Effect of Different Piperacillin-Tazobactam Dosage Regimens on Synergy of the Combination with Tobramycin against Pseudomonas aeruginosa for the Pharmacokinetics of Critically Ill Patients in a Dynamic Infection Model
Abstract
1. Introduction
2. Results
2.1. Static-Concentration Time-Kill Studies
2.2. Dynamic In Vitro Infection Model
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates, Antibiotics, Media and Susceptibility Testing
4.2. Static-Concentration Time-Kill Experiments
4.3. Dynamic In Vitro Infection Model
4.4. Pharmacodynamic Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kollef, M.H.; Chastre, J.; Fagon, J.Y.; Francois, B.; Niederman, M.S.; Rello, J.; Torres, A.; Vincent, J.L.; Wunderink, R.G.; Go, K.W.; et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit. Care Med. 2014, 42, 2178–2187. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Kim, H.A.; Ryu, S.Y.; Lee, E.J.; Lee, M.S.; Kim, J.; Park, S.Y.; Yang, K.S.; Kim, S.W.; Antibiotic Stewardship Study, G. Tree-structured survival analysis of patients with Pseudomonas aeruginosa bacteremia: A multicenter observational cohort study. Diagn. Microbiol. Infect. Dis. 2017, 87, 180–187. [Google Scholar] [CrossRef] [PubMed]
- MacVane, S.H. Antimicrobial Resistance in the Intensive Care Unit: A Focus on Gram-Negative Bacterial Infections. J. Intensive Care Med. 2017, 32, 25–37. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Healthcare-associated infections acquired in intensive care units. In ECDC. Annual Epidemiological Report for 2017; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- U.S. Department of Health and Human Services, CDC. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 30 November 2021).
- Thaden, J.T.; Park, L.P.; Maskarinec, S.A.; Ruffin, F.; Fowler, V.G., Jr.; van Duin, D. Results from a 13-Year Prospective Cohort Study Show Increased Mortality Associated with Bloodstream Infections Caused by Pseudomonas aeruginosa Compared to Other Bacteria. Antimicrob. Agents Chemother. 2017, 61, e02671-16. [Google Scholar] [CrossRef]
- Livermore, D.M. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 34, 634–640. [Google Scholar] [CrossRef]
- Murray, J.L.; Kwon, T.; Marcotte, E.M.; Whiteley, M. Intrinsic Antimicrobial Resistance Determinants in the Superbug Pseudomonas aeruginosa. mBio 2015, 6, e01603-15. [Google Scholar] [CrossRef]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of Bolus dosing versus continuous infusion of Piperacillin and Tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic resistance—What’s dosing got to do with it? Crit. Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Bassetti, M.; Blasi, F.; Goossens, H.; Rello, J.; Sotgiu, G.; Tavoschi, L.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. A Systematic Review of the Effect of Delayed Appropriate Antibiotic Treatment on the Outcomes of Patients With Severe Bacterial Infections. Chest 2020, 158, 929–938. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Nation, R.L. Key Challenges in Providing Effective Antibiotic Therapy for Critically Ill Patients with Bacterial Sepsis and Septic Shock. Clin Pharm. 2021, 109, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Lodise, T.P. Saving lives with optimal antimicrobial chemotherapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 245–247. [Google Scholar] [CrossRef]
- Craig, W.A.; Ebert, S.C. Continuous infusion of beta-lactam antibiotics. Antimicrob. Agents Chemother. 1992, 36, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- MacVane, S.H.; Kuti, J.L.; Nicolau, D.P. Prolonging beta-lactam infusion: A review of the rationale and evidence, and guidance for implementation. Int. J. Antimicrob. Agents 2014, 43, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Obrink-Hansen, K.; Juul, R.V.; Storgaard, M.; Thomsen, M.K.; Hardlei, T.F.; Brock, B.; Kreilgaard, M.; Gjedsted, J. Population pharmacokinetics of piperacillin in the early phase of septic shock: Does standard dosing result in therapeutic plasma concentrations? Antimicrob. Agents Chemother. 2015, 59, 7018–7026. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent beta-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef]
- Lee, Y.R.; Miller, P.D.; Alzghari, S.K.; Blanco, D.D.; Hager, J.D.; Kuntz, K.S. Continuous Infusion Versus Intermittent Bolus of Beta-Lactams in Critically Ill Patients with Respiratory Infections: A Systematic Review and Meta-analysis. Eur. J. Drug Metab. Pharm. 2018, 43, 155–170. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Clinical outcomes of continuous vs intermittent meropenem infusion for the treatment of sepsis: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2020, 29, 993–1000. [Google Scholar] [CrossRef]
- Wu, C.C.; Su, Y.C.; Wu, K.S.; Wu, T.H.; Yang, C.S. Loading dose and efficacy of continuous or extended infusion of beta-lactams compared with intermittent administration in patients with critical illnesses: A subgroup meta-analysis and meta-regression analysis. J. Clin. Pharm. 2021, 46, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, S.; Barton, S.; Nabhani-Gebara, S. Comparing clinical outcomes of piperacillin-tazobactam administration and dosage strategies in critically ill adult patients: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 430. [Google Scholar] [CrossRef]
- Yadav, R.; Rogers, K.E.; Bergen, P.J.; Bulitta, J.B.; Kirkpatrick, C.M.J.; Wallis, S.C.; Paterson, D.L.; Nation, R.L.; Lipman, J.; Roberts, J.A.; et al. Optimization and evaluation of piperacillin-tobramycin combination dosage regimens against Pseudomonas aeruginosa for patients with altered pharmacokinetics via the hollow-fiber Infection model and mechanism-based modeling. Antimicrob. Agents Chemother. 2018, 62, e00078-18. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Duffull, S.B.; Kirkpatrick, C.M.; Begg, E.J. Comparison of two Bayesian approaches to dose-individualization for once-daily aminoglycoside regimens. Br. J. Clin. Pharm. 1997, 43, 125–135. [Google Scholar] [CrossRef]
- Boselli, E.; Breilh, D.; Cannesson, M.; Xuereb, F.; Rimmele, T.; Chassard, D.; Saux, M.C.; Allaouchiche, B. Steady-state plasma and intrapulmonary concentrations of piperacillin/tazobactam 4 g/0.5 g administered to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 2004, 30, 976–979. [Google Scholar] [CrossRef]
- Stanford Health Care Antimicrobial Dosing Reference Guide. Available online: https://med.stanford.edu/bugsanddrugs/guidebook.html (accessed on 18 November 2021).
- Dhaese, S.A.M.; Roberts, J.A.; Carlier, M.; Verstraete, A.G.; Stove, V.; De Waele, J.J. Population pharmacokinetics of continuous infusion of piperacillin in critically ill patients. Int. J. Antimicrob. Agents 2018, 51, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Busse, D.; Simon, P.; Petroff, D.; Dorn, C.; Schmitt, L.; Bindellini, D.; Kratzer, A.; Dietrich, A.; Zeitlinger, M.; Huisinga, W.; et al. Similar Piperacillin/Tazobactam Target Attainment in Obese versus Nonobese Patients despite Differences in Interstitial Tissue Fluid Pharmacokinetics. Pharmaceutics 2021, 13, 1380. [Google Scholar] [CrossRef] [PubMed]
- Stankowicz, M.S.; Ibrahim, J.; Brown, D.L. Once-daily aminoglycoside dosing: An update on current literature. Am. J. Health Syst. Pharm. 2015, 72, 1357–1364. [Google Scholar] [CrossRef]
- Udy, A.A.; Lipman, J.; Jarrett, P.; Klein, K.; Wallis, S.C.; Patel, K.; Kirkpatrick, C.M.; Kruger, P.S.; Paterson, D.L.; Roberts, M.S.; et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care 2015, 19, 28. [Google Scholar] [CrossRef]
- Cabezudo, I.; Pfaller, M.A.; Barrett, M.; Bale, M.; Wenzel, R.P. In vitro comparison of mezlocillin and piperacillin plus tobramycin or gentamicin versus 100 gram-negative nosocomial bloodstream isolates. Am. J. Infect. Control 1990, 18, 250–256. [Google Scholar] [CrossRef]
- Dundar, D.; Otkun, M. In-vitro efficacy of synergistic antibiotic combinations in multidrug resistant Pseudomonas aeruginosa strains. Yonsei Med. J. 2010, 51, 111–116. [Google Scholar] [CrossRef][Green Version]
- Fass, R.J. Comparative in vitro activities of beta-lactam-tobramycin combinations against Pseudomonas aeruginosa and multidrug-resistant gram-negative enteric bacilli. Antimicrob. Agents Chemother. 1982, 21, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.A.; Nascimento, M.M.; Vitali, L.H.; Martinez, R. In vitro activity of antimicrobial combinations against multidrug-resistant Pseudomonas aeruginosa. Rev. Soc. Bras. Med. Trop. 2013, 46, 299–303. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Ogake, N.; Takahata, M.; Minami, S. In vitro interaction of piperacillin and imipenem/cilastatin combined with aminoglycosides against Pseudomonas aeruginosa. Jpn. J. Antibiot. 2000, 53, 194–200. [Google Scholar] [PubMed]
- Rees, V.E.; Bulitta, J.; Nation, R.; T Tsuji, B.; Sörgel, F.; Landersdorfer, C. Shape does matter: Short high-concentration exposure minimizes resistance emergence for fluoroquinolones in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2015, 70, 818–826. [Google Scholar] [CrossRef]
- Kristoffersson, A.N.; David-Pierson, P.; Parrott, N.J.; Kuhlmann, O.; Lave, T.; Friberg, L.E.; Nielsen, E.I. Simulation-Based Evaluation of PK/PD Indices for Meropenem Across Patient Groups and Experimental Designs. Pharm. Res. 2016, 33, 1115–1125. [Google Scholar] [CrossRef]
- Rees, V.E.; Bulitta, J.B.; Oliver, A.; Tsuji, B.T.; Rayner, C.R.; Nation, R.L.; Landersdorfer, C.B. Resistance suppression by high-intensity, short-duration aminoglycoside exposure against hypermutable and non-hypermutable Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2016, 71, 3157–3167. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Rees, V.E.; Yadav, R.; Rogers, K.E.; Kim, T.H.; Bergen, P.J.; Cheah, S.E.; Boyce, J.D.; Peleg, A.Y.; Oliver, A.; et al. Optimization of a meropenem-tobramycin combination dosage regimen against hypermutable and nonhypermutable Pseudomonas aeruginosa via mechanism-based modeling and the hollow-fiber infection model. Antimicrob. Agents Chemother. 2018, 62, e02055-17. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Nation, R.L. Limitations of Antibiotic MIC-Based PK-PD Metrics: Looking Back to Move Forward. Front. Pharm. 2021, 12, 770518. [Google Scholar] [CrossRef]
- Croisier, D.; Martha, B.; Piroth, L.; Chavanet, P. In vivo efficacy of humanised intermittent versus continuous ceftazidime in combination with tobramycin in an experimental model of pseudomonal pneumonia. Int. J. Antimicrob. Agents 2008, 32, 494–498. [Google Scholar] [CrossRef]
- Luyt, C.E.; Brechot, N.; Trouillet, J.L.; Chastre, J. Antibiotic stewardship in the intensive care unit. Crit. Care 2014, 18, 480. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–12. [Google Scholar] [CrossRef]
- Barreto, E.F.; Webb, A.J.; Pais, G.M.; Rule, A.D.; Jannetto, P.J.; Scheetz, M.H. Setting the Beta-Lactam Therapeutic Range for Critically Ill Patients: Is There a Floor or Even a Ceiling? Crit. Care Explor. 2021, 3, e0446. [Google Scholar] [CrossRef]
- Thabet, P.; Joshi, A.; MacDonald, E.; Hutton, B.; Cheng, W.; Stevens, A.; Kanji, S. Clinical and pharmacokinetic/dynamic outcomes of prolonged infusions of beta-lactam antimicrobials: An overview of systematic reviews. PLoS ONE 2021, 16, e0244966. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Liebchen, U.; Paal, M.; Taubert, M.; Vogeser, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J. Intensive Care 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Meletiadis, J.; Voss, A.; Turnidge, J. Variation of MIC measurements: The contribution of strain and laboratory variability to measurement precision-authors’ response. J. Antimicrob. Chemother. 2019, 74, 1761–1762. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W. Soup with or without meatballs: Impact of nutritional factors on the MIC, kill-rates and growth-rates. Eur. J. Pharm. Sci. 2018, 125, 23–27. [Google Scholar] [CrossRef]
- Friberg, L.E. Pivotal Role of Translation in Anti-Infective Development. Clin. Pharmacol. Ther. 2021, 109, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Wicha, S.G.; Märtson, A.-G.; Nielsen, E.I.; Koch, B.C.P.; Friberg, L.E.; Alffenaar, J.-W.; Minichmayr, I.K.; International Society of Anti-Infective Pharmacology. ESCMID PK/PD of Anti-Infectives Study Group. From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef]
- Cabot, G.; Ocampo-Sosa, A.A.; Tubau, F.; Macia, M.D.; Rodríguez, C.; Moya, B.; Zamorano, L.; Suárez, C.; Peña, C.; Martínez-Martínez, L.; et al. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: Prevalence and impact on resistance in a Spanish multicenter study. Antimicrob. Agents Chemother. 2011, 55, 1906–1911. [Google Scholar] [CrossRef]
- Poole, K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011, 2, 65. [Google Scholar] [CrossRef]
- Yadav, R.; Bulitta, J.B.; Schneider, E.K.; Shin, B.S.; Velkov, T.; Nation, R.L.; Landersdorfer, C.B. Aminoglycoside concentrations required for synergy with carbapenems against Pseudomonas aeruginosa determined via mechanistic studies and modeling. Antimicrob. Agents Chemother. 2017, 61, e00722-17. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Clarke, A.J.; Beveridge, T.J. Surface action of gentamicin on Pseudomonas aeruginosa. J. Bacteriol. 1993, 175, 5798–5805. [Google Scholar] [CrossRef]
- Yadav, R.; Landersdorfer, C.B.; Nation, R.L.; Boyce, J.D.; Bulitta, J.B. Novel Approach To Optimize Synergistic Carbapenem-Aminoglycoside Combinations against Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 2286. [Google Scholar] [CrossRef] [PubMed]
- Zahedi Bialvaei, A.; Rahbar, M.; Hamidi-Farahani, R.; Asgari, A.; Esmailkhani, A.; Mardani Dashti, Y.; Soleiman-Meigooni, S. Expression of RND efflux pumps mediated antibiotic resistance in Pseudomonas aeruginosa clinical strains. Microb. Pathog. 2021, 153, 104789. [Google Scholar] [CrossRef]
- Jeannot, K.; Sobel, M.L.; El Garch, F.; Poole, K.; Plésiat, P. Induction of the MexXY efflux pump in Pseudomonas aeruginosa is dependent on drug-ribosome interaction. J. Bacteriol. 2005, 187, 5341–5346. [Google Scholar] [CrossRef]
- Masuda, N.; Sakagawa, E.; Ohya, S.; Gotoh, N.; Tsujimoto, H.; Nishino, T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000, 44, 3322–3327. [Google Scholar] [CrossRef]
- Morita, Y.; Tomida, J.; Kawamura, Y. MexXY multidrug efflux system of Pseudomonas aeruginosa. Front. Microbiol. 2012, 3, 408. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; M100; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
- Bergen, P.J.; Bulitta, J.B.; Sime, F.B.; Lipman, J.; McGregor, M.J.; Millen, N.; Paterson, D.L.; Kirkpatrick, C.M.J.; Roberts, J.A.; Landersdorfer, C.B. Differences in suppression of regrowth and resistance despite similar initial bacterial killing for meropenem and piperacillin/tazobactam against Pseudomonas aeruginosa and Escherichia coli. Diagn. Microbiol. Infect. Dis. 2018, 91, 69–76. [Google Scholar] [CrossRef]
- Yadav, R.; Bulitta, J.B.; Nation, R.L.; Landersdorfer, C.B. Optimization of synergistic combination regimens against carbapenem- and aminoglycoside-resistant clinical Pseudomonas aeruginosa isolates via mechanism-based pharmacokinetic/pharmacodynamic modeling. Antimicrob. Agents Chemother. 2017, 61, e01011-16. [Google Scholar] [CrossRef]
- Bergen, P.J.; Bulitta, J.B.; Kirkpatrick, C.M.; Rogers, K.E.; McGregor, M.J.; Wallis, S.C.; Paterson, D.L.; Lipman, J.; Roberts, J.A.; Landersdorfer, C.B. Effect of different renal function on antibacterial effects of piperacillin against Pseudomonas aeruginosa evaluated via the hollow-fibre infection model and mechanism-based modelling. J. Antimicrob. Chemother. 2016, 71, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Ulldemolins, M.; Roberts, M.S.; McWhinney, B.; Ungerer, J.; Paterson, D.L.; Lipman, J. Therapeutic drug monitoring of beta-lactams in critically ill patients: Proof of concept. Int. J. Antimicrob. Agents 2010, 36, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger-Arnaiz, J.A.; Ahmad-Diaz, F.; Miralbes-Torner, M.; Aragones-Eroles, A.; Cano-Marron, M.; Palomar-Martinez, M. Usefulness of therapeutic drug monitoring of piperacillin and meropenem in routine clinical practice: A prospective cohort study in critically ill patients. Eur. J. Hosp. Pharm. 2020, 27, e30–e35. [Google Scholar] [CrossRef] [PubMed]
- Tait, J.R.; Bilal, H.; Kim, T.H.; Oh, A.; Peleg, A.Y.; Boyce, J.D.; Oliver, A.; Bergen, P.J.; Nation, R.L.; Landersdorfer, C.B. Pharmacodynamics of ceftazidime plus tobramycin combination dosage regimens against hypermutable Pseudomonas aeruginosa isolates at simulated epithelial lining fluid concentrations in a dynamic in vitro infection model. J. Glob. Antimicrob. Resist. 2021, 26, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Bergen, P.J.; Rogers, K.E.; Kirkpatrick, C.M.J.; Wallis, S.C.; Huang, Y.; Bulitta, J.B.; Paterson, D.L.; Lipman, J.; Nation, R.L.; et al. Meropenem-tobramycin combination regimens combat carbapenem-resistant Pseudomonas aeruginosa in the hollow-fiber infection model simulating augmented renal clearance in critically ill patients. Antimicrob. Agents Chemother. 2019, 64, e01679-19. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Naicker, S.; Islam, K.; Cottrell, K.; Wallis, S.C.; Lipman, J.; Harris, P.N.A.; Sime, F.B.; Roberts, J.A. Pharmacodynamic evaluation of intermittent versus extended and continuous infusions of piperacillin/tazobactam in a hollow-fibre infection model against Klebsiella pneumoniae. J. Antimicrob. Chemother. 2020, 75, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
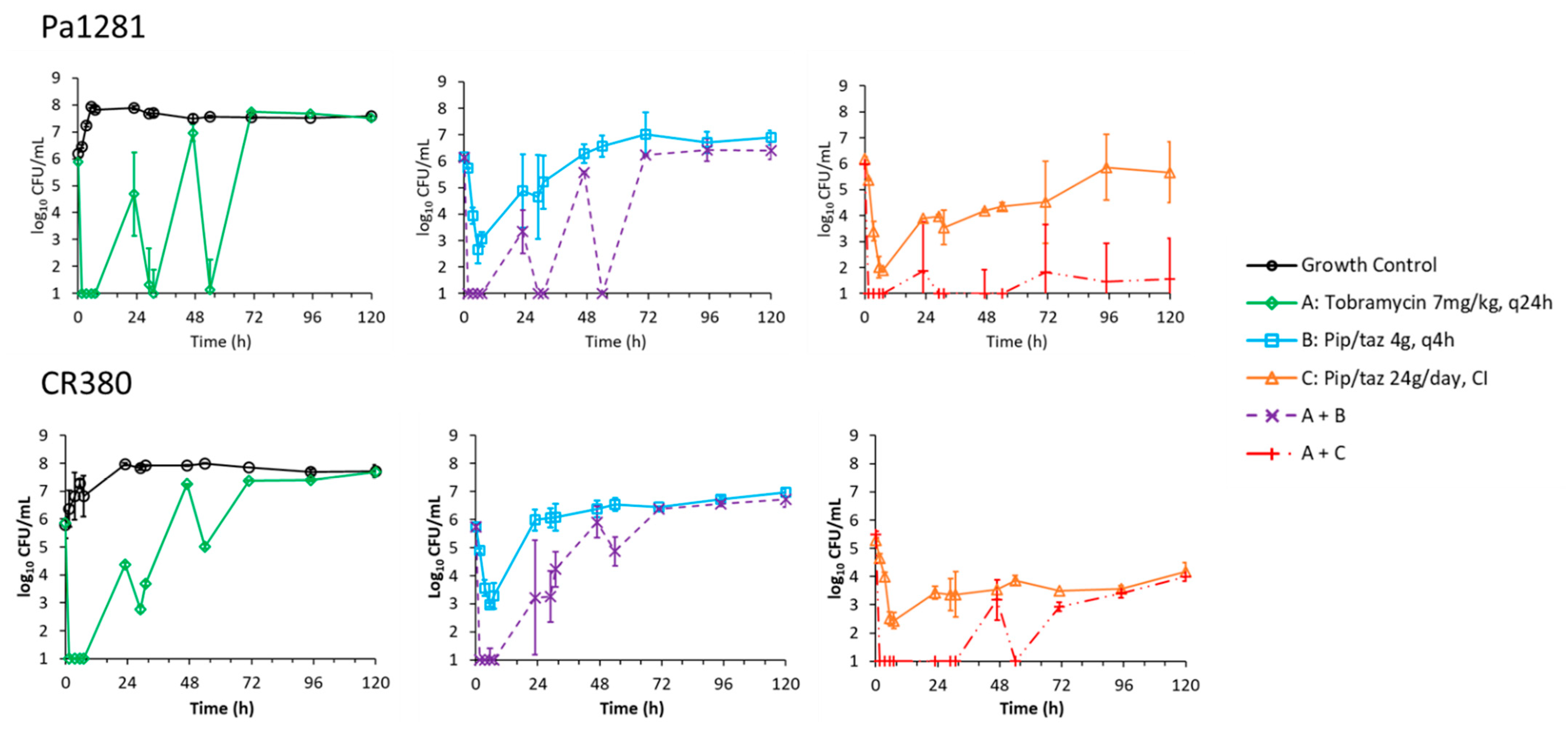

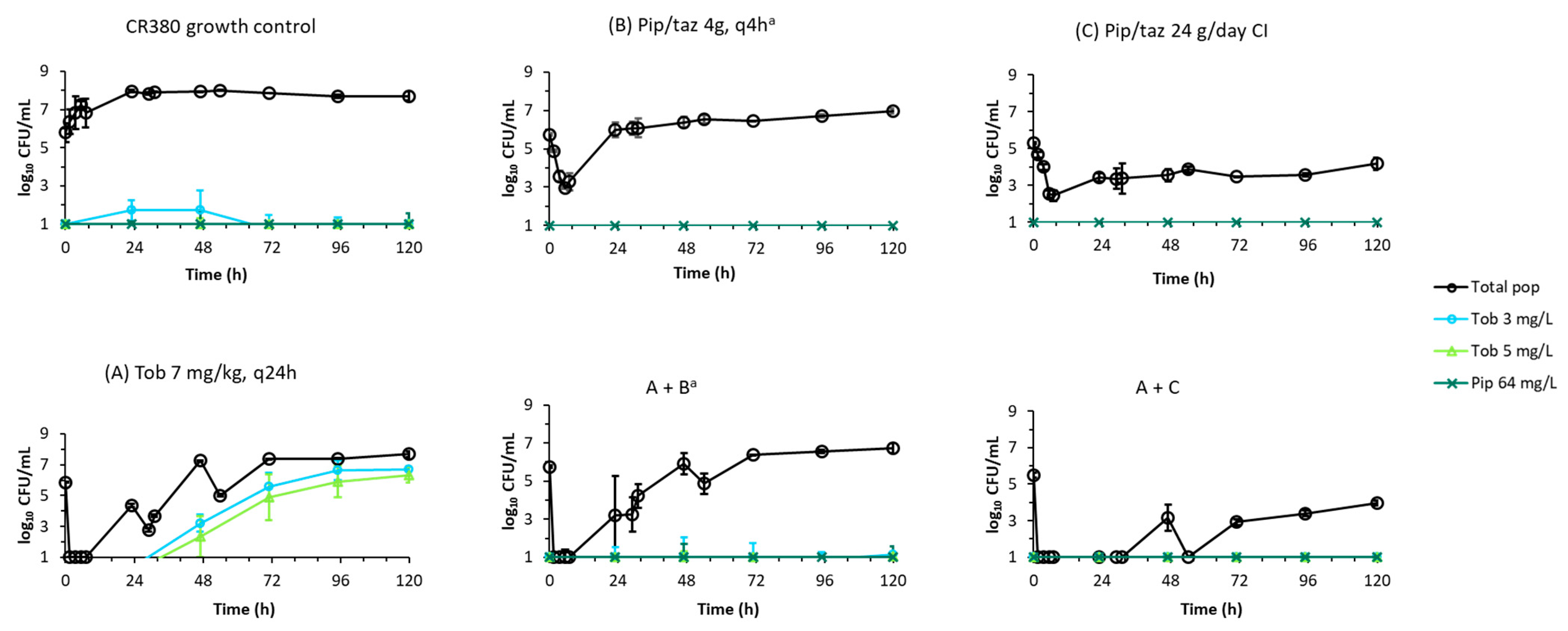
| Isolate | Piperacillin-Tazobactam MIC | Tobramycin MIC | Comment |
|---|---|---|---|
| Pa1281 | 4 | 0.5 | |
| CR382 | 16 | 1 | CR |
| CR379 | 32 | 1 | CR, MDR |
| CR380 | 32 | 1 | CR, MDR |
| Isolate | Time (h) | A: Tob 7 mg/kg, q24 h | B: Pip/taz 4 g, q4 h | C: Pip/taz 24 g/day, CI | A + B | A + C |
|---|---|---|---|---|---|---|
| CR380 | 1.5 | −5.86 | −0.86 | −0.62 | −5.75 | −5.50 |
| 3.5 | −5.86 | −2.18 | −1.29 | −5.75 | −5.50 | |
| 5.5 | −5.86 | −2.78 | −2.75 | −5.42 | −5.50 | |
| 7 | −5.86 | −2.47 | −2.84 | −5.75 | −5.50 | |
| 23 | −1.50 | 0.24 | −1.85 | −2.53 | −5.50 | |
| 29 | −3.09 | 0.31 | −1.92 | −2.50 | −5.50 | |
| 31 | −2.18 | 0.34 | −1.91 | −1.52 | −5.50 | |
| 47 | 1.40 | 0.64 | −1.73 | 0.16 | −2.33 | |
| 54 | −0.84 | 0.79 | −1.42 | −0.88 | −5.50 | |
| 71 | 1.51 | 0.70 | −1.79 | 0.63 | −2.57 | |
| 95 | 1.53 | 0.97 | −1.71 | 0.81 | −2.10 | |
| 120 | 1.82 | 1.22 | −1.11 | 0.97 | −1.51 | |
| Pa1281 | 1.5 | −5.89 | −0.44 | −0.81 | −6.13 | −5.97 |
| 3.5 | −5.89 | −2.24 | −2.79 | −6.13 | −5.97 | |
| 5.5 | −5.89 | −3.51 | −4.18 | −6.13 | −5.97 | |
| 7 | −5.89 | −3.12 | −4.29 | −6.13 | −5.97 | |
| 23 | −1.20 | −1.29 | −2.30 | −2.79 | −4.10 | |
| 29 | −4.56 | −1.53 | −2.21 | −6.13 | −5.97 | |
| 31 | −5.00 | −0.96 | −2.65 | −6.13 | −5.97 | |
| 47 | 1.07 | 0.11 | −2.01 | −0.55 | −5.05 | |
| 54 | −4.76 | 0.40 | −1.84 | −6.13 | −5.97 | |
| 71 | 1.85 | 0.84 | −1.67 | 0.10 | −4.15 | |
| 95 | 1.79 | 0.54 | −0.33 | 0.30 | −4.51 | |
| 120 | 1.62 | 0.72 | −0.52 | 0.26 | −4.41 |
| Isolate, Antibiotic | Regimen | fCmax/fCmin or fCss (mg/L) | fAUC24 (mg∗h/L) | fCmax/MIC | fT>MIC (%) | fT>4× MIC (%) | fT>10× MIC (%) | fAUC24/MIC |
|---|---|---|---|---|---|---|---|---|
| Pa1281 | ||||||||
| Piperacillin-tazobactam | 4 g q4 h a | 117/23.1 | 1477 | 29.25 | 100 | 100 | 75 | 369.25 |
| 24 g/day CI a | 58 | 1477 | 100 | 100 | 100 | 369.25 | ||
| Tobramycin | 7 mg/kg q24 h | 24.7/0.0619 | 112 | 59.4 | 70 | 224 | ||
| CR380 | ||||||||
| Piperacillin-tazobactam | 4 g q4 h a | 117/23.1 | 1477 | 3.66 | 90 | 0 | 0 | 46.16 |
| 24 g/day CI a | 58 | 1477 | 100 | 0 | 0 | 46.16 | ||
| Tobramycin | 7 mg/kg q24 h | 24.7/0.0619 | 112 | 24.7 | 58 | 112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tait, J.R.; Bilal, H.; Rogers, K.E.; Lang, Y.; Kim, T.-H.; Zhou, J.; Wallis, S.C.; Bulitta, J.B.; Kirkpatrick, C.M.J.; Paterson, D.L.; et al. Effect of Different Piperacillin-Tazobactam Dosage Regimens on Synergy of the Combination with Tobramycin against Pseudomonas aeruginosa for the Pharmacokinetics of Critically Ill Patients in a Dynamic Infection Model. Antibiotics 2022, 11, 101. https://doi.org/10.3390/antibiotics11010101
Tait JR, Bilal H, Rogers KE, Lang Y, Kim T-H, Zhou J, Wallis SC, Bulitta JB, Kirkpatrick CMJ, Paterson DL, et al. Effect of Different Piperacillin-Tazobactam Dosage Regimens on Synergy of the Combination with Tobramycin against Pseudomonas aeruginosa for the Pharmacokinetics of Critically Ill Patients in a Dynamic Infection Model. Antibiotics. 2022; 11(1):101. https://doi.org/10.3390/antibiotics11010101
Chicago/Turabian StyleTait, Jessica R., Hajira Bilal, Kate E. Rogers, Yinzhi Lang, Tae-Hwan Kim, Jieqiang Zhou, Steven C. Wallis, Jürgen B. Bulitta, Carl M. J. Kirkpatrick, David L. Paterson, and et al. 2022. "Effect of Different Piperacillin-Tazobactam Dosage Regimens on Synergy of the Combination with Tobramycin against Pseudomonas aeruginosa for the Pharmacokinetics of Critically Ill Patients in a Dynamic Infection Model" Antibiotics 11, no. 1: 101. https://doi.org/10.3390/antibiotics11010101
APA StyleTait, J. R., Bilal, H., Rogers, K. E., Lang, Y., Kim, T.-H., Zhou, J., Wallis, S. C., Bulitta, J. B., Kirkpatrick, C. M. J., Paterson, D. L., Lipman, J., Bergen, P. J., Roberts, J. A., Nation, R. L., & Landersdorfer, C. B. (2022). Effect of Different Piperacillin-Tazobactam Dosage Regimens on Synergy of the Combination with Tobramycin against Pseudomonas aeruginosa for the Pharmacokinetics of Critically Ill Patients in a Dynamic Infection Model. Antibiotics, 11(1), 101. https://doi.org/10.3390/antibiotics11010101









