Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review
Abstract
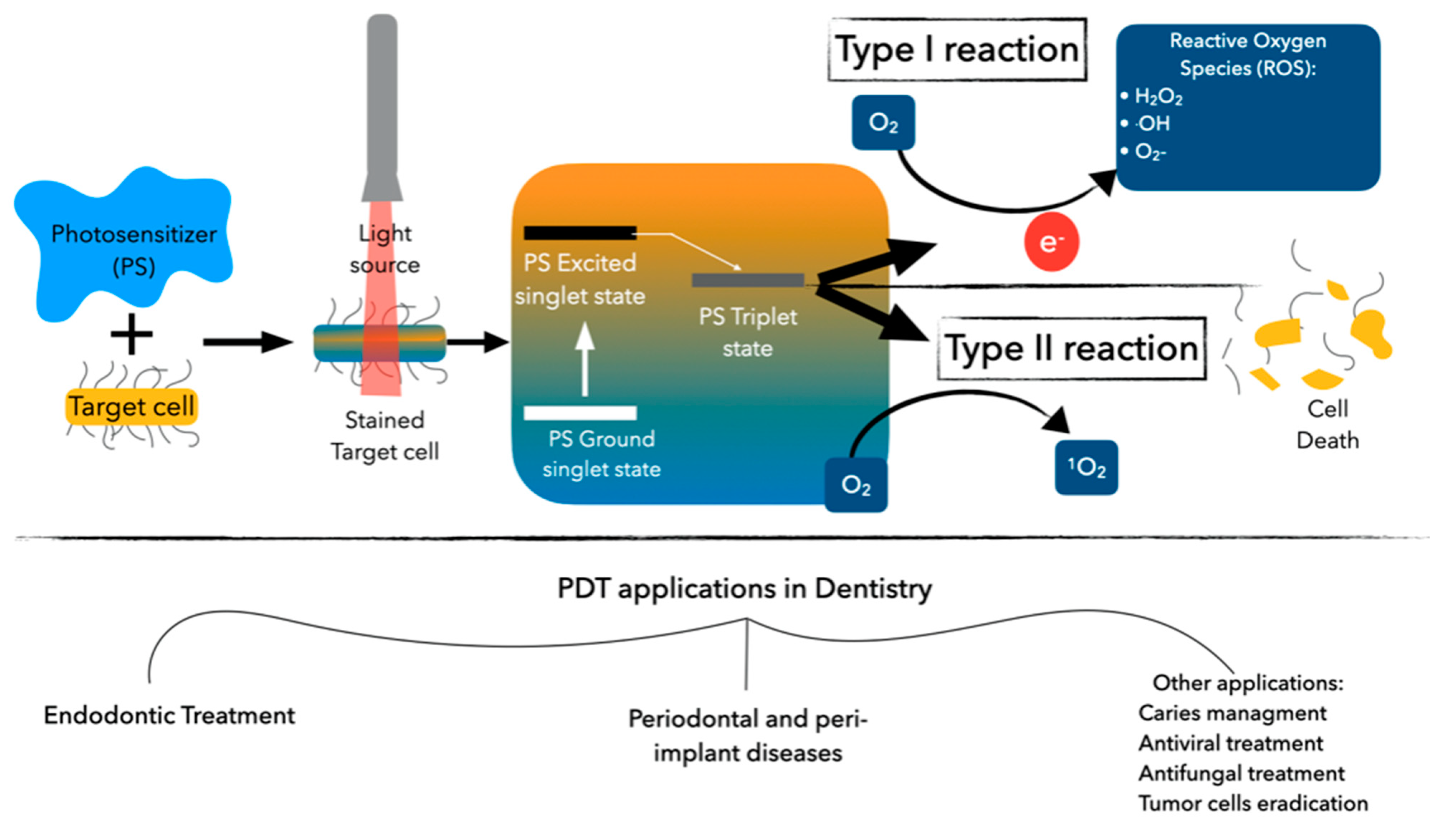
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirsch, J.; Basche, S.; Neunzehn, J.; Dede, M.; Dannemann, M.; Hannig, C.; Weber, M.-T. Is it really penetration? Locomotion of devitalized Enterococcus faecalis cells within dentinal tubules of bovine teeth. Arch. Oral Biol. 2017, 83, 289–296. [Google Scholar] [CrossRef]
- Rana, V.; Baba, S.M.; Pandey, A. Bacteriology of Infected Deciduous Root Canal—A Review. People’s J. Sci. Res. 2009, 2, 45–48. [Google Scholar]
- Hani, F.; Dina, D.; Ziad, S.; Anis, C.; Hassan, B. Endodontic consideration in pediatric dentistry: A clinical perspective. Dent. Hor. 2009, 1, 5–10. [Google Scholar]
- Mohammadi, Z. Sodium hypochlorite in endodontics: An update review. Int. Dent. J. 2008, 58, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Gould, K.; Şen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A. Antibiotics in Endodontics: A review. Int. Endod. J. 2017, 50, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Vallano, A.; Izarra, A. Principios de terapéutica antimicrobiana. Medicine 2006, 9, 3196–3203. [Google Scholar] [CrossRef]
- Marra, F.; George, D.; Chong, M.; Sutherland, S.; Patrick, D.M. Antibiotic prescribing by dentists has increased: Why? J. Am. Dent. Assoc. 2016, 147, 320–327. [Google Scholar] [CrossRef]
- Maestre-Vera, J.R. Opciones terapéuticas en la infección de origen odontogénico. Med. Oral. Patol. Oral. Cir. Bucal. 2004, 9, 19–31. [Google Scholar]
- Epstein, J.B.; Chong, S.; Le, N.D. A survey of antibiotic use in dentistry. J. Am. Dent. Assoc. 2000, 131, 1600–1609. [Google Scholar] [CrossRef]
- Pallasch, T.J. Global antibiotic resistance and its impact on the dental community. J. N. J. Dent. Assoc. 2000, 71, 14–15. [Google Scholar]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontology 2000, 51, 109–140. [Google Scholar] [CrossRef]
- De Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral. Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Romanos, G.E. Does photodynamic therapy enhance standard antibacterial therapy in dentistry? Photomed. Laser Surg. 2013, 31, 512–518. [Google Scholar] [CrossRef]
- Gluckman, J.L. Hematoporphyrin photodynamic therapy: Is there truly a future in head and neck oncology? Reflections on a 5-year experience. Laryngoscope 1991, 101, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- Silva, M.; Paulo, M.; Cardoso, M.; Martins, M.; Noites, R. The use of systemic antibiotics in endodontics: A cross-sectional study. J. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2017, 58, 205–2011. [Google Scholar] [CrossRef][Green Version]
- Mainjot, A.; D’Hoore, W.; Vanheusden, A.; Van Nieuwenhuysen, J.P. Antibiotic prescribing in dental practice in Belgium. Int. Endod. J. 2009, 42, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Núñez, A.; Cisneros-Cabello, R.; Velasco-Ortega, E.; Llamas-Carreras, J.M.; Tórres-Lagares, D.; Segura-Egea, J.J. Antibiotic use by members of the Spanish Endodontic Society. J. Endod. 2009, 35, 1198–1203. [Google Scholar] [CrossRef]
- Dormoy, J.; Vuillemin, M.O.; Rossi, S.; Boivin, J.M.; Guillet, J. Perceptions of Antibiotic Use and Resistance: Are Antibiotics the Dentists’ Anxiolytics? Antibiotics 2021, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, C.M.; Lee, E.H.; Martin, M.J.; Flannagan, S.E. Antibiotic resistance gene transfer between Streptococcus gordonii and Enterococcus faecalis in root canals of teeth ex vivo. J. Endod. 2008, 34, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Williams, D.W.; Yanagisawa, M.; Iwahara, K.; Shimizu, C.; Nakagawa, K.; Yamamoto, E.; Karasawa, T. Antimicrobial susceptibility of 800 anaerobic isolates from patients with dentoalveolar infection to 13 oral antibiotics. Oral. Microbiol. Immunol. 2007, 22, 285–288. [Google Scholar] [CrossRef]
- Dahlén, G.; Samuelsson, W.; Molander, A.; Reit, C. Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral. Microbiol. Immunol. 2000, 15, 309–312. [Google Scholar] [CrossRef]
- Jungermann, G.B.; Burns, K.; Nandakumar, R.; Tolba, M.; Venezia, R.A.; Fouad, A.F. Antibiotic resistance in primary and persistent endodontic infections. J. Endod. 2011, 37, 1337–1344. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Jafarzadeh, H.; Shalavi, S.; Yaripour, S.; Sharifi, F.; Kinoshita, J.I. A Review on Triple Antibiotic Paste as a Suitable Material Used in Regenerative Endodontics. Iran. Endod. J. 2018, 13, 1–6. [Google Scholar]
- Camacho-Alonso, F.; Salmerón-Lozano, P.; Martínez-Beneyto, Y. Effects of photodynamic therapy, 2% chlorhexidine, triantibiotic mixture, propolis and ozone on root canals experimentally infected with Enterococcus faecalis: An in vitro study. Odontology 2017, 105, 338–346. [Google Scholar] [CrossRef]
- Gajdács, M.; Spengler, G.; Urbán, E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik’s Cube of Clinical Microbiology? Antibiotics 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Meire, M.A.; De Prijck, K.; Coenye, T.; Nelis, H.J.; De Moor, R.J. Effectiveness of different laser systems to kill Enterococcus faecalis in aqueous suspension and in an infected tooth model. Int. Endod. J. 2009, 42, 351–359. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. An in vivo evaluation of microbial diversity before and after the photo-activated disinfection in primary endodontic infections: Traditional phenotypic and molecular approaches. Photodiagn. Photodyn. Ther. 2018, 22, 19–25. [Google Scholar] [CrossRef]
- Kosarieh, E.; Khavas, S.S.; Rahimi, A.; Chiniforush, N.; Gutknecht, N. The comparison of penetration depth of two different photosensitizers in root canals with and without smear layer: An in vitro study. Photodiagn. Photodyn. Ther. 2016, 13, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Giusti, J.S.; Santos-Pinto, L.; Pizzolito, A.C.; Helmerson, K.; Carvalho-Filho, E.; Kurachi, C.; Bagnato, V.S. Antimicrobial photodynamic action on dentin using a light-emitting diode light source. Photomed. Laser Surg. 2008, 26, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Mello, I.; Franco, G.C.; de Medeiros, J.M.; Dos Santos, S.S.; Habitante, S.M. Effectiveness of photodynamic therapy against Enterococcus faecalis, with and without the use of an intracanal optical fiber: An in vitro study. Photomed. Laser Surg. 2011, 29, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.; Fontana, C.R.; Ruggiero, K.; Riahi, R.; Vera, A.; Doukas, A.G. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Lasers Surg. Med. 2007, 39, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Nuñez, S.C.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J. Endod. 2008, 34, 138–142. [Google Scholar] [CrossRef]
- Garcez, A.S.; Hamblin, M.R. Methylene blue and hydrogen peroxide for photodynamic inactivation in root canal—A new protocol for use in endodontics. Eur. Endod. J. 2017, 2, 29. [Google Scholar] [CrossRef]
- Sjogren, U.; Figdor, D.; Persson, S.; Sundqvist, G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int. Endod. J. 1997, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Martinelli, M.; Cocchi, A.; Arbia, D.; Fantetti, L.; Roncucci, G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob. Agents Chemother. 2010, 54, 637–642. [Google Scholar] [CrossRef]
- Lauro, F.M.; Pretto, P.; Covolo, L.; Jori, G.; Bertoloni, G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 2002, 1, 468–470. [Google Scholar] [CrossRef]
- López-Jiménez, L.; Fusté, E.; Martínez-Garriga, B.; Arnabat-Domínguez, J.; Vinuesa, T.; Viñas, M. Effects of photodynamic therapy on Enterococcus faecalis biofilms. Lasers Med. Sci. 2015, 30, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. The effect of tissue inhibitors on the antibacterial activity of chitosan nanoparticles and photodynamic therapy. J. Endod. 2012, 38, 1275–1278. [Google Scholar] [CrossRef]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus faecalis elimination in root canals using silver nanoparticles, photodynamic therapy, diode laser, or laser-activated nanoparticles: An in vitro study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Singh, F.; Papamanou, D.A.; Song, X.; Patel, C.; Holewa, C.; Patel, N.; Klepac-Ceraj, V.; Fontana, C.R.; Kent, R.; et al. Endodontic photodynamic therapy ex vivo. J. Endod. 2011, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hoedke, D.; Enseleit, C.; Gruner, D.; Dommisch, H.; Schlafer, S.; Dige, I.; Bitter, K. Effect of photodynamic therapy in combination with various irrigation protocols on an endodontic multispecies biofilm ex vivo. Int. Endod. J. 2018, 51 (Suppl. 1), e23–e34. [Google Scholar] [CrossRef]
- Garcez, A.S.; Nuñez, S.C.; Hamblim, M.R.; Suzuki, H.; Ribeiro, M.S. Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: A preliminary report. J. Endod. 2010, 36, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Zorita-García, M.; Alonso-Ezpeleta, L.Ó.; Cobo, M.; Del Campo, R.; Rico-Romano, C.; Mena-Álvarez, J.; Zubizarreta-Macho, Á. Photodynamic therapy in endodontic root canal treatment significantly increases bacterial clearance, preventing apical periodontitis. Quintessence Int. 2019, 50, 782–789. [Google Scholar] [PubMed]
- Garcez, A.S.; Neto, J.G.A.; Sellera, D.P.; Fregnani, E. Effects of antimicrobial photodynamic therapy and surgical endodontic treatment on the bacterial load reduction and periapical lesion healing. Three years follow up. Photodiagn. Photodyn. Ther. 2015, 12, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.C.S.; Antunes, H.S.; Pérez, A.R.; Gonçalves, L.S.; Antunes, F.E.; Siqueira, J.F., Jr.; Rôças, I.N. Molecular Analysis of the Antibacterial Effects of Photodynamic Therapy in Endodontic Surgery: A Case Series. J. Endod. 2018, 44, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Abu Hasna, A.; Pereira Santos, D.; Gavlik de Oliveira, T.R.; Pinto, A.B.A.; Pucci, C.R.; Lage-Marques, J.L. Apicoectomy of Perforated Root Canal Using Bioceramic Cement and Photodynamic Therapy. Int. J. Dent. 2020, 2020, 6677588. [Google Scholar] [CrossRef]
- Moreira, M.S.; de Freitas Archilla, J.R.; Lascala, C.A.; Ramalho, K.M.; Gutknecht, N.; Marques, M.M. Post-Treatment Apical Periodontitis Successfully Treated with Antimicrobial Photodynamic Therapy Via Sinus Tract and Laser Phototherapy: Report of Two Cases. Photomed. Laser Surg. 2015, 33, 524–528. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, R.G.; Colombo, A.P.V. Clinical and microbiological effectiveness of photodynamic therapy on primary endodontic infections: A 6-months randomized clinical trial. Clin. Oral. Investig. 2018, 22, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Conejero, M.J.; Almenar, A.; Forner, L.; Sanz, J.L.; Llena, C. Retrospective clinical evaluation of root canal treatment with or without photodynamic therapy for necrotic teeth and teeth subjected to retreatment. J. Oral. Sci. 2021, 63, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Rabello, D.G.D.; Corazza, B.J.M.; Ferreira, L.L.; Santamaria, M.P.; Gomes, A.P.M.; Martinho, F.C. Does supplemental photodynamic therapy optimize the disinfection of bacteria and endotoxins in one-visit and two-visit root canal therapy? A randomized clinical trial. Photodiagn. Photodyn. Ther. 2017, 19, 205–211. [Google Scholar] [CrossRef]
- Asnaashari, M.; Ashraf, H.; Rahmati, A.; Amini, N. A comparison between effect of photodynamic therapy by LED and calcium hydroxide therapy for root canal disinfection against Enterococcus faecalis: A randomized controlled trial. Photodiagn. Photodyn. Ther. 2017, 17, 226–232. [Google Scholar] [CrossRef]
| Author | Year | Study Design | Sample Size (n) | Study Groups | Endodontic Pathology | PS and Concentration | Pre-Irradiation Time | Light Source and Wavelength | Power | Power Density | Irradiation Time | Outcome Evaluation | Follow-Up | Clinical Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Miranda et al. | 2017 | Randomized controlled clinical trial | 32 patients (16 in each group) | Control group (CMD + calcium hydroxide), PDT group (CMD+PDT+Cal hydroxide) | Primary endodontic infections | MB (25 μg/mL) | 5 min | Diode laser (660 nm) | 100 mW | Not reported | 5 min | PAI | Baseline, 3 and 6 months | Statistically significant improvement Periapical Index Score at 6 months |
| Rabello et al. | 2017 | Case series | 24 patients (12 in each group) | 1 visit PDT and 2 visits calcium hydroxide and PDT | Primary endodontic infection | MB (0.1 mg/mL) | 60 s | Diode laser (660 nm) | 60 mW | Not reported | 2 min | Microbiological endodoxins analysis | No follow up was reported | PDT significant reduction of 1 visit compared to control group but not significant in 2 visits with calcium hydroxide |
| Asnaashari et al. | 2016 | Case series | 20 patients | 2 sessions with calcium hydroxide intracanal dressing or a single visit with adjunctive aPDT | Persistent endodontic infection | TBO (0.1 mg/mL) | 5 min | LED (620–640 nm) | Not reported | 2–4 mW/cm2 | 60 s | Microbial reduction by culture samples | No follow up was reported | Decrease in number of colonies was more evident in aPDT group |
| Garcez et al. | 2010 | Quasi-controlled clinical trial | 30 patients | No groups. 3 microbiological culture samples were taken in the same tooth of each patient | Persistent endodontic infection | Conjugate between polyethylenimine (PEI) and chlorin (e6) (60 μmol/L) | 2 min | Diode laser (660 nm) | 40 mW | Not reported | 4 min | Microbial reduction by culture samples | No follow up was reported | 10 root canals after CMD (eliminated microorganism, while all 30 had complete elimination after PDT) |
| Garcez et al. | 2015 | Case series | 28 teeth (from 22 patients) | Endodontic surgery (apicoectomy) with PDT | Persistent endodontic infection | MB (60 μM) | 3 min | Diode laser (660 nm) | 40 mW | Not reported | 3 min | -Microbial reduction by culture samples. -Radiographic follow up. | 36 months radiographic follow up | -Significant reduction in bacterial culture samples after aPDT application -average periapical radiographic lesion reduction of 78% |
| Vieira et al. | 2018 | Randomized controlled clinical trial | 19 teeth treated in 16 patients | Endodontic surgery (apicoectomy) with PDT | Persistent endodontic infection | MB (0.01%) | Not reported | Diode laser (660 nm) | 40 mW | Not reported | 3 min | Evaluate healing: (complete, incomplete, uncertain, unsatisfactory) Radiographic and clinical evaluation: rigid and loose | 12–21 months (mean of 16 months) | Statistically significant bacterial reduction after PDT and 93% success rate (loose criteria) and 73% success using rigid criteria |
| Abu Hasna et al. | 2020 | Case report | 1 patient with endodontic retreatment and apicoectomy using adjunctive aPDT | - | Persistent endodontic infection | MB (0.005%) | 5 min | Diode laser (660 nm) | Not reported | 100 mW/cm2 | 2 min | Clinical and radiographic outcome | 30 days and 12 months | Twelve-month cone beam computed tomography follow-up showed bone neoformation at the periapical area indicating success of the treatment |
| Zorita García et al. | 2019 | Quasi-controlled clinical trial | 42 posterior single rooted teeth (33 patients) | No groups. 3 microbiological culture samples were taken in the same tooth of each patient | Primary endodontic infection | TBO (concentration was not reported) | 2 min | LED (630 ± 20 nm) | Not reported | 2000 mW/cm2 | 2 cycles of 30 s each | Microbial reduction by culture samples | No follow up was reported | Significant reduction in CFU/tooth was achieved after aPDT application in all cases. |
| Moreira et al. | 2015 | Case reports | 2 cases with failed endodontic retreatments and with persistent sinus tract. | - | Persistent endodontic infection | MB (0.01%) | 4 min | AsGaAl diode laser (660 nm) | 40 mW | Not reported | 63 s | -Clinical sinus tract healing. -CBCT. | 2 and 4 years of follow-up, respectively | Healing of the sinus tract was achieved with periapical bone repair confirmed by CBCT after seven to ten sessions via sinus tract. |
| Conejero et al. | 2021 | Retrospective study | 100 teeth treated with conventional CMD and 114 teeth received adjunctive aPDT | CMD or CMD + aPDT | Primary and persistent infections | TBO (0.1 mg/mL) | Not reported | LED (360 nm) | Not reported | 2000 mW/cm2 | 30 s | -Radiographic PAI index score. -Clinical signs and symptoms. | No follow up (retrospective study) | -Success rate for CMD group was 94.7% and for CMD + aPDT was 97.2%. -Periapical healing in CMD group 20.35 ± 22.1 months, CMD + aPDT group was 15 ± 9.33 months. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkarim-Elafifi, H.; Parada-Avendaño, I.; Arnabat-Dominguez, J. Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review. Antibiotics 2021, 10, 1106. https://doi.org/10.3390/antibiotics10091106
Abdelkarim-Elafifi H, Parada-Avendaño I, Arnabat-Dominguez J. Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review. Antibiotics. 2021; 10(9):1106. https://doi.org/10.3390/antibiotics10091106
Chicago/Turabian StyleAbdelkarim-Elafifi, Haitham, Isabel Parada-Avendaño, and Josep Arnabat-Dominguez. 2021. "Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review" Antibiotics 10, no. 9: 1106. https://doi.org/10.3390/antibiotics10091106
APA StyleAbdelkarim-Elafifi, H., Parada-Avendaño, I., & Arnabat-Dominguez, J. (2021). Photodynamic Therapy in Endodontics: A Helpful Tool to Combat Antibiotic Resistance? A Literature Review. Antibiotics, 10(9), 1106. https://doi.org/10.3390/antibiotics10091106







