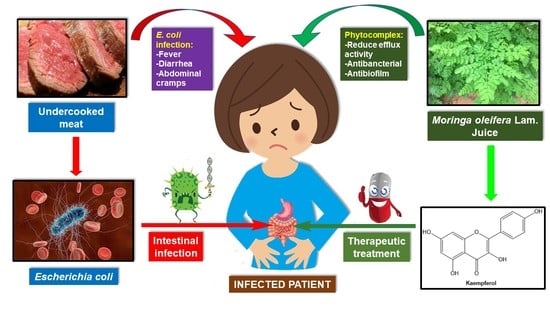
Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties
Abstract
1. Introduction
2. Significant Gatherings of Antimicrobial Mixtures from Plants
2.1. Phenolics and Polyphenols
2.1.1. Simple Phenols and Phenolic Acids
2.1.2. Quinones
2.1.3. Flavones, Flavonoids, and Flavonols
2.1.4. Tannins
2.1.5. Coumarins
2.2. Terpenoids and Essential Oils
2.3. Alkaloids
2.4. Lectins and Polypeptides
3. Some Phytochemicals That Have Antimicrobial Activity
3.1. Kaempferol
3.2. Juglone
3.3. Xanthopurpurin and Vanillic Acid
4. Antimicrobial Activity of Medicinal Plants
| Scientific Name | Plant Parts | Active Principle | Antimicrobial Activity | MIC Value |
|---|---|---|---|---|
| Aloe vera | S | Latex | Salmonella and Streptococcus spp. | P. aeruginosa: ≤400 μg/mL [103] |
| Malus sylvestris | FR | ‘Phloretin’ a flavonoid | General antimicrobial | P. aeruginosa: 7.81 μg/mL [104] |
| Withania somnifera | R and L | ‘Withaferin A’ a lactone | Antibacterial Antifungal | S. aureus: 250 μg/mL [105] |
| Aegel marmelos | L, FR and R | Essential oils/terpenoids | Antifungal | A. fumigatus: 15.625 μg/mL |
| Barberis vulgaris | R and SB | ‘Barberine’ an alkaloid | M. tuberculosis, Vibrio cholera, Plasmodium and Trypanosomes | P. aeruginosa: 16 μg/mL Proteus vulgaris: 32 μg/mL E. coli: 32 μg/mL [106] |
| Ocimum sanctum | a. L and Sb. R | a. Essential oils b. Root extract | a. Salmonella, Ringworm, common cold virus b. In malarial fever to bring sweating | S. aureus: 128 μg/mL [107] |
| Laurus nobilis | Leaves | Essential oils | Antibacterial and antifungal | E. coli: >22.5 mg/mL, P. aeruginosa: 22 mg/mL [108] |
| Piper nigrum | S | ‘Piperine’ an alkaloid | Fungi, Micrococci, E. coli | C. albicans: 3.125 mg/mL [109] |
| Bacopa monnieri | WP | ‘Brahmine’ an alkaloid | Anthelmintic property | UTI and RTI bacteria: 2.5 mg/mL E. coli: 2.5 μg/mL [110] |
| Acorus calamus | RH and L | Volatile oils | Enteric bacteria Insecticidal | S. aureus and E. coli: 5–10 mg/mL |
| Ricinus communis | S | Castor oil | Antifungal (in dermatitis) | S. aureus: 62.5 μg/mL [111] |
| Cinnamomum verum | BA and L | Essential oils | General antimicrobial | S. aureus: 0.5 μL/disc [112] |
| Cinchona officinalis | BA | ‘Quinine’ an alkaloid | Antimalarial | Helicobacter pylori: 0.1 ng/ML [113] |
| Capsicum annum | FR | ‘Capsaicin’ a terpenoid | Antibacterial | From 10 to 20 μg/mL [114] |
| Hydnocarpus kurzii | Essential oil | Mycobacterium leprae | Thyme: 1.25 mg/mL [115] | |
| Coriandrum sativum | WP, L, S | Antibacterial Antifungal | Candida albicans: 0.02 mg/mL, E. coli: 0.64 mg/mL [116] | |
| Eucalyptus globulus | L | Tannins and terpenoids /essential oils | Antibacterial Antiviral Antifungal | S. aureus: 64 mg/mL, S. pyogenes: 32 mg/mL, S. pneumoniae: 16 mg/mL, Haemophilus influenzae: 16 mg/mL [117] |
| Allium sativum | B | Sulfated terpenoids | General antimicrobial | (Methanolic extract) S. aureus: 1.25 mg/mL S. pneumonia: 0.312 mg/mL P. aeruginosa: 1.25 mg/mL K. pneumoniae: 0.312 mg/mL(Ethanolic extract) S. aureus: 2.5 mg/mL S. pneumonia: 0.312 mg/mL P. aeruginosa: 0.625 mg/mL K. pneumoniae: 0.156 mg/mL [118] |
| Piper longum | FR and L | Piperin | Antibacterial | B. cereus and E. coli: 12.5 mg/mL [102] |
| Glycyrrhiza glabra | 1. ‘Glycyrrhizin’ a terpenoid 2. ‘Glabrol’ an alcohol | 1. HIV virus and other viruses 2. M. tuberculosis S. aureus | S. aureus: 50 mg/mL [119] | |
| Calendula officinalis | L FL | Essential oils/Terpenoids | General antimicrobial | S. mutans: 3.12 μg/mL [120] |
| Mentha arvensis | L | ‘Menthol’ an alcohol | AntisepticMouth wash | Acinetobacter baumannii: 23.5 μg/mL [121] |
| Azadirachtus indica | R, LB, FR, FL | 1.‘Azadirachtin’ 2. ‘Nimbin’ 3. ‘Nimbidin’ 4. ‘Gedunin’ 5. ‘Salannin’ 6. ‘Quercetin’ | 1. Repellant and antifeedant 2. Antifungal 3. Antibacterial, antifungal 4. Anti-malarial, antifungal 5. Repellant 6. Antibacterial, antiprotozoal | Streptococcus sp.: 125 μg/mL S. aureus: 250 μg/mL Enterococcus faecalis: 500 μg/mL [122] |
| Oleo europoea | ‘Hexanal’ an aldehyde | General antimicrobial | S. cerevisiae: 24 μg/mL, [123] | |
| Allium cepa | B | ‘Allicin’ a sulfoxide and other sulfated terpenoids | Antibacterial Antifungal | S. aureus: 7 μg/mL [124] |
| Citrus sinensis | FP, L | Terpenoids | Antifungal | Streptococcus sp.:12.4 mg/mL |
| Carica papaya | LA, FR | Terpenoids, organic acids, and alkaloids | General antimicrobial | S. aureus: 1250 μg/mL |
| Butea monosperma | S, L | Tannins | Round worm, Ring worm, Dhobi-itch | Acinetobacter sp: 2.62 mg/mL [125] |
| Mentha piperita | WP | 1. ‘Menthol’ an alcohol 2. Peppermint oil terpenoid | 1. General antimicrobial 2. Mouth freshener | Klebsiella pneumonia: 0.4 ± 0.02(v/v) [126] |
| Papaver somniferum | ‘Opium’ an alkaloid | General antimicrobial | K. pneumonia: 2.2 mg/mL C. albicans: 1.1 mg/mL [127] | |
| Solanum tuberosum | T | Potato starch | Antibacterial Antifungal | S. aureus: 0.62 mg/mL, S. pyogenes:1.25 mg/mL [101] |
| Rauwolfia serpentine | R | ‘Reserpine’ an alkaloid | General antimicrobial | S. aureus: 30 mg/mL [101] |
| Pterocarpus santalinus | W | Terpenoids | Antibacterial, Antiseptic Against skin infections and inflammations | S. aureus: 4 mg/mL |
| Catharanthus roseus | Ajmalicine, serpentine, reserpine (alkaloids) | General antimicrobial Anti-cancer | E. coli: 12.5 µg/mL | |
| Santalum album | W | Terpenoids, saponins, phenolic compounds | Antibacterial Skin infections TB of gallbladder | S. aureus: 0.078 µg/mL [128] |
| Centratherum anthelmintium | S | Anthelmintic | E. coli: 0.0020 µg/mL P. aeruginosa: 0.006 µg/mL | |
| Sida cardifolia | WP and R with ginger | Antimicrobial | C. albicans: 8.33 µg/mL | |
| Thymus vulgaris | ‘Caffeic acid’, ‘thymol’ and tannins | Antibacterial, antiviral, antifungal | S. aureus: 0.312 mg/mL | |
| Tamarindus indica | PF | GIT infections and toxicity | E. coli: 15 mg/mL and Shigella flexnerri: 10 mg/mL [129] | |
| Curcuma longa | R, RH and L | ‘Curcumin’, turmeric oil, terpenoids | Antibacterial, antiprotozoal, Anthelmintic | S. aureus: 190 mg/mL |
| Salix alba | ‘Salicin’, tannins, and essential oils | General antimicrobial | S. aureus: 100 mg/mL | |
| Gaultheria fragrantissima | Tannins and polyphenols | Hook worms, mosquito and fly repellant, anticancer drug | Inhibited growth and aflatoxin B1: 7 at 1.0 and 0.7 µL/mL, respectively [130] | |
| Artemisia maritime | Immature F and L | Anthelmintic (worms and round worms), GIT infections | B. subtilis, S. aureus, Salmonella sp: 0.09mg/mL [131] | |
| Terminalia chebula Terminalia belerica Embilica officinalis | S and FR | S. aureus E. coli P. aeroginosa M. tuberculosis Common cold virus | S. typhimirium: 1 mg/mL, MRSA: 0.25 mg/mL [132] |
| Plant | Part Used | Chemical Compounds | Inhibited Microorganisms |
|---|---|---|---|
| Cymbopogon citrates Allium sativum | FR B | Ethanolic compounds Isothiocyanate | Enterobacteriaceae, S. aureus Enteriobacteriacae, Candida spp. [134] |
| Thymus vulgaris | AE | Thymol, Linalol, Carvacrol | L. monocytogens, E. coli, S. typhimirium, S. aureus [134] |
| Pimpinella anisum | S | Trans-anethole | S. typhimirium, E. coli [135] |
| Origanum vulgare | AE | Carvacrol, Thymol, γ-Terpinene | L. monocytogens, E. coli, Adeno virus, Polio virus [136] |
| Feoniculum vulgare | S | Trans-anethole | Alternaria alternata, Fusarium oxysporium, Aspergillus flavus [137] |
| Cinnamomum zeylanicum | BA | Cinnamaldehyde | Enterobacteriacae [138] |
| Amomum kerervanh | S | Ethanolic compounds | Enteriobacteriacae [139] |
| Syzygium aromaticum Zingiber officinale | FB RH | Eugenol, Eugenylacetate Ethanolic compounds | Enteriobactericae A. fumigatus, Candida spp., Adeno virus, Polio virus [140] |
| Artemisa arborescens | L | β-Triketone | Herpes simplex virus [141] |
| Rosmarinus officinalis | FL | Benzaylacetate, Linalool, α-pipene | E. coli, S. typhimirium, B. cerus, S. aureus [142] |
| Thymus vulgaris, Mentha piperita | AE | 1,8- Cineole, Eugenol | S. aureus, S. typhimirium Vibrio parahaemolyticus [143] |
| Salvia officinalis | AE | 1,8-Cineole, α-pipene | S. aureus, E. coli [144] |
| Verbana officinalis | AE | Borneol, Geranoil | S. aureus, E. coli, S. typhimirium, L. monocytogens [145] |
5. Antifungal Activity of Medicinal Plants
6. Antimicrobial Fractions or Compounds Isolated from Agaricus bisporus
7. Phytotherapy
7.1. Phytotherapy in Bacterial Infection
7.1.1. Respiratory Tract Infection
7.1.2. Urinary Tract Infection
7.1.3. Cutaneous Infection
7.1.4. Digestive Infection
7.2. Phytotherapy in Viral Infection
7.3. Phytotherapy in Parasitosis
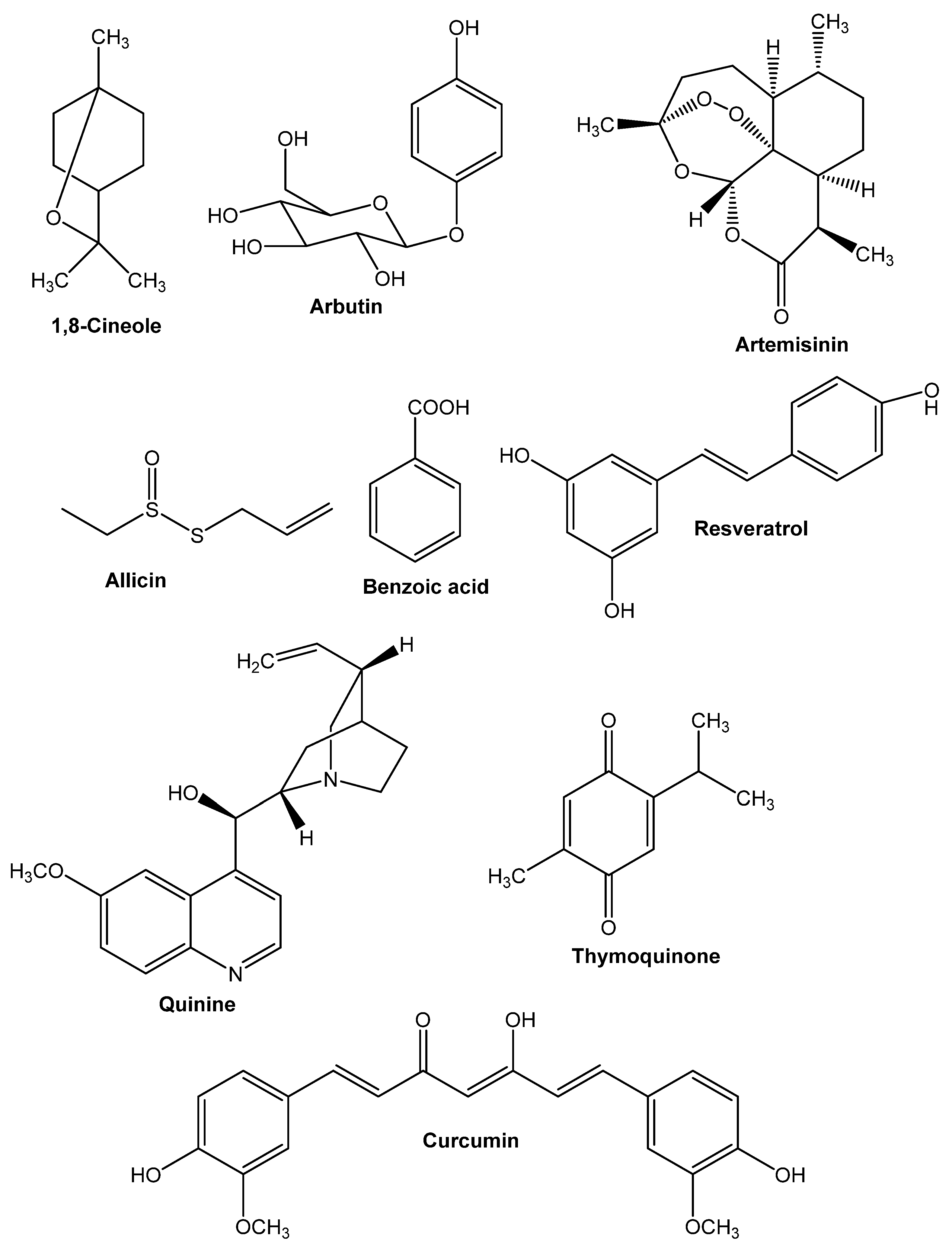
8. Active Compounds from Medicinal Plants
8.1. 1,8-Cineole
8.2. Arbutin
8.3. Allicin
8.4. Artemisinin
8.5. Benzoicacid
8.6. Curcumin
8.7. Quinine
8.8. Resveratrol
8.9. Thymoquinone
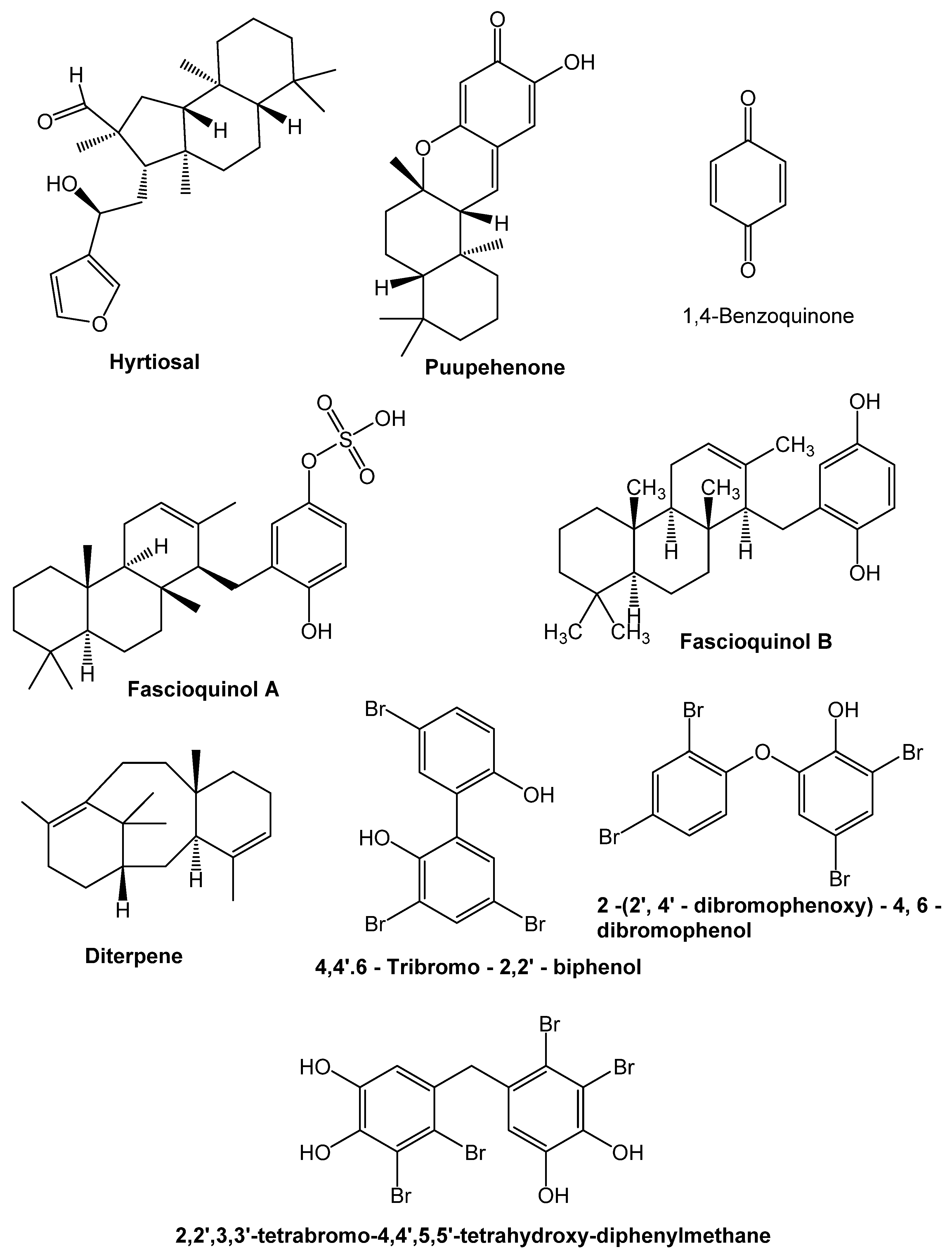
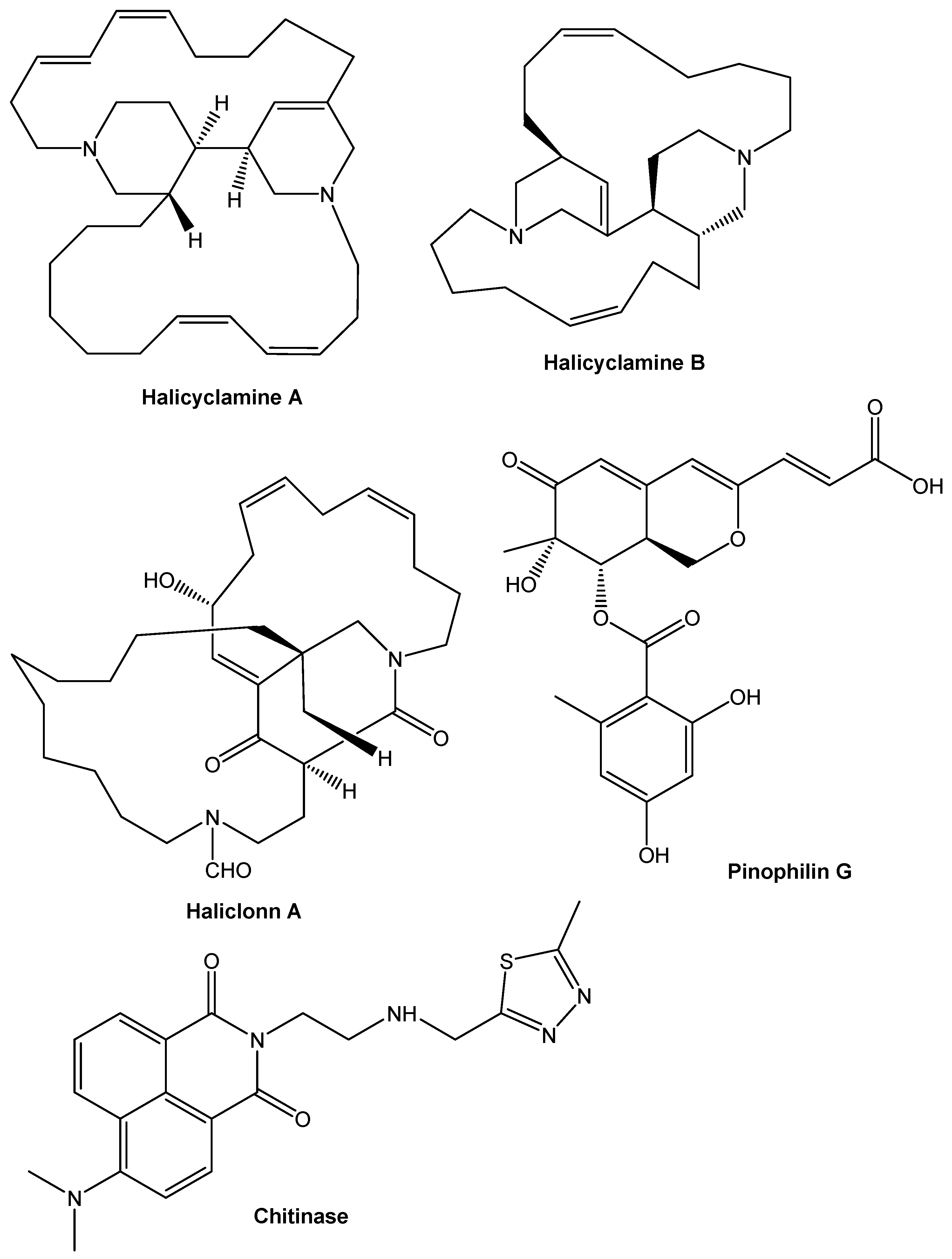
9. Chemical Compounds Having Antimicrobial Properties Derived from Marine Source
9.1. Terpenoids
9.2. Phenolic Compounds
9.3. Alkaloids
9.4. Polysaccharides
9.5. Fatty Acids
9.6. Fungi
9.7. Actinobacteria
9.8. Cyanobacteria
10. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homsy, J.; King, R.; Balaba, D.; Kabatesi, D. Traditional health practitioners are key to scaling up comprehensive care for HIV/AIDS in sub-Saharan Africa. Aids 2004, 18, 1723–1725. [Google Scholar] [CrossRef]
- Ionescu, M.I. Are herbal products an alternative to antibiotics? In Bacterial Pathogenesis and Antibacterial Control; IntechOpen: London, UK, 2017. [Google Scholar]
- Di Pierro, F.; Rapacioli, G.; Ferrara, T.; Togni, S. Use of a standardized extract from Echinacea angustifolia (Polinacea [R]) for the prevention of respiratory tract infections. Altern. Med. Rev. 2012, 17, 36–42. [Google Scholar] [PubMed]
- Chapman, A.D. Numbers of Living Species in Australia and the World; Department of the Environment, Water, Heritage and the Arts: Canberra, ACT, Australia, 2009. [Google Scholar]
- Smith, J.P., Jr. Medical Plants-General References; Humboldt State University: Arcata, CA, USA, 2017. [Google Scholar]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M.; Saki, K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Med. 2014, 7, S348–S354. [Google Scholar] [CrossRef]
- Biswasroy, P.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Recent advancement in topical nanocarriers for the treatment of psoriasis. AAPS PharmSciTech 2021, 22, 164. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Ezzat, S.M. The use of aromatic plants and their therapeutic potential as antiviral agents: A hope for finding anti-COVID 19 essential oils. J. Essent. Oil Res. 2021, 33, 105–113. [Google Scholar] [CrossRef]
- Alves, M.; Young, C.; Bozzetto, K.; Poole-Warren, L.; Martens, P. Degradable, click poly (vinyl alcohol) hydrogels: Characterization of degradation and cellular compatibility. Biomed. Mater. 2012, 7, 024106. [Google Scholar] [CrossRef]
- Ramić, A.; Skočibušić, M.; Odžak, R.; Čipak Gašparović, A.; Milković, L.; Mikelić, A.; Sović, K.; Primožič, I.; Hrenar, T. Antimicrobial activity of quasi-enantiomeric cinchona alkaloid derivatives and prediction model developed by machine learning. Antibiotics 2021, 10, 659. [Google Scholar] [CrossRef]
- Al Ramahi, M.; Keszthelyi-Szabó, G.; Beszédes, S. Corrigendum: Water Science and Technology 81 (6), 1231–1241: Improving biogas production performance of dairy activated sludge via ultrasound disruption prior to microwave disintegration. Water Sci. Technol. 2020, 82, 1720. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z. Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chin. Med. J. 2014, 127, 1344–1350. [Google Scholar] [PubMed]
- Yesilada, E. Contribution of traditional medicine in the healthcare system of the Middle East. Chin. J. Integr. Med. 2011, 17, 95–98. [Google Scholar] [CrossRef]
- Ghirga, F.; Quaglio, D.; Mori, M.; Cammarone, S.; Iazzetti, A.; Goggiamani, A.; Ingallina, C.; Botta, B.; Calcaterra, A. A unique high-diversity natural product collection as a reservoir of new therapeutic leads. Org. Chem. Front. 2021, 8, 996–1025. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol enhances the antimicrobial effect of polymyxin B on Klebsiella pneumoniae and Escherichia coli isolates with polymyxin B resistance. BMC Microbiol. 2020, 20, 306. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Fahrenholtz, C.G.; Bonanno, L.S.; Martin, J.B. Tranexamic acid as adjuvant treatment for postpartum hemorrhage: A systematic review protocol. JBI Evid. Synth. 2019, 17, 1565–1572. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Prev. Biomark. 2002, 11, 1025–1032. [Google Scholar]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Goud, J.V.; Suryam, A.; Charya, M.S. Biomolecular and phytochemical analyses of three aquatic angiosperms. Afr. J. Microbiol. Res. 2009, 3, 418–421. [Google Scholar]
- Vojtovič, D.; Luhová, L.; Petřivalský, M. Something smells bad to plant pathogens: Production of hydrogen sulfide in plants and its role in plant defence responses. J. Adv. Res. 2020, 27, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P. Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Medicinal Herbs; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Katerere, D.R.; Luseba, D. Ethnoveterinary Botanical Medicine: Herbal Medicines for Animal Health; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Khandelwal, K.R. Practical Pharmacognosy; Pragati Books Private Limited: Pune, India, 2008. [Google Scholar]
- Saran, S.; Menon, S.; Shailajan, S.; Pokharna, P. Validated RP-HPLC method to estimate eugenol from commercial formulations like Caturjata Churna, Lavangadi Vati, Jatiphaladi Churna, Sitopaladi Churna and clove oil. J. Pharm. Res. 2013, 6, 53–60. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 2001, 14, 227. [Google Scholar] [CrossRef]
- Marcó, M.B.; Mercanti, D.J. Bacteriophages in dairy plants. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 97, pp. 1–54. [Google Scholar]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. N. J. Sci. 2014, 2014, 28. [Google Scholar] [CrossRef]
- Motley, T.J. The ethnobotany of sweet flag, Acorus calamus (Araceae). Econ. Bot. 1994, 48, 397–412. [Google Scholar] [CrossRef]
- El-Najjar, N.; Gali-Muhtasib, H.; Ketola, R.A.; Vuorela, P.; Urtti, A.; Vuorela, H. The chemical and biological activities of quinones: Overview and implications in analytical detection. Phytochem. Rev. 2011, 10, 353–370. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Chandra, A.K.; De, N. Goitrogenic/antithyroidal potential of green tea extract in relation to catechin in rats. Food Chem. Toxicol. 2010, 48, 2304–2311. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Chemical diversity and activity profiles of HIV-1 reverse transcriptase inhibitors from plants. Rev. Bras. Farmacogn. 2019, 29, 504–528. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hamada, S.; Ooshima, T. Molecular analysis of the inhibitory effects of oolong tea polyphenols on glucan-binding domain of recombinant glucosyltransferases from Streptococcus mutans MT8148. FEMS Microbiol. Lett. 2003, 228, 73–80. [Google Scholar] [CrossRef]
- De Oliveira, D.R.; Tintino, S.R.; Braga, M.F.B.M.; Boligon, A.A.; Athayde, M.L.; Coutinho, H.D.M.; de Menezes, I.R.A.; Fachinetto, R. In vitro antimicrobial and modulatory activity of the natural products silymarin and silibinin. Biomed. Res. Int. 2015, 2015, 292797. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shahzad, A.; Malik, A.; Sahai, A. Recent Trends in Biotechnology and Therapeutic Applications of Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Pasetto, S.; Pardi, V.; Murata, R.M. Anti-HIV-1 activity of flavonoid myricetin on HIV-1 infection in a dual-chamber in vitro model. PLoS ONE 2014, 9, e115323. [Google Scholar] [CrossRef] [PubMed]
- Pengsuparp, T.; Cai, L.; Constant, H.; Fong, H.H.; Lin, L.-Z.; Kinghorn, A.D.; Pezzuto, J.M.; Cordell, G.A.; Ingolfsdöttir, K.; Wagner, H. Mechanistic evaluation of new plant-derived compounds that inhibit HIV-1 reverse transcriptase. J. Nat. Prod. 1995, 58, 1024–1031. [Google Scholar] [CrossRef]
- Watanbe, H.; Miyaji, C.; Makino, M.; Abo, T. Therapeutic effects of glycyrrhizin in mice infected with LP-BM5 murine retrovirus and mechanisms involved in the prevention of disease progression. Biotherapy 1996, 9, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.M.; Madany, M. Coumarin pretreatment alleviates salinity stress in wheat seedlings. Plant Physiol. Biochem. 2015, 88, 27–35. [Google Scholar] [CrossRef]
- Jaafar, H.Z.; Ibrahim, M.H.; Karimi, E. Phenolics and flavonoids compounds, phenylanine ammonia lyase and antioxidant activity responses to elevated CO2 in Labisia pumila (Myrisinaceae). Molecules 2012, 17, 6331–6347. [Google Scholar] [CrossRef]
- Scervino, J.; Ponce, M.; Erra-Bassells, R.; Bompadre, M.; Vierheilig, H.; Ocampo, J.; Godeas, A. Glycosidation of apigenin results in a loss of its activity on different growth parameters of arbuscular mycorrhizal fungi from the genus Glomus and Gigaspora. Soil Biol. Biochem. 2006, 38, 2919–2922. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Yadav, H.; Yadav, M.; Jain, S.; Bhardwaj, A.; Singh, V.; Parkash, O.; Marotta, F. Antimicrobial property of a herbal preparation containing Dalbergia sissoo and Datura stramonium with cow urine against pathogenic bacteria. Int. J. Immunopathol. Pharmacol. 2008, 21, 1013–1020. [Google Scholar] [CrossRef]
- Savic, I.; Nikolic, V.; Savic-Gajic, I.; Nikolic, L.B.; Moder, K.; Hopkins, M. Optimization of quercetin extraction from green tea (Camellia sinensis) using central composite design, and the pharmacological activity of the extract. Chem. Biochem. Eng. Q. 2016, 30, 103–115. [Google Scholar] [CrossRef]
- Kirsch, G.; Abdelwahab, A.B.; Chaimbault, P. Natural and synthetic coumarins with effects on inflammation. Molecules 2016, 21, 1322. [Google Scholar] [CrossRef]
- Polassi, M.R.; Oliveira, T.d.S.; Carvalho, A.C.d.; Medeiros, L.S.d.M.; Veiga, T.A.M.; Graeff, C.F.d.O.; González, A.H.M.; Marcucci, M.C.; Grecco, S.d.S.; D’Alpino, P.H.P. Influence of dentin priming with tannin-rich plant extracts on the longevity of bonded composite restorations. Sci. World J. 2021, 2021, 1614643. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021, 113870. [Google Scholar] [CrossRef]
- Prabhu, S.; Molath, A.; Choksi, H.; Kumar, S.; Mehra, R. Classifications of polyphenols and their potential application in human health and diseases. Int. J. Physiol. Nutr. Phys. Educ. 2021, 6, 293–301. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.Á.; Butassi, E.; Ribas, J.C.; Svetaz, L.A.; Cortés, J.C. Natural products targeting the synthesis of β (1, 3)-D-glucan and chitin of the fungal cell wall. Existing drugs and recent findings. Phytomedicine 2021, 88, 153556. [Google Scholar] [CrossRef]
- Bilgin, H.M.; Atmaca, M.; Obay, B.D.; Özekinci, S.; Taşdemir, E.; Ketani, A. Protective effects of coumarin and coumarin derivatives against carbon tetrachloride-induced acute hepatotoxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 325–330. [Google Scholar] [CrossRef]
- Hoult, J.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Tripathi, R.; Sen, M.; Dutta, G. The Use of Artemisinin Derivative Suppositories as Life-Saving Remedy for Critical Malaria Patients; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2021. [Google Scholar]
- Soine, T.O. Naturally occurring coumarins and related physiological activities. J. Pharm. Sci. 1964, 53, 231–264. [Google Scholar] [CrossRef] [PubMed]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Parages, M.L.; O’Gara, F. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.; Rahimi, E.; Moosavi, S. Antimicrobial activity of essential oils of three herbs against Listeria monocytogenes on chicken frankfurters. Acta Agric. Slov. 2010, 95, 219. [Google Scholar] [CrossRef][Green Version]
- Reguera, R.M.; Elmahallawy, E.K.; García-Estrada, C.; Carbajo-Andrés, R.; Balaña-Fouce, R. DNA topoisomerases of Leishmania parasites; druggable targets for drug discovery. Curr. Med. Chem. 2019, 26, 5900–5923. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Gray, A.I.; Waterman, P.G. A new antibacterial sesquiterpene from Premna oligotricha. J. Nat. Prod. 1993, 56, 140–143. [Google Scholar] [CrossRef]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial peptides: Features, action, and their resistance mechanisms in bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Aureli, P.; Costantini, A.; Zolea, S. Antimicrobial activity of some plant essential oils against Listeria monocytogenes. J. Food Prot. 1992, 55, 344–348. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Wu, R.; Jiang, D.; Bai, B.; Tan, D.; Yan, T.; Sun, X.; Zhang, Q.; Wu, Z. Antibacterial activity of juglone against Staphylococcus aureus: From apparent to proteomic. Int. J. Mol. Sci. 2016, 17, 965. [Google Scholar] [CrossRef]
- Ji, J.; Shankar, S.; Royon, F.; Salmieri, S.; Lacroix, M. Essential oils as natural antimicrobials applied in meat and meat products—A review. Crit. Rev. Food Sci. Nutr. 2021, 10, 1–17. [Google Scholar]
- Sharma, V.; Ichikawa, M.; Freeze, H.H. Mannose metabolism: More than meets the eye. Biochem. Biophys. Res. Commun. 2014, 453, 220–228. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
- Singh, G.; Passsari, A.K.; Leo, V.V.; Mishra, V.K.; Subbarayan, S.; Singh, B.P.; Kumar, B.; Kumar, S.; Gupta, V.K.; Lalhlenmawia, H. Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Front. Plant Sci. 2016, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Wu, R.; Jiang, D.; Bai, B.; Tan, D.; Yan, T.; Sun, X.; Zhang, Q.; Wu, Z. Proteomic analysis of the antibacterial mechanism of action of juglone against Staphylococcus aureus. Nat. Prod. Commun. 2016, 11, 1934578X1601100632. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in oxidative stress and cell signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef]
- Tullus, K.; Marks, S.D. Vasculitis in children and adolescents. Pediatr. Drugs 2009, 11, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gong, X.; Tan, J.Y.; Kang, L.; Li, D.; Yang, J.; Du, G. In vitro antiviral activity of Rubia cordifolia aerial part extract against rotavirus. Front. Pharmacol. 2016, 7, 308. [Google Scholar] [CrossRef]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018. [Google Scholar]
- Tian, X.-R.; Feng, J.-T.; Ma, Z.-Q.; Xie, N.; Zhang, J.; Zhang, X.; Tang, H.-F. Three new glycosides from the whole plant of Clematis lasiandra Maxim and their cytotoxicity. Phytochem. Lett. 2014, 10, 168–172. [Google Scholar] [CrossRef]
- Simmonds, M.S. Flavonoid–insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Li, P.; Luo, H.; Meng, J.; Sun, W.; Wang, X.; Lu, S.; Peng, Y.; Zhou, L. Effects of oligosaccharides from endophytic Fusarium oxysporum Dzf17 on activities of defense-related enzymes in Dioscorea zingiberensis suspension cell and seedling cultures. Electron. J. Biotechnol. 2014, 17, 156–161. [Google Scholar] [CrossRef]
- Morsy, N. Phytochemical analysis of biologically active constituents of medicinal plants. Main Group Chem. 2014, 13, 7–21. [Google Scholar] [CrossRef]
- Qi, X.; Wang, E.; Xing, M.; Zhao, W.; Chen, X. Rhizosphere and non-rhizosphere bacterial community composition of the wild medicinal plant Rumex patientia. World J. Microbiol. Biotechnol. 2012, 28, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Köberl, M.; Ramadan, E.M.; Adam, M.; Cardinale, M.; Hallmann, J.; Heuer, H.; Smalla, K.; Berg, G. Bacillus and Streptomyces were selected as broad-spectrum antagonists against soilborne pathogens from arid areas in Egypt. FEMS Microbiol. Lett. 2013, 342, 168–178. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shukla, S.D. Pro-and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes. Eur. J. Pharmacol. 2005, 508, 31–45. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Chebotar, V.; Tikhonovich, I.; Kamilova, F.; Validov, S.Z. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fertil. Soils 2011, 47, 197–205. [Google Scholar] [CrossRef]
- Malfanova, N.; Kamilova, F.; Validov, S.; Shcherbakov, A.; Chebotar, V.; Tikhonovich, I.; Lugtenberg, B. Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microb. Biotechnol. 2011, 4, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Kumar, G.; Kanaujia, N.; Bafana, A. Functional and phylogenetic diversity of root-associated bacteria of Ajuga bracteosa in Kangra valley. Microbiol. Res. 2012, 167, 220–225. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, P.; Gawai, K.; Kodam, K.; Kuchekar, B.; Chabukswar, A.; Jagdale, S. Antibacterial activity of extracts of Piper longum. J. Pharm. Toxicol. 2007, 2, 574–579. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, H.; Acharya, A.; Singh, M.; Bhatia, D.; Mukerjee, A. Investigation of comparative antimicrobial activity of aloe vera gel and juice. Pharmacologyonline 2008, 1, 239–243. [Google Scholar]
- Jelodarian, S.; Ebrahimabadi, A.H.; Kashi, F.J. Evaluation of antimicrobial activity of Malus domestica fruit extract from Kashan area. Avicenna J. Phytomed. 2013, 3, 1. [Google Scholar]
- Bokaeian, M.; Saeidi, S. Evolution of antimicrobial activity of leaf extract of Withania somnifera against antibiotic resistanct Staphylococcus aureus. Zahedan J. Res. Med. Sci. 2015, 17, e1016. [Google Scholar] [CrossRef]
- Dashti, Z.; Shariatifar, N.; Nafchi, A. Study on antibacterial and antioxidant activity of Berberis vulgaris aqueous extracts from Iran. Int. J. Pharm. Sci. Res. 2014, 5, 705–708. [Google Scholar]
- Rahman, M.; Khan, M.; Jamal, M.A. Anti-bacterial evaluation and minimum inhibitory concentration analysis of oxalis corniculata and ocimum santum against bacterial pathogens. Biotechnology 2010, 9. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A comparative study of the in vitro antimicrobial and synergistic effect of essential oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with antimicrobial drugs: New approach for health promoting products. Antibiotics 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Toma, Z. Antimicrobial Activity of Piperine purified from piper nigrum. J. Basrah Res. 2010, 36, 54–61. [Google Scholar]
- Phongpaichit, S.; Pujenjob, N.; Rukachaisirikul, V.; Ongsakul, M. Antimicrobial activities of the crude methanol extract of Acorus calamus Linn. Songklanakarin J. Sci. Technol. 2005, 27, 517–523. [Google Scholar]
- Al-Mamun, M.A.; Akter, Z.; Uddin, M.J.; Ferdaus, K.; Hoque, K.; Ferdousi, Z.; Reza, M.A. Characterization and evaluation of antibacterial and antiproliferative activities of crude protein extracts isolated from the seed of Ricinus communis in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Vazirian, M.; Alehabib, S.; Jamalifar, H.; Fazeli, M.; Najarian Toosi, A.; Khanavi, M. Antimicrobial effect of cinnamon (Cinnamomum verum J. Presl) bark essential oil in cream-filled cakes and pastries. Res. J. Pharmacogn. 2015, 2, 11–16. [Google Scholar]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Casimir, O.A.; Martin, D.K.; Philippe, E.K.; Augustin, A.A.; Parfait, K.E.J. Chemical composition, antioxidant and antimicrobial activities of Capsicum annuum var. annuum concentrated extract obtained by reverse osmosis. GSC Biol. Pharm. Sci. 2018, 5, 116–125. [Google Scholar]
- Radaelli, M.; Silva, B.P.d.; Weidlich, L.; Hoehne, L.; Flach, A.; Costa, L.A.M.A.d.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]
- Jeya, K.; Veerapagu, M.; Sangeetha, V. Antimicrobial and antioxidant properties of Coriandrum sativum L. seed essential oil. Am. J. Essent. Oils Nat. Prod. 2019, 7, 6–10. [Google Scholar]
- Salari, M.; Amine, G.; Shirazi, M.; Hafezi, R.; Mohammadypour, M. Antibacterial effects of Eucalyptus globulus leaf extract on pathogenic bacteria isolated from specimens of patients with respiratory tract disorders. Clin. Microbiol. Infect. 2006, 12, 194–196. [Google Scholar] [CrossRef]
- Mohsenipour, Z.; Hassanshahian, M. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J. Microbiol. 2015, 8, e18971. [Google Scholar] [CrossRef]
- Sedighinia, F.; Afshar, A.S. Antibacterial activity of Glycyrrhiza glabra against oral pathogens: An in vitro study. Avicenna J. Phytomed. 2012, 2, 118. [Google Scholar] [PubMed]
- Shankar, S.; Bardvalli, S.G.; Jyotirmayee, R.; Bhushan, K.; Kumar, S. Efficacy of calendula officinalis extract (marigold flower) as an antimicrobial agent against oral microbes: An invitro study in comparison with chlorhexidine digluconate. J. Clin. Diagn. Res. 2017, 11, 5–10. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.-G.; Liang, W.; Mei, J.; Di, Y.-Y.; Lan, H.-H.; Yang, Y.; Wang, W.-W.; Luo, Y.-Y.; Wang, H.-Z. Antibacterial activity and mode of action of Mentha arvensis ethanol extract against multidrug-resistant Acinetobacter baumannii. Trop. J. Pharm. Res. 2015, 14, 2099–2106. [Google Scholar] [CrossRef]
- Mistry, K.S.; Sanghvi, Z.; Parmar, G.; Shah, S. The antimicrobial activity of Azadirachta indica, Mimusops elengi, Tinospora cardifolia, Ocimum sanctum and 2% chlorhexidine gluconate on common endodontic pathogens: An in vitro study. Eur. J. Dent. 2014, 8, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Korukluoglu, M.; Sahan, Y.; Yigit, A.; Karakas, R. Antifungal activity of olive leaf (Olea Europaea L.) extracts from the Trilye region of Turkey. Ann. Microbiol. 2006, 56, 359–362. [Google Scholar] [CrossRef]
- Shetty, S.B.; Mahin-Syed-Ismail, P.; Varghese, S.; Thomas-George, B.; Kandathil-Thajuraj, P.; Baby, D.; Haleem, S.; Sreedhar, S.; Devang-Divakar, D. Antimicrobial effects of Citrus sinensis peel extracts against dental caries bacteria: An in vitro study. J. Clin. Exp. Dent. 2016, 8, e71. [Google Scholar] [CrossRef]
- Rahman, S.; Imran, M.; Muhammad, N.; Khan, N.U.; Chisthi, A.K.; Khan, A.F.; Sadozai, S.; Khan, S.M. Antibacetial screening of leaves and stem of Carica papaya. J. Med. Plant Res. 2011, 5, 5167–5171. [Google Scholar]
- Singh, R.; Shushni, M.A.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Ismaili, A.; Sohrabi, S.; Azadpour, M.; Heydari, R.; Rashidipour, M. Evaluation of the antimicrobial activity of alkaloid extracts of four Papaver species. Herb. Med. J. 2017, 2, 146–152. [Google Scholar] [CrossRef]
- Misra, B.B.; Dey, S. Comparative phytochemical analysis and antibacterial efficacy of in vitro and in vivo extracts from E ast I ndian sandalwood tree (Santalum album L.). Lett. Appl. Microbiol. 2012, 55, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Doughari, J. Antimicrobial activity of Tamarindus indica Linn. Trop. J. Pharm. Res. 2006, 5, 597–603. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, P.; Dkhar, M.; Kayang, H.; Raghuwanshi, R.; Dubey, N.K. Assessment of chemically characterised Gaultheria fragrantissima Wall. essential oil and its major component as safe plant based preservative for millets against fungal, aflatoxin contamination and lipid peroxidation during storage. J. Food Sci. Technol. 2018, 55, 111–119. [Google Scholar] [CrossRef]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.-K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. BioMed Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, M.P.J.; Manoraj, A.; Thevanesam, V.; Ekanayake, A.; Kumar, N.S.; Liyanapathirana, V.; Abeyratne, E.; Bandara, B.R. Terminalia bellirica fruit extracts: In-vitro antibacterial activity against selected multidrug-resistant bacteria, radical scavenging activity and cytotoxicity study on BHK-21 cells. BMC Complement. Altern. Med. 2018, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. J. Issues ISSN 2014, 2350, 1588. [Google Scholar]
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ban, X.; Zeng, H.; Huang, B.; He, J.; Wang, Y. In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control 2011, 22, 1992–1999. [Google Scholar] [CrossRef]
- Ultee, A.; Smid, E. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Hammer, K.; Carson, C.; Riley, T. In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. J. Antimicrob. Chemother. 2002, 50, 195–199. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M. Evaluation of common antibacterial screening methods utilized in essential oil research. J. Essent. Oil Res. 2003, 15, 428–433. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Sinico, C.; De Logu, A.; Lai, F.; Valenti, D.; Manconi, M.; Loy, G.; Bonsignore, L.; Fadda, A.M. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur. J. Pharm. Biopharm. 2005, 59, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J. Chemical composition of essential oils of thymus and mentha speciesand their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef]
- Guleria, S.; Kumar, A. Azadirachta indica leaf extract induces resistance in sesame against Alternaria leaf spot disease. J. Cell Mol. Biol. 2006, 5, 81–86. [Google Scholar]
- Gholibeikian, M.; Bamoniri, A.; HoushdarTehrani, M.H.; Mirjalili, B.B.F.; Bijanzadeh, H.R. Structure-activity relationship studies of Longicalcynin A analogues, as anticancer cyclopeptides. Chem. Biol. Interact. 2020, 315, 108902. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.A.; Gunnarsson, T.G.; Potts, P.M.; Sutherland, W.J.; Gill, J.A. Sex-biases in distribution and resource use at different spatial scales in a migratory shorebird. Ecol. Evol. 2013, 3, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Rezaeian, S.; Pourianfar, H.R. Antimicrobial properties of the button mushroom, Agaricus bisporus: A mini-review. Int. J. Adv. Res. 2016, 4, 426–429. [Google Scholar]
- Pinna, S.; Gévry, M.-F.; Côté, M.; Sirois, L. Factors influencing fructification phenology of edible mushrooms in a boreal mixed forest of Eastern Canada. For. Ecol. Manag. 2010, 260, 294–301. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antifungal activity of mushroom (basidiomycetes) extracts and isolated compounds. Curr. Top. Med. Chem. 2013, 13, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Yamaç, M.; Bilgili, F. Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm. Biol. 2006, 44, 660–667. [Google Scholar] [CrossRef]
- Ngai, P.H.; Zhao, Z.; Ng, T. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea. Peptides 2005, 26, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.; Cheung, R.C.; Ye, X.; Wang, H.; Lam, S.; Lin, P.; Chan, Y.; Fang, E.F.; Ngai, P.H. Proteins with antifungal properties and other medicinal applications from plants and mushrooms. Appl. Microbiol. Biotechnol. 2010, 87, 1221–1235. [Google Scholar] [CrossRef]
- Le Grand, A. Anti-infective phytotherapies of the tree-savannah, Senegal (occidental Africa). III: A review of phytochemical substances and the antimicrobial activity of 43 species. J. Ethnopharmacol. 1989, 25, 315–338. [Google Scholar] [CrossRef]
- Ginja, C.; Gama, L.T.; Cortés, O.; Burriel, I.M.; Vega-Pla, J.L.; Penedo, C.; Sponenberg, P.; Cañón, J.; Sanz, A.; do Egito, A.A.; et al. Author correction: The genetic ancestry of American Creole cattle inferred from uniparental and autosomal genetic markers. Sci. Rep. 2020, 10, 16930. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic antimicrobial activity of Camellia sinensis and Juglans regia against multidrug-resistant bacteria. PLoS ONE 2015, 10, e0118431. [Google Scholar] [CrossRef]
- Owen, L.; Grootveld, M.; Arroo, R.; Ruiz-Rodado, V.; Price, P.; Laird, K. A multifactorial comparison of ternary combinations of essential oils in topical preparations to current antibiotic prescription therapies for the control of acne vulgaris-associated bacteria. Phytother. Res. 2017, 31, 410–417. [Google Scholar] [CrossRef]
- El-Massry, K.; Farouk, A.; Abou-Zeid, M. Free radical scavenging activity and lipoxygenase inhibition of rosemary (Rosmarinus officinalis L) volatile oil. J. Essent. Oil Bear. Plants 2008, 11, 536–543. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; DeLampasona, M.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A. Essential Oil as antimicrobial agents: Efficacy, stability, and safety issues for food application. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020; pp. 1–33. [Google Scholar]
- Rodgers, A.; Mokoena, M.; Durbach, I.; Lazarus, J.; de Jager, S.; Ackermann, H.; Breytenbach, I.; Okada, A.; Usami, M.; Hirose, Y. Do teas rich in antioxidants reduce the physicochemical and peroxidative risk factors for calcium oxalate nephrolithiasis in humans? Pilot studies with Rooibos herbal tea and Japanese green tea. Urolithiasis 2016, 44, 299–310. [Google Scholar] [CrossRef]
- Vučić, D.M.; Petković, M.R.; Rodić-Grabovac, B.B.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. In vitro activity of heather [Calluna vulgaris (L.) Hull] extracts on selected urinary tract pathogens. Bosn. J. Basic Med. Sci. 2014, 14, 234. [Google Scholar] [CrossRef][Green Version]
- Schulz, V.; Hänsel, R.; Blumenthal, M.; Tyler, V.E. Rational Phytotherapy: A Reference Guide for Physicians and Pharmacists; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Shams-Ghahfarokhi, M.; Shokoohamiri, M.-R.; Amirrajab, N.; Moghadasi, B.; Ghajari, A.; Zeini, F.; Sadeghi, G.; Razzaghi-Abyaneh, M. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia 2006, 77, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Navid, M.H.; Laszczyk-Lauer, M.; Reichling, J.; Schnitzler, P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 2014, 21, 1273–1280. [Google Scholar] [CrossRef]
- Weatherhead, J.E.; Hotez, P.J.; Mejia, R. The global state of helminth control and elimination in children. Pediatr. Clin. 2017, 64, 867–877. [Google Scholar] [CrossRef]
- Akhondian, J.; Parsa, A.; Rakhshande, H. The effect of Nigella sativa L. (black cumin seed) on intractable pediatric seizures. Med. Sci. Monit. 2007, 13, CR555–CR559. [Google Scholar]
- Nakasugi, T.; Murakawa, T.; Shibuya, K.; Morimoto, M. Deodorizing substance in black cumin (Nigella sativa L.) seed oil. J. Oleo Sci. 2017, 66, 877–882. [Google Scholar] [CrossRef][Green Version]
- Abamor, E.S.; Allahverdiyev, A.M. A nanotechnology based new approach for chemotherapy of cutaneous leishmaniasis: TIO2@ AG nanoparticles–Nigella sativa oil combinations. Exp. Parasitol. 2016, 166, 150–163. [Google Scholar] [CrossRef]
- Han, Y.; Yang, B.; Zhang, F.; Miao, X.; Li, Z. Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with South China Sea sponge Craniella australiensis. Mar. Biotechnol. 2009, 11, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Edwards, H.; Lawson, E.; Barry, B. Molecular interactions between the penetration enhancer 1, 8-cineole and human skin. J. Raman Spectrosc. 2006, 37, 361–366. [Google Scholar] [CrossRef]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031. [Google Scholar]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. Nobel for Antimalarial Drug Highlights East-West Divide; American Association for the Advancement of Science: Washington, DC, USA, 2015. [Google Scholar]
- Callaway, E.; Cyranoski, D. Anti-parasite drugs sweep Nobel prize in medicine 2015. Nat. News 2015, 526, 174. [Google Scholar] [CrossRef]
- Fairhurst, R.M.; Dondorp, A.M. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol. Spectr. 2016, 4, EI10-0013-2016. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Risinger, A.L.; Nair, S.; Peng, J.; Anderson, T.J.; Du, L.; Powell, D.R.; Mooberry, S.L.; Cichewicz, R.H. Identification of compounds with efficacy against malaria parasites from common North American plants. J. Nat. Prod. 2016, 79, 490–498. [Google Scholar] [CrossRef]
- Wolf, R.; Baroni, A.; Greco, R.; Donnarumma, G.; Ruocco, E.; Tufano, M.A.; Ruocco, V. Quinine sulfate and bacterial invasion. Ann. Clin. Microbiol. Antimicrob. 2002, 1, 1–5. [Google Scholar] [CrossRef]
- Kharal, S.A.; Hussain, Q.; Ali, S. Quinine is bactericidal. J. Pak. Med. Assoc. 2009, 59, 208–212. [Google Scholar] [PubMed]
- Bottari, N.B.; Baldissera, M.D.; Tonin, A.A.; Rech, V.C.; Nishihira, V.S.; Thomé, G.R.; Schetinger, M.R.C.; Morsch, V.M.; Camillo, G.; Vogel, F.F. Sulfamethoxazole-trimethoprim associated with resveratrol for the treatment of toxoplasmosis in mice: Influence on the activity of enzymes involved in brain neurotransmission. Microb. Pathog. 2015, 79, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef]
- Duan, G.-J.; Zhu, J.; Wan, J.-Y.; Li, X.; Ge, X.-D.; Liu, L.-M.; Liu, Y.-S. A synthetic MD-2 mimetic peptide attenuates lipopolysaccharide-induced inflammatory responses in vivo and in vitro. Int. Immunopharmacol. 2010, 10, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Lin, W.; Proksch, P. Bioactive sesterterpenes and triterpenes from marine sponges: Occurrence and pharmacological significance. Mar. Drugs 2010, 8, 313–346. [Google Scholar] [CrossRef]
- Lee, D.; Shin, J.; Yoon, K.-M.; Kim, T.-I.; Lee, S.-H.; Lee, H.-S.; Oh, K.-B. Inhibition of Candida albicans isocitrate lyase activity by sesterterpene sulfates from the tropical sponge Dysidea sp. Bioorgan. Med. Chem. Lett. 2008, 18, 5377–5380. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Shen, L.; Yu, Z.; Chen, J.; Guo, Y.; Tang, Y.; Shen, X.; Jiang, H. Hyrtiosal, from the marine sponge Hyrtios erectus, inhibits HIV-1 integrase binding to viral DNA by a new inhibitor binding site. ChemMedChem Chem. Enabling Drug Discov. 2008, 3, 173–180. [Google Scholar]
- Xu, W.-H.; Ding, Y.; Jacob, M.R.; Agarwal, A.K.; Clark, A.M.; Ferreira, D.; Liang, Z.-S.; Li, X.-C. Puupehanol, a sesquiterpene-dihydroquinone derivative from the marine sponge Hyrtios sp. Bioorgan. Med. Chem. Lett. 2009, 19, 6140–6143. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, X.M. A new sesquiterpenoid hydroquinone from the marine sponge Dysidea arenaria. Molecules 2008, 13, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; McCluskey, A.; Robertson, M.J.; MacGregor, K.A.; Gordon, C.P.; Guenther, J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela hooperi. Org. Biomol. Chem. 2011, 9, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Skildum, A.; Stromquist, E.; Rose-Hellekant, T.; Chang, L.C. Bioactive polybrominated diphenyl ethers from the marine sponge Dysidea sp. J. Nat. Prod. 2008, 71, 262–264. [Google Scholar] [CrossRef]
- Hanif, N.; Tanaka, J.; Setiawan, A.; Trianto, A.; De Voogd, N.J.; Murni, A.; Tanaka, C.; Higa, T. Polybrominated diphenyl ethers from the Indonesian sponge Lamellodysidea herbacea. J. Nat. Prod. 2007, 70, 432–435. [Google Scholar] [CrossRef]
- Oh, K.-B.; Lee, J.H.; Chung, S.-C.; Shin, J.; Shin, H.J.; Kim, H.-K.; Lee, H.-S. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorgan. Med. Chem. Lett. 2008, 18, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Vynne, N.G.; Månsson, M.; Nielsen, K.F.; Gram, L. Bioactivity, chemical profiling, and 16S rRNA-based phylogeny of Pseudoalteromonas strains collected on a global research cruise. Mar. Biotechnol. 2011, 13, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Sobou, M.; Vilchéze, C.; Baughn, A.; Hashizume, H.; Pruksakorn, P.; Ishida, S.; Matsumoto, M.; Jacobs, W.R., Jr.; Kobayashi, M. Halicyclamine A, a marine spongean alkaloid as a lead for anti-tuberculosis agent. Bioorgan. Med. Chem. 2008, 16, 6732–6736. [Google Scholar] [CrossRef]
- Takayama, K.; Iwata, M.; Hisamichi, H.; Okamoto, Y.; Aoki, M.; Niwa, A. Synthetic studies on selective type 4 phosphodiesterase (PDE 4) inhibitors. 1. Structure—Activity relationships and pharmacological evaluation of 1,8-Naphthyridin-2(1H)-one derivatives. Chem. Pharm. Bull. 2002, 50, 1050–1059. [Google Scholar] [CrossRef][Green Version]
- Jang, K.H.; Kang, G.W.; Jeon, J.-E.; Lim, C.; Lee, H.-S.; Sim, C.J.; Oh, K.-B.; Shin, J. Haliclonin A, a new macrocyclic diamide from the sponge Haliclona sp. Org. Lett. 2009, 11, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Tsuda, M.; Kubota, T.; Mikami, Y.; Fromont, J.; Kobayashi, J.I. Nagelamide J, a novel dimeric bromopyrrole alkaloid from a sponge Agelas species. Org. Lett. 2007, 9, 2369–2371. [Google Scholar] [CrossRef]
- Araki, A.; Kubota, T.; Tsuda, M.; Mikami, Y.; Fromont, J.; Kobayashi, J.I. Nagelamides K and L, dimeric bromopyrrole alkaloids from sponge Agelas species. Org. Lett. 2008, 10, 2099–2102. [Google Scholar] [CrossRef]
- Kubota, T.; Araki, A.; Ito, J.; Mikami, Y.; Fromont, J.; Kobayashi, J.i. Nagelamides M and N, new bromopyrrole alkaloids from sponge Agelas species. Tetrahedron 2008, 64, 10810–10813. [Google Scholar] [CrossRef]
- Araki, A.; Kubota, T.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J.I. Nagelamides Q and R, novel dimeric bromopyrrole alkaloids from sponges Agelas sp. Org. Lett. 2009, 11, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.-M.; Peng, J.; Dunbar, D.C.; Schinazi, R.F.; de Castro Andrews, A.G.; Cuevas, C.; Garcia-Fernandez, L.F.; Kelly, M.; Hamann, M.T. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron 2007, 63, 11179–11188. [Google Scholar] [CrossRef]
- Takishima, S.; Ishiyama, A.; Iwatsuki, M.; Otoguro, K.; Yamada, H.; Omura, S.; Kobayashi, H.; van Soest, R.W.; Matsunaga, S. Merobatzelladines A and B, anti-infective tricyclic guanidines from a marine sponge Monanchora sp. Org. Lett. 2009, 11, 2655–2658. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.-B.; Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Okuyama, S.; Hori, K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J. Biol. Chem. 2007, 282, 11021–11029. [Google Scholar] [CrossRef]
- Tianero, M.D.B.; Hanif, N.; de Voogd, N.J.; van Soest, R.W.; Tanaka, J. A new antimicrobial fatty acid from the calcareous sponge Paragrantia cf. waguensis. Chem. Biodivers. 2009, 6, 1374–1377. [Google Scholar] [CrossRef]
- Taniguchi, M.; Uchio, Y.; Yasumoto, K.; Kusumi, T.; Ooi, T. Brominated unsaturated fatty acids from marine sponge collected in Papua New Guinea. Chem. Pharm. Bull. 2008, 56, 378–382. [Google Scholar] [CrossRef]
- Keffer, J.L.; Plaza, A.; Bewley, C.A. Motualevic Acids A–F, Antimicrobial acids from the sponge Siliquariaspongia sp. Org. Lett. 2009, 11, 1087–1090. [Google Scholar] [CrossRef]
- Desbois, A.P.; Lebl, T.; Yan, L.; Smith, V.J. Isolation and structural characterisation of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2008, 81, 755–764. [Google Scholar] [CrossRef]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, X.M.; Cui, C.M.; Feng, C.; Wang, B.G. New sphingolipids with a previously unreported 9-methyl-C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids 2007, 42, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Li, X.-M.; Zhang, Y.; Li, C.-S.; Cui, C.-M.; Wang, B.-G. Comazaphilones A—F, azaphilone derivatives from the marine sediment-derived fungus Penicillium commune QSD-17. J. Nat. Prod. 2011, 74, 256–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-M.; Shang, Z.; Li, C.-S.; Ji, N.-Y.; Wang, B.-G. Meroterpenoid and diphenyl ether derivatives from Penicillium sp. MA-37, a fungus isolated from marine mangrove rhizospheric soil. J. Nat. Prod. 2012, 75, 1888–1895. [Google Scholar] [CrossRef]
- Wang, J.; Ding, W.; Li, C.; Huang, S.; She, Z.; Lin, Y. A new polysubstituted benzaldehyde from the co-culture broth of two marine fungi (Strains Nos. E33 and K38). Chem. Nat. Compd. 2013, 49, 799–802. [Google Scholar] [CrossRef]
- Wang, M.L.; Lu, C.H.; Xu, Q.Y.; Song, S.Y.; Hu, Z.Y.; Zheng, Z.H. Four new citrinin derivatives from a marine-derived Penicillium sp. fungal strain. Molecules 2013, 18, 5723–5735. [Google Scholar] [CrossRef]
- Julianti, E.; Lee, J.-H.; Liao, L.; Park, W.; Park, S.; Oh, D.-C.; Oh, K.-B.; Shin, J. New polyaromatic metabolites from a marine-derived fungus Penicillium sp. Org. Lett. 2013, 15, 1286–1289. [Google Scholar] [CrossRef]
- Hodges, T.W.; Slattery, M.; Olson, J.B. Unique actinomycetes from marine caves and coral reef sediments provide novel PKS and NRPS biosynthetic gene clusters. Mar. Biotechnol. 2012, 14, 270–280. [Google Scholar] [CrossRef]
- Guo, X.; Liu, N.; Li, X.; Ding, Y.; Shang, F.; Gao, Y.; Ruan, J.; Huang, Y. Red soils harbor diverse culturable actinomycetes that are promising sources of novel secondary metabolites. Appl. Environ. Microbiol. 2015, 81, 3086–3103. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, R.W.; Harrigan, B.L.; Davidson, B.S. Kahakamides A and B, new neosidomycin metabolites from a marine-derived actinomycete. Tetrahedron Lett. 2001, 42, 5133–5135. [Google Scholar] [CrossRef]
- Raju, R.; Piggott, A.M.; Khalil, Z.; Bernhardt, P.V.; Capon, R.J. Heronamycin A: A new benzothiazine ansamycin from an Australian marine-derived Streptomyces sp. Tetrahedron Lett. 2012, 53, 1063–1065. [Google Scholar] [CrossRef]
- McArthur, K.A.; Mitchell, S.S.; Tsueng, G.; Rheingold, A.; White, D.J.; Grodberg, J.; Lam, K.S.; Potts, B.C. Lynamicins A—E, chlorinated bisindole pyrrole antibiotics from a novel marine Actinomycete. J. Nat. Prod. 2008, 71, 1732–1737. [Google Scholar] [CrossRef]
- El-Gendy, M.M.; Shaaban, M.; El-Bondkly, A.-M.; Shaaban, K. Bioactive benzopyrone derivatives from new recombinant fusant of marine Streptomyces. Appl. Biochem. Biotechnol. 2008, 150, 85–96. [Google Scholar] [CrossRef]
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Alvarenga, D.O.; Fiore, M.F.; Varani, A.M. A metagenomic approach to cyanobacterial genomics. Front. Microbiol. 2017, 8, 809. [Google Scholar] [CrossRef]
- Swain, S.S.; Padhy, R.N.; Singh, P.K. Anticancer compounds from cyanobacterium Lyngbya species: A review. Antonie Van Leeuwenhoek 2015, 108, 223–265. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Jaśkiewicz, M. Microalgal food supplements from the perspective of Polish consumers: Patterns of use, adverse events, and beneficial effects. J. Appl. Phycol. 2017, 29, 1841–1850. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Chlipala, G.; Orjala, J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 2009, 72, 894–899. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mannan Mithi, F.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics 2021, 10, 1076. https://doi.org/10.3390/antibiotics10091076
Rahman MM, Rahaman MS, Islam MR, Hossain ME, Mannan Mithi F, Ahmed M, Saldías M, Akkol EK, Sobarzo-Sánchez E. Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics. 2021; 10(9):1076. https://doi.org/10.3390/antibiotics10091076
Chicago/Turabian StyleRahman, Md. Mominur, Md. Saidur Rahaman, Md. Rezaul Islam, Md. Emon Hossain, Faria Mannan Mithi, Muniruddin Ahmed, Marianela Saldías, Esra Küpeli Akkol, and Eduardo Sobarzo-Sánchez. 2021. "Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties" Antibiotics 10, no. 9: 1076. https://doi.org/10.3390/antibiotics10091076
APA StyleRahman, M. M., Rahaman, M. S., Islam, M. R., Hossain, M. E., Mannan Mithi, F., Ahmed, M., Saldías, M., Akkol, E. K., & Sobarzo-Sánchez, E. (2021). Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics, 10(9), 1076. https://doi.org/10.3390/antibiotics10091076