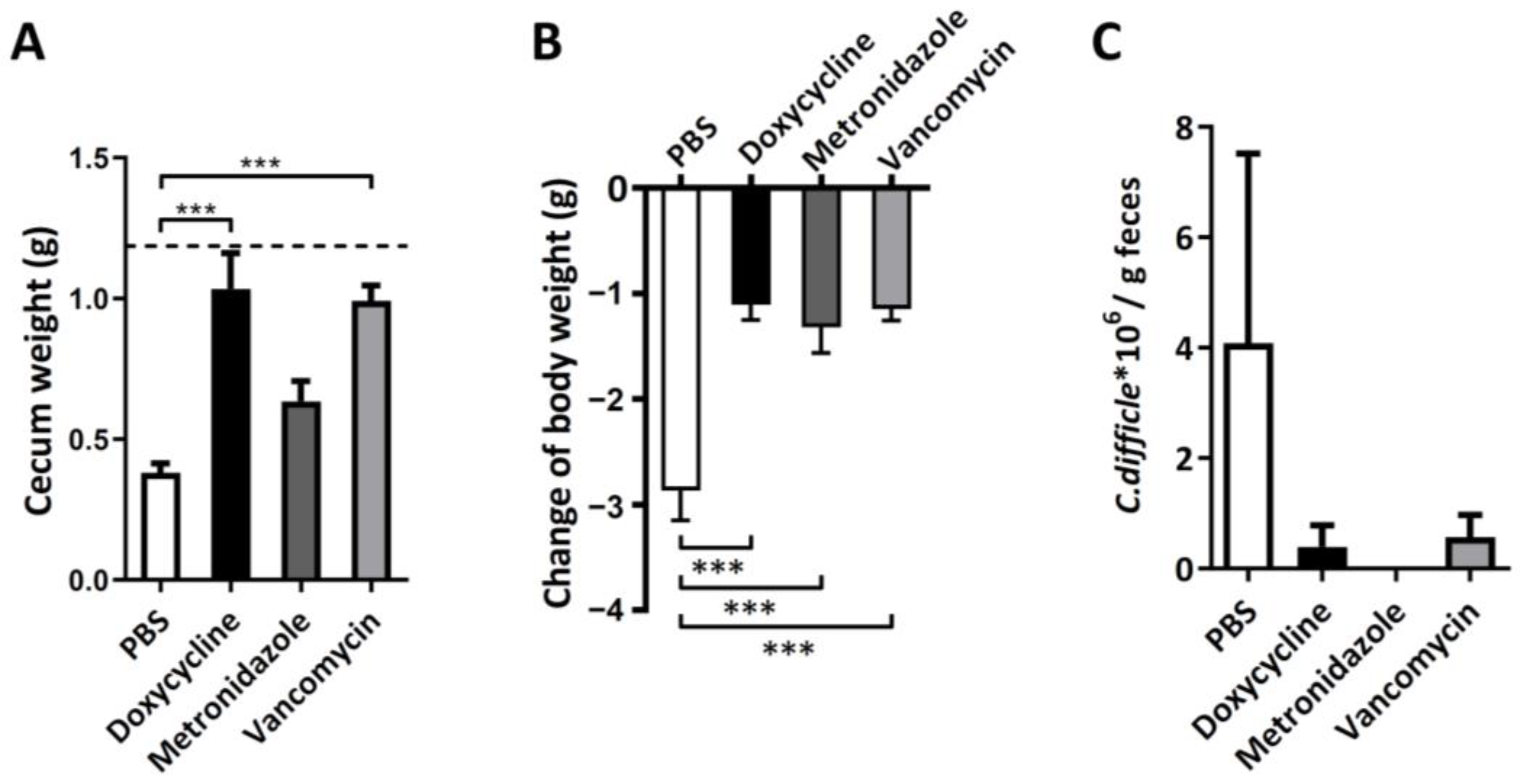
3.2. Therapeutic Effect of Oral Doxycycline on C. difficile Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuijper, E.J.; van Dissel, J.T.; Wilcox, M.H. Clostridium difficile: Changing epidemiology and new treatment options. Curr. Opin. Infect. Dis. 2007, 20, 376–383. [Google Scholar] [CrossRef]
- Zhang, R.F.; Man, Y.X.; Bai, Y.Y.; Shao, C.H.; Liu, C.M.; Wang, C.H.; Lei, Y.X.; Wang, Y.; Jin, Y. Molecular characterization of Clostridioides difficile ribotype 027 in a major Chinese hospital. J. Microbiol. Immunol. Infect. 2021, 54, 1179–1183. [Google Scholar] [CrossRef]
- Lee, J.C.; Hung, Y.P.; Tsai, B.Y.; Tsai, P.J.; Ko, W.C. Severe Clostridium difficile infections in intensive care units: Diverse clinical presentations. J. Microbiol. Immunol. Infect. 2020, 54, 1111–1117. [Google Scholar] [CrossRef]
- Chang, T.H.; Hsu, W.Y.; Yang, T.I.; Lu, C.Y.; Hsueh, P.R.; Chen, J.M.; Lee, P.I.; Huang, L.M.; Chang, L.Y. Increased age and proton pump inhibitors are associated with severe Clostridium difficile infections in children. J. Microbiol. Immunol. Infect. 2020, 53, 578–584. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lee, J.C.; Tsai, B.Y.; Wu, J.L.; Liu, H.C.; Liu, H.C.; Lin, H.J.; Tsai, P.J.; Ko, W.C. Risk factors of Clostridium difficile-associated diarrhea in hospitalized adults: Vary by hospitalized duration. J. Microbiol. Immunol. Infect. 2019, 54, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Tsai, C.S.; Tsai, B.Y.; Tsai, P.J.; Lee, Y.T.; Lee, J.C.; Liu, H.C.; Hsueh, P.R.; Lee, C.C.; Ko, W.C. Clostridioides difficile infection in patients with hematological malignancy: A multicenter study in Taiwan. J. Microbiol. Immunol. Infect. 2021, 54, 1101–1110. [Google Scholar] [CrossRef]
- Lin, T.C.; Hung, Y.P.; Ko, W.C.; Ruan, J.W. Fecal microbiota transplantation for Clostridium difficile infection in Taiwan: Establishment and implementation. J. Microbiol. Immunol. Infect. 2019, 52, 841–850. [Google Scholar] [CrossRef]
- Wu, K.S.; Syue, L.S.; Cheng, A.; Yen, T.Y.; Chen, H.M.; Chiu, Y.H.; Hsu, Y.L.; Chiu, C.H.; Su, T.Y.; Tsai, W.L.; et al. Recommendations and guidelines for the treatment of Clostridioides difficile infection in Taiwan. J. Microbiol. Immunol. Infect. 2020, 53, 191–208. [Google Scholar] [CrossRef]
- Chang, F.C.; Liu, C.P.; Sun, F.J.; Lin, C.C. Optimizing laboratory workflow for the diagnosis of Clostridiodes difficile infection in a medical center in Northern Taiwan. J. Microbiol. Immunol. Infect. 2021, 54, 284–289. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef]
- Li, Y.; Figler, R.A.; Kolling, G.; Bracken, T.C.; Rieger, J.; Stevenson, R.W.; Linden, J.; Guerrant, R.L.; Warren, C.A. Adenosine A2A receptor activation reduces recurrence and mortality from Clostridium difficile infection in mice following vancomycin treatment. BMC Infect. Dis. 2012, 12, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koon, H.W.; Wang, J.; Mussatto, C.C.; Ortiz, C.; Lee, E.C.; Tran, D.H.; Chen, X.; Kelly, C.P.; Pothoulakis, C. Fidaxomicin and OP-1118 Inhibit Clostridium difficile Toxin A- and B-Mediated Inflammatory Responses via Inhibition of NF-kappaB Activity. Antimicrob. Agents Chemother. 2018, 62, e01513-17. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Golledge, C.L.; Riley, T.V. Clostridium difficile-associated diarrhoea after doxycycline malaria prophylaxis. Lancet 1995, 345, 1377–1378. [Google Scholar] [CrossRef]
- Baxter, R.; Ray, G.T.; Fireman, B.H. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect. Control Hosp. Epidemiol. 2008, 29, 44–50. [Google Scholar] [CrossRef]
- Doernberg, S.B.; Winston, L.G.; Deck, D.H.; Chambers, H.F. Does doxycycline protect against development of Clostridium difficile infection? Clin. Infect. Dis. 2012, 55, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.B.; Smith, C.B.; Martello, J.L.; Slain, D. Role of doxycycline in Clostridium difficile infection acquisition. Ann. Pharmacother. 2014, 48, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Loffler, B.; Ackermann, G. Antimicrobial phenotypes and molecular basis in clinical strains of Clostridium difficile. Diagn. Microbiol. Infect. Dis. 2007, 59, 1–5. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Keates, A.C.; Wang, C.C.; Pasha, A.; Valenick, L.; Kelly, C.P.; Nikulasson, S.T.; LaMont, J.T.; Pothoulakis, C. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J. Immunol. 1998, 160, 6039–6045. [Google Scholar]
- Hung, Y.P.; Tsai, P.J.; Lee, Y.T.; Tang, H.J.; Lin, H.J.; Liu, H.C.; Lee, J.C.; Tsai, B.Y.; Hsueh, P.R.; Ko, W.C. Nationwide surveillance of ribotypes and antimicrobial susceptibilities of toxigenic Clostridium difficile isolates with an emphasis on reduced doxycycline and tigecycline susceptibilities among ribotype 078 lineage isolates in Taiwan. Infect. Drug Resist. 2018, 11, 1197–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, Y.P.; Ko, W.C.; Chou, P.H.; Chen, Y.H.; Lin, H.J.; Liu, Y.H.; Tsai, H.W.; Lee, J.C.; Tsai, P.J. Proton-Pump Inhibitor Exposure Aggravates Clostridium difficile-Associated Colitis: Evidence From a Mouse Model. J. Infect. Dis. 2015, 212, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Jump, R.L.; Li, Y.; Pultz, M.J.; Kypriotakis, G.; Donskey, C.J. Tigecycline exhibits inhibitory activity against Clostridium difficile in the colon of mice and does not promote growth or toxin production. Antimicrob. Agents Chemother. 2011, 55, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Borriello, S.P.; Barclay, F.E. An in-vitro model of colonisation resistance to Clostridium difficile infection. J. Med. Microbiol. 1986, 21, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Gustafsson, J.K.; Sjoberg, K.E.; Petersson, J.; Holm, L.; Sjovall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238. [Google Scholar] [CrossRef] [Green Version]
- Verdu, E.F.; Bercik, P.; Cukrowska, B.; Farre-Castany, M.A.; Bouzourene, H.; Saraga, E.; Blum, A.L.; Corthesy-Theulaz, I.; Tlaskalova-Hogenova, H.; Michetti, P. Oral administration of antigens from intestinal flora anaerobic bacteria reduces the severity of experimental acute colitis in BALB/c mice. Clin. Exp. Immunol. 2000, 120, 46–50. [Google Scholar] [CrossRef]
- Hansen, A.K.; Malm, S.A.; Metzdorff, S.B. The cre-inducer doxycycline lowers cytokine and chemokine transcript levels in the gut of mice. J. Appl. Genet. 2017, 58, 535–538. [Google Scholar] [CrossRef]
- Boynton, F.D.D.; Ericsson, A.C.; Uchihashi, M.; Dunbar, M.L.; Wilkinson, J.E. Doxycycline induces dysbiosis in female C57BL/6NCrl mice. BMC Res. Notes 2017, 10, 644. [Google Scholar] [CrossRef] [Green Version]
- Angelakis, E.; Million, M.; Kankoe, S.; Lagier, J.C.; Armougom, F.; Giorgi, R.; Raoult, D. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob. Agents Chemother. 2014, 58, 3342–3347. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.H.; Wu, C.J.; Lee, H.C.; Yan, J.J.; Chang, C.M.; Lee, N.Y.; Chen, P.L.; Lee, C.C.; Hung, Y.P.; Ko, W.C. Clostridium difficile infection at a medical center in southern Taiwan: Incidence, clinical features and prognosis. J. Microbiol. Immunol. Infect. 2010, 43, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Hung, Y.P.; Lin, H.J.; Wu, T.C.; Liu, H.C.; Lee, J.C.; Lee, C.I.; Wu, Y.H.; Wan, L.; Tsai, P.J.; Ko, W.C. Risk factors of fecal toxigenic or non-toxigenic Clostridium difficile colonization: Impact of Toll-like receptor polymorphisms and prior antibiotic exposure. PLoS ONE 2013, 8, e69577. [Google Scholar] [CrossRef] [PubMed]
- Riggs, M.M.; Sethi, A.K.; Zabarsky, T.F.; Eckstein, E.C.; Jump, R.L.; Donskey, C.J. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin. Infect. Dis. 2007, 45, 992–998. [Google Scholar] [CrossRef]
- Ryan, J.; Murphy, C.; Twomey, C.; Paul Ross, R.; Rea, M.C.; MacSharry, J.; Sheil, B.; Shanahan, F. Asymptomatic carriage of Clostridium difficile in an Irish continuing care institution for the elderly: Prevalence and characteristics. Ir. J. Med. Sci. 2010, 179, 245–250. [Google Scholar] [CrossRef]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; van den Brink, M.R.; Kamboj, M.; et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef]
- Hung, Y.P.; Tsai, P.J.; Hung, K.H.; Liu, H.C.; Lee, C.I.; Lin, H.J.; Wu, Y.H.; Wu, J.J.; Ko, W.C. Impact of toxigenic Clostridium difficile colonization and infection among hospitalized adults at a district hospital in southern Taiwan. PLoS ONE 2012, 7, e42415. [Google Scholar] [CrossRef]
- Kouassi, K.A.; Dadie, A.T.; N’Guessan, K.F.; Dje, K.M.; Loukou, Y.G. Clostridium perfringens and Clostridium difficile in cooked beef sold in Cote d’Ivoire and their antimicrobial susceptibility. Anaerobe 2014, 28, 90–94. [Google Scholar] [CrossRef]
- Simango, C.; Mwakurudza, S. Clostridium difficile in broiler chickens sold at market places in Zimbabwe and their antimicrobial susceptibility. Int. J. Food Microbiol. 2008, 124, 268–270. [Google Scholar] [CrossRef]
- Simango, C. Prevalence of Clostridium difficile in the environment in a rural community in Zimbabwe. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 1146–1150. [Google Scholar] [CrossRef]
- McKee, H.K.; Kajiwara, C.; Yamaguchi, T.; Ishii, Y.; Shimizu, N.; Ohara, A.; Tateda, K. Clostridioides difficile toxins enhanced the in vitro production of CXC chemokine ligand 2 and tumor necrosis factor-alpha via Toll-like receptors in macrophages. J. Med. Microbiol. 2021, 70, 001342. [Google Scholar] [CrossRef]
- McDermott, A.J.; Falkowski, N.R.; McDonald, R.A.; Frank, C.R.; Pandit, C.R.; Young, V.B.; Huffnagle, G.B. Role of interferon-gamma and inflammatory monocytes in driving colonic inflammation during acute Clostridium difficile infection in mice. Immunology 2017, 150, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Khande, H.; Periasamy, H.; Mokale, S. Immunomodulatory Effect of Doxycycline Ameliorates Systemic and Pulmonary Inflammation in a Murine Polymicrobial Sepsis Model. Inflammation 2020, 43, 1035–1043. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, X.; Wu, D.; Jin, X.; Liu, R.; Hu, X.; Fu, Y.; Ding, Z.; Zhang, N.; Cao, Y. Doxycycline Attenuates Leptospira-Induced IL-1beta by Suppressing NLRP3 Inflammasome Priming. Front. Immunol. 2017, 8, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, B.-Y.; Lai, Y.-H.; Chiu, C.-W.; Hsu, C.-Y.; Chen, Y.-H.; Chen, Y.-L.; Tsai, P.-J.; Hung, Y.-P.; Ko, W.-C. Effect of Doxycycline in Decreasing the Severity of Clostridioides difficile Infection in Mice. Antibiotics 2022, 11, 116. https://doi.org/10.3390/antibiotics11010116
Tsai B-Y, Lai Y-H, Chiu C-W, Hsu C-Y, Chen Y-H, Chen Y-L, Tsai P-J, Hung Y-P, Ko W-C. Effect of Doxycycline in Decreasing the Severity of Clostridioides difficile Infection in Mice. Antibiotics. 2022; 11(1):116. https://doi.org/10.3390/antibiotics11010116
Chicago/Turabian StyleTsai, Bo-Yang, Yi-Hsin Lai, Chun-Wei Chiu, Chih-Yu Hsu, Yi-Hsuan Chen, Yueh-Lin Chen, Pei-Jane Tsai, Yuan-Pin Hung, and Wen-Chien Ko. 2022. "Effect of Doxycycline in Decreasing the Severity of Clostridioides difficile Infection in Mice" Antibiotics 11, no. 1: 116. https://doi.org/10.3390/antibiotics11010116
APA StyleTsai, B.-Y., Lai, Y.-H., Chiu, C.-W., Hsu, C.-Y., Chen, Y.-H., Chen, Y.-L., Tsai, P.-J., Hung, Y.-P., & Ko, W.-C. (2022). Effect of Doxycycline in Decreasing the Severity of Clostridioides difficile Infection in Mice. Antibiotics, 11(1), 116. https://doi.org/10.3390/antibiotics11010116





