Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
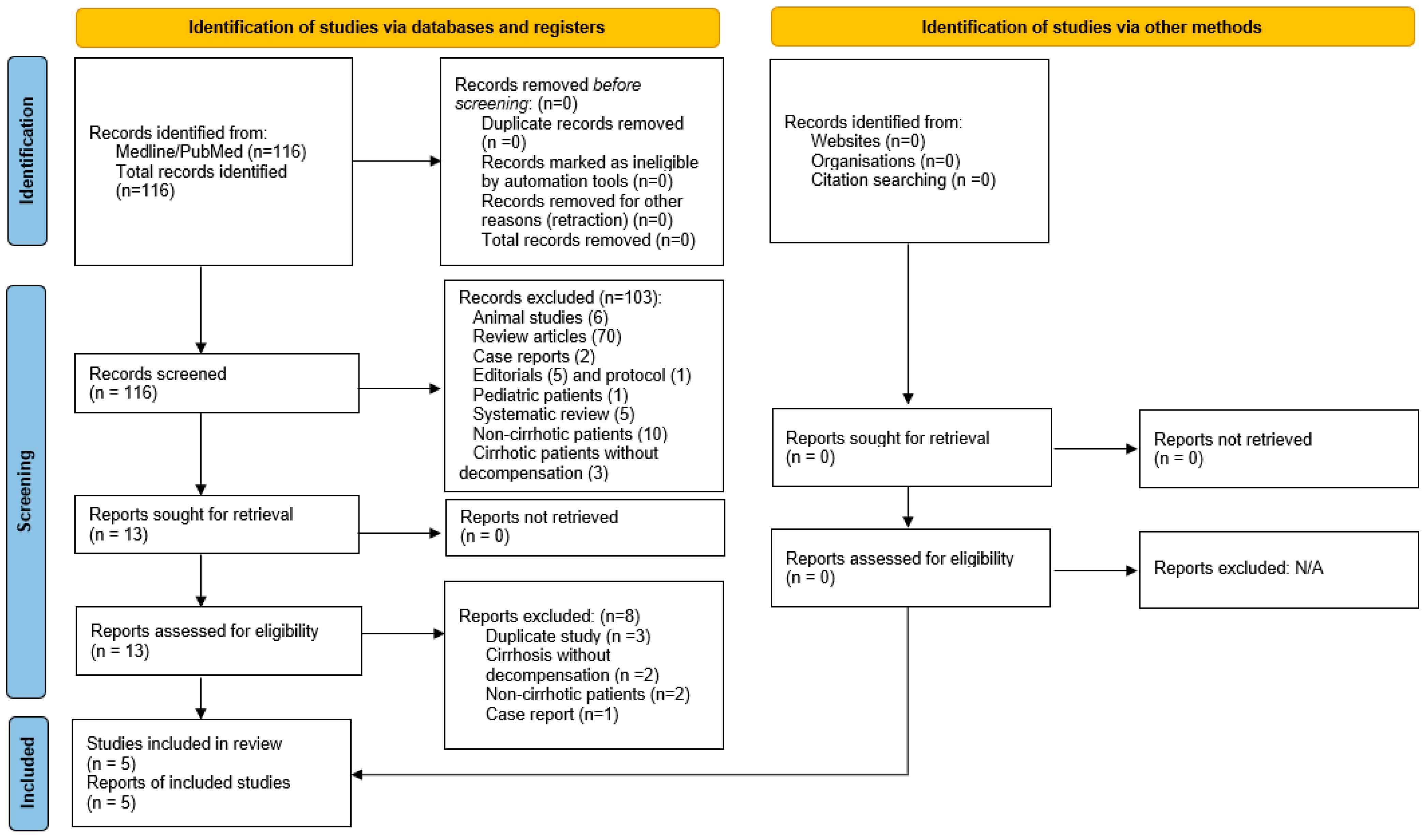
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Study Outcomes
2.5. Quality Assessment
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut-liver axis, cirrhosis and portal hypertension: The chicken and the egg. Hepatol. Int. 2018, 12 (Suppl. 1), 24–33. [Google Scholar] [CrossRef] [PubMed]
- Paratore, M.; Santopaolo, F.; Cammarota, G.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Fecal microbiota transplantation in patients with HBV infection or other chronic liver diseases: Update on current knowledge and future perspectives. J. Clin. Med. 2021, 10, 2605. [Google Scholar] [CrossRef] [PubMed]
- Hassouneh, R.; Bajaj, J.S. Gut microbiota modulation and fecal transplantation: An overview on innovative strategies for hepatic encephalopathy treatment. J. Clin. Med. 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Kimer, N.; Bendtsen, F.; Petersen, A.M. Fecal microbiota transplantation in hepatic encephalopathy: A systematic review. Scand. J. Gastroenterol. 2021, 56, 560–569. [Google Scholar] [CrossRef]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103, Correction in J. Hepatol. 2018, 68, 1336. [Google Scholar] [CrossRef] [Green Version]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.J.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018, 67, 1920–1941. [Google Scholar] [CrossRef] [Green Version]
- Tun, K.; Hong, A.; Batra, K.; Naga, Y.; Ohning, G. A systematic review on the efficacy and safety of fecal microbiota transplantation in treatment of hepatic encephalopathy and Clostridioides difficile infection in patients with cirrhosis. Cureus 2022, 14, e25537. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Salzman, N.H.; Acharya, C.; Sterling, R.K.; White, M.B.; Gavis, E.A.; Fagan, A.; Hayward, M.; Holtz, M.L.; Matherly, S.; et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: A phase 1, randomized, placebo-controlled trial. Hepatology 2019, 70, 1690–1703, Correction in Hepatology 2020, 72, 1501. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Alhaffar, D.; Saha, S.; Khanna, S.; Bohm, M.; Phelps, E.; Ghabril, M.; Orman, E.; Sashidhar, S.; Rogers, N.; et al. Fecal microbiota transplantation is safe and effective in patients with Clostridioides difficile infection and cirrhosis. Clin. Gastroenterol. Hepatol. 2021, 19, 1627–1634. [Google Scholar] [CrossRef]
- Mehta, R.; Kabrawala, M.; Nandwani, S.; Kalra, P.; Patel, C.; Desai, P.; Parekh, K. Preliminary experience with single fecal microbiota transplant for treatment of recurrent overt hepatic encephalopathy—A case series. Indian J. Gastroenterol. 2018, 37, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, M.; Reigadas, E.; Valerio, M.; Vázquez-Cuesta, S.; Pajares, J.A.; Matilla, A.; Muñoz, P.; Bouza, E. Is it reasonable to perform fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with liver cirrhosis? Rev. Esp. Quim. 2019, 32, 205–207. [Google Scholar] [CrossRef]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J.; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2014, 20 (Suppl. 2), 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.W.; Wang, K.; Yu, Y.Q.; Wang, H.B.; Li, Y.H.; Xu, J.M. Psychometric hepatic encephalopathy score for diagnosis of minimal hepatic encephalopathy in China. World J. Gastroenterol. 2013, 19, 8745–8751. [Google Scholar] [CrossRef]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Cunha-Silva, M.; Neto, F.L.P.; de Araújo, P.S.; Pazinato, L.V.; Greca, R.D.; Secundo, T.M.; Imbrizi, M.R.; Monici, L.T.; Sevá-Pereira, T.; Mazo, D.F. EncephalApp Stroop Test validation for the screening of minimal hepatic encephalopathy in Brazil. Ann. Hepatol. 2022, 27, 100543. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013 Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014, 22 (Suppl. 2), S41–S410. [CrossRef]
- Michailidis, L.; Currier, A.C.; Le, M.; Flomenhoft, D.R. Adverse events of fecal microbiota transplantation: A meta-analysis of high-quality studies. Ann. Gastroenterol. 2021, 34, 802–814. [Google Scholar] [CrossRef]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778, Correction in JAMA 2015, 313, 729. [Google Scholar] [CrossRef] [Green Version]
- Shogbesan, O.; Poudel, D.R.; Victor, S.; Jehangir, A.; Fadahunsi, O.; Shogbesan, G.; Donato, A. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1394379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammarota, G.; Masucci, L.; Ianiro, G.; Bibbò, S.; Dinoi, G.; Costamagna, G.; Sanguinetti, M.; Gasbarrini, A. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015, 41, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Bloom, P.P.; Donlan, J.; Torres Soto, M.; Daidone, M.; Hohmann, E.; Chung, R.T. Fecal microbiota transplant improves cognition in hepatic encephalopathy and its effect varies by donor and recipient. Hepatol. Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
| Author/ Year | Study Design | Quality Assessment | Quality Score | Dates | Sample Size | # Pts FMT | Follow-up Period | Pt qualifications | Etiology Cirrhosis | Exclusions | Indication for FMT | FMT Method |
| Bajaj 2017 * [8] | RCT | RoB 2 | 8 | 10/2015–7/2016 | 20 | 10 | 5 months | Age > 18, cirrhosis with recurrent HE at least 2 documented episodes requiring therapy | Hepatitis C virus, Non-alcoholic fatty liver disease, alcohol, others | MELD > 17, allergies to pre FMT abx, antimicrobials, immunosuppressive, CDI, pregnancy, EtOH, unable to give informed consent | HE | Enema |
| Bajaj 2019 * [9] | RCT | RoB 2 | 8 | 7/2017–5/2018 | 20 | 10 | 5 months | Cirrhosis w recurrent HE at least 2 episodes within last year on lactulose and rifaximin | Hepatitis C virus, Non-alcoholic steatohepatitis, alcohol, others | MELD > 17, unable consent, current abx, contraindication to endoscopic procedure | HE | Open-biome capsule |
| Cheng 2020 [10] | Retro-spective study | NIH quality assessment | 9 | 1/2012–11/2018 | 63 | 24 # | 12 weeks | Decompensated cirrhosis (ascites, varices, variceal hemorrhage, HE) | Hepatitis C virus, alcohol, Non-Alcoholic SteatoHepatitis, others | Patients with <12 week follow up and history of liver transplantation | Recurrent, severe CDI | Capsule Colonoscopy PEG |
| Mehta 2018 [11] | Case series | NIH quality assessment | 7 | 8/2017–10/2017 | 10 | 10 | 20 weeks | Hepatic encephalopathy more or equal to 2 episodes of grade 2–4 HE in last 6mo | Alcohol, Non-alcoholic steatohepatitis, Hepatitis C virus | Active EtOH, positive CDI, on immunosuppressive or antimicrobial | HE | Colonoscopy |
| Olmedo 2019 [12] | Case series | NIH quality assessment | 8 | 2013–2017 | 4 | 4 ** | 4–11 months | Cirrhosis (CP C or esophageal varices bleeding) and CDI | Alcohol, Hepatitis C virus | None | Recurrent severe CDI | Colonoscopy or NG tube |
| Study | Death | SAE | AE | Unrelated |
|---|---|---|---|---|
| Bajaj 2017 [8] | None | None | Unknown | 2– day 85 for AKI and day 1115 for chest pain was neg ACS |
| Bajaj 2019 [9] | None | None | 1 UTI from Klebsiella pneumoniae 2 months post; 1 pneumonia and receiving alpha 1 antitrypsin infusions | 1– post TIPS complication HE not related to FMT |
| Cheng 2020 [10] | None | None | Unknown | 2– bleeding portal hypertensive gastropathy 23 days after FMT; hepatic encephalopathy 56 days after FMT |
| Mehta 2018 [11] | 1– bronchopneumonia 2 months after FMT | 1– SBP at week 4 | Unknown | 1– SBP at week 8 |
| Olmedo 2019 [12] | 1– death 7 days post FMT from cholangitis | 1– Escherichia. Coli bacteremia 3 days post FMT without other cause | Unknown | None |
| Study | Indication for FMT | Definition of efficacy | Outcome |
|---|---|---|---|
| Bajaj 2017 [8] | HE | No recurrence of HE | significant less HE episodes at 5 months (0% vs. 50% p = 0.03); PHES score improvement (−3.1 vs. 0.00 p = 0.01); MELD score no clinically significant difference (0.78) |
| Bajaj 2019 [9] | HE | EncephalApp and no recurrence of HE | EncephalApp performance improved post FMT only (p = 0.02); 3 patients had no recurrence of HE. 1 patient had HE recurrence |
| Cheng 2020 [10] | Recurrent, severe, fulminant CDI | No recurrence of CDI | 18 out of 24 patients with decompensated cirrhosis who received FMT had resolution of CDI. 6 patients had recurrent CDI at follow up |
| Mehta 2018 [11] | HE | No recurrence of HE, CTP, MELD | 7 out of 10 patients had no recurrence of HE; statistically significant reduction in CTP score (9.5 9–10.75) vs. 8 (7–8) and MELD 18 (16.25–19) vs. 15 (14–16) |
| Olmedo 2019 [12] | Recurrent severe CDI | Not well defined | 3 out of 3 patients had resolution of CDI. 1 patient expired from cholangitis within 7 days of FMT and was excluded from efficacy review |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, A.S.; Tun, K.M.; Hong, J.M.; Batra, K.; Ohning, G. Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy. Antibiotics 2022, 11, 838. https://doi.org/10.3390/antibiotics11070838
Hong AS, Tun KM, Hong JM, Batra K, Ohning G. Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy. Antibiotics. 2022; 11(7):838. https://doi.org/10.3390/antibiotics11070838
Chicago/Turabian StyleHong, Annie S., Kyaw Min Tun, Jenny M. Hong, Kavita Batra, and Gordon Ohning. 2022. "Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy" Antibiotics 11, no. 7: 838. https://doi.org/10.3390/antibiotics11070838
APA StyleHong, A. S., Tun, K. M., Hong, J. M., Batra, K., & Ohning, G. (2022). Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy. Antibiotics, 11(7), 838. https://doi.org/10.3390/antibiotics11070838







