Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies
Abstract
1. Introduction
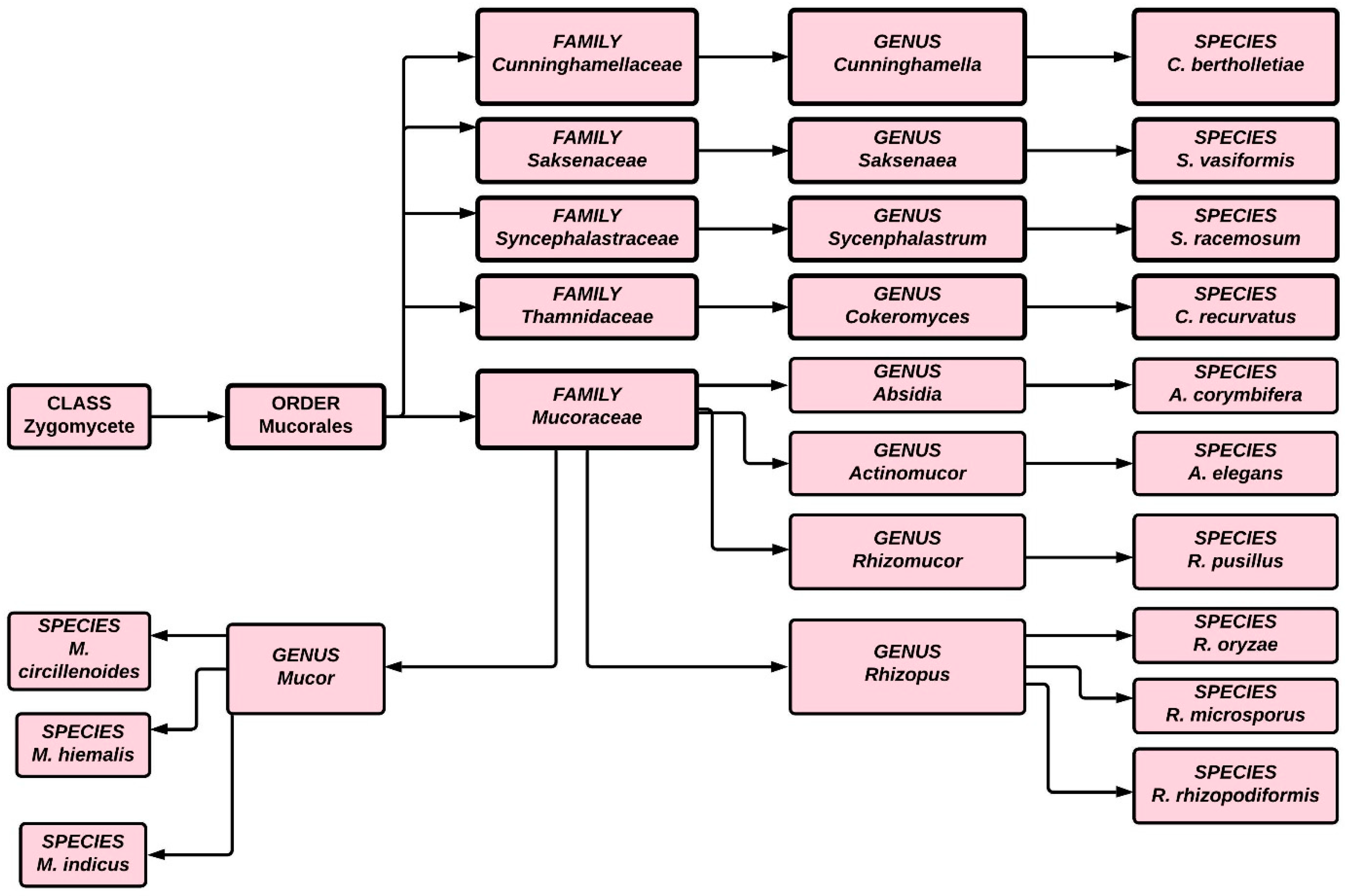
2. Manifestation of Mucormycosis
3. Epidemiology
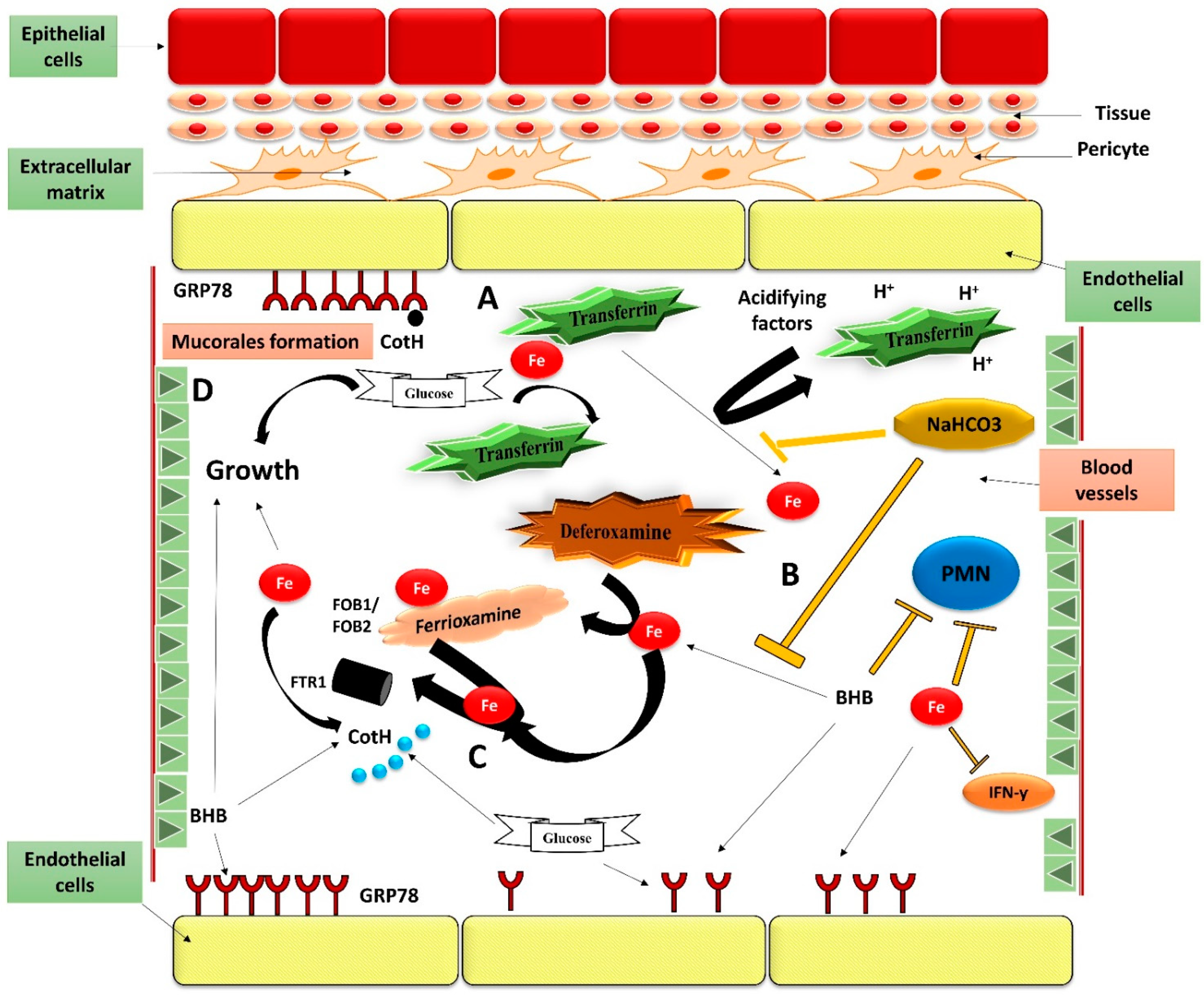
4. Pathogenesis Mechanism
4.1. Phagocytes
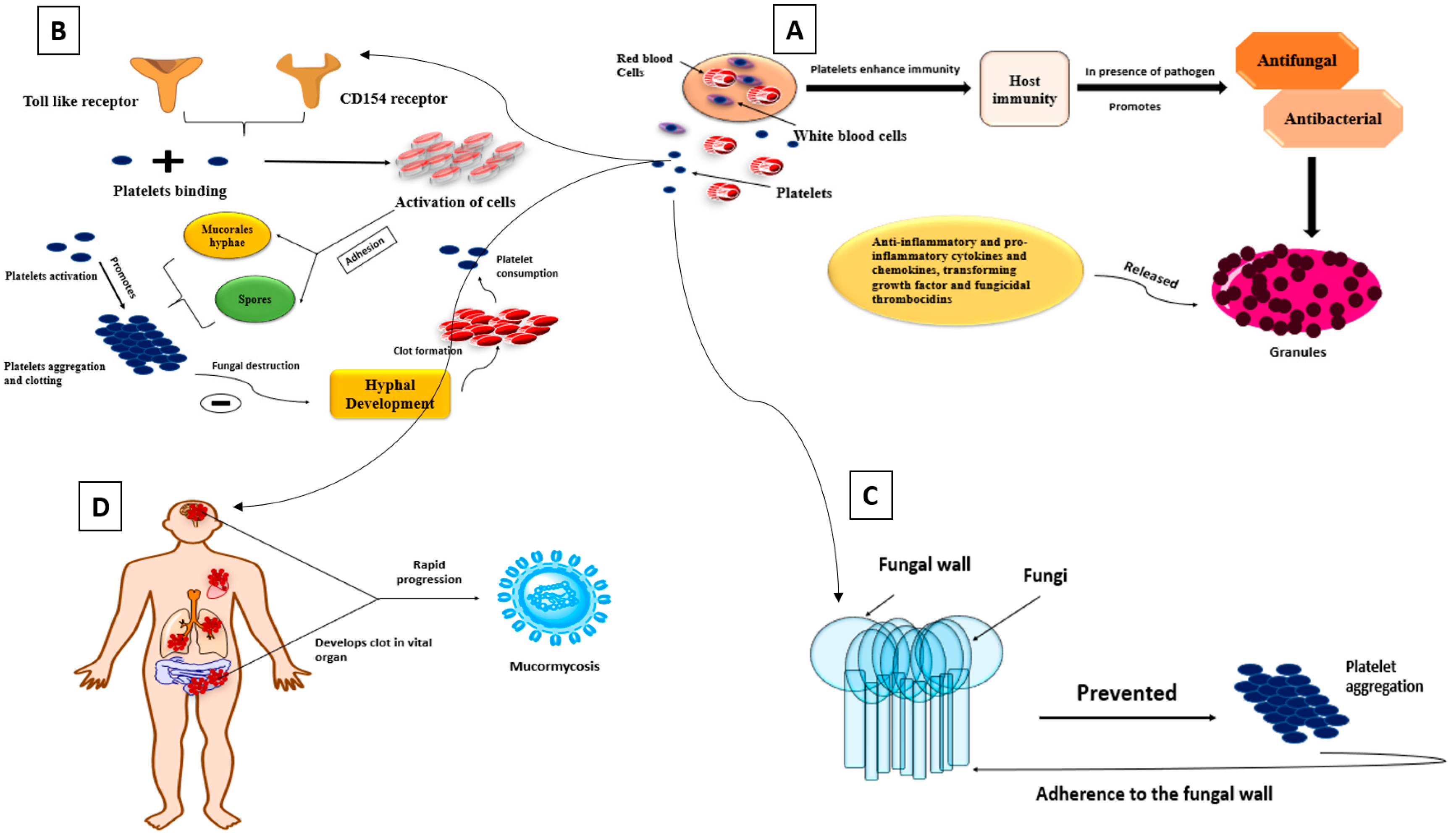
4.2. Platelets
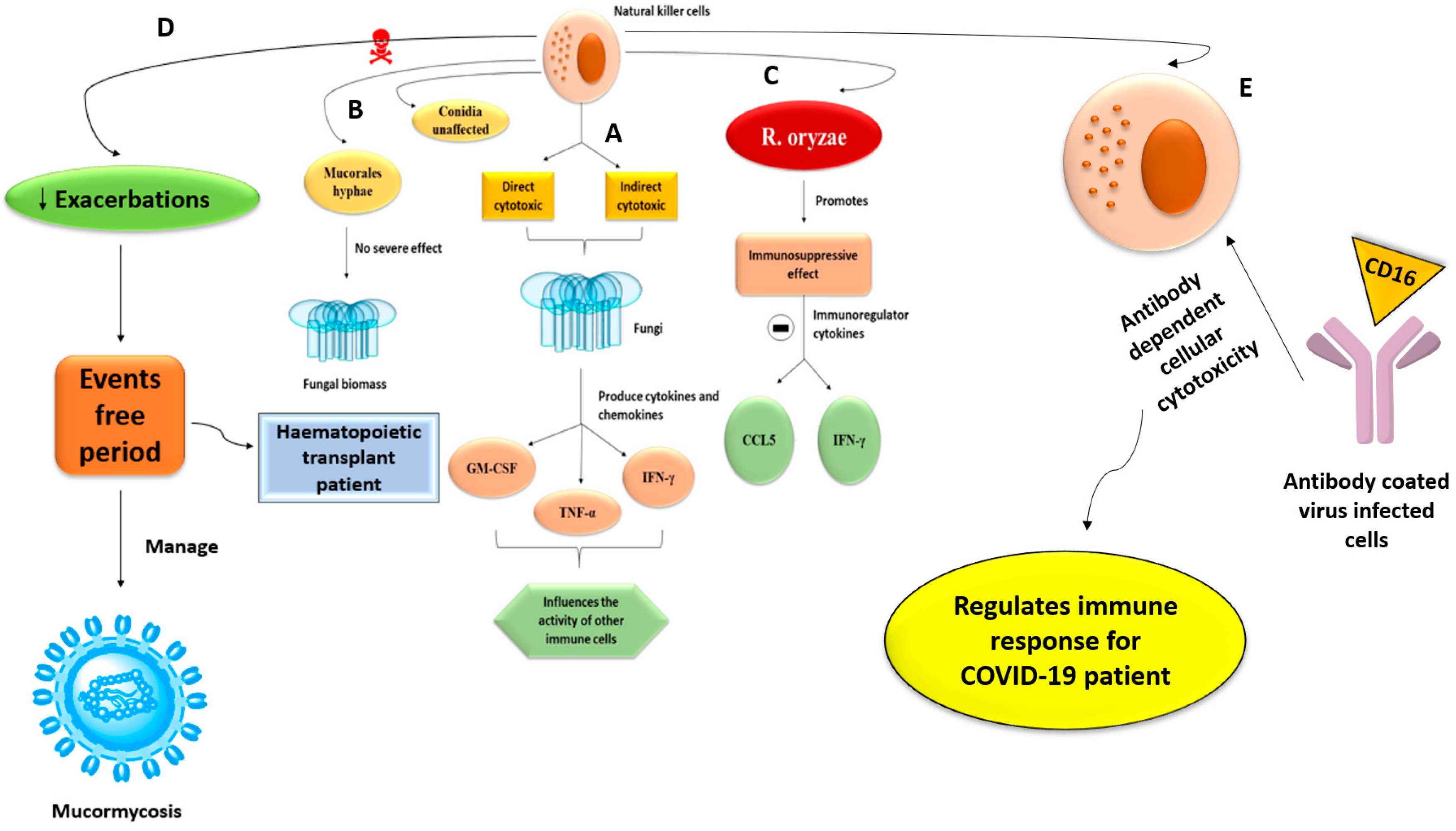
4.3. Natural Killer Cells
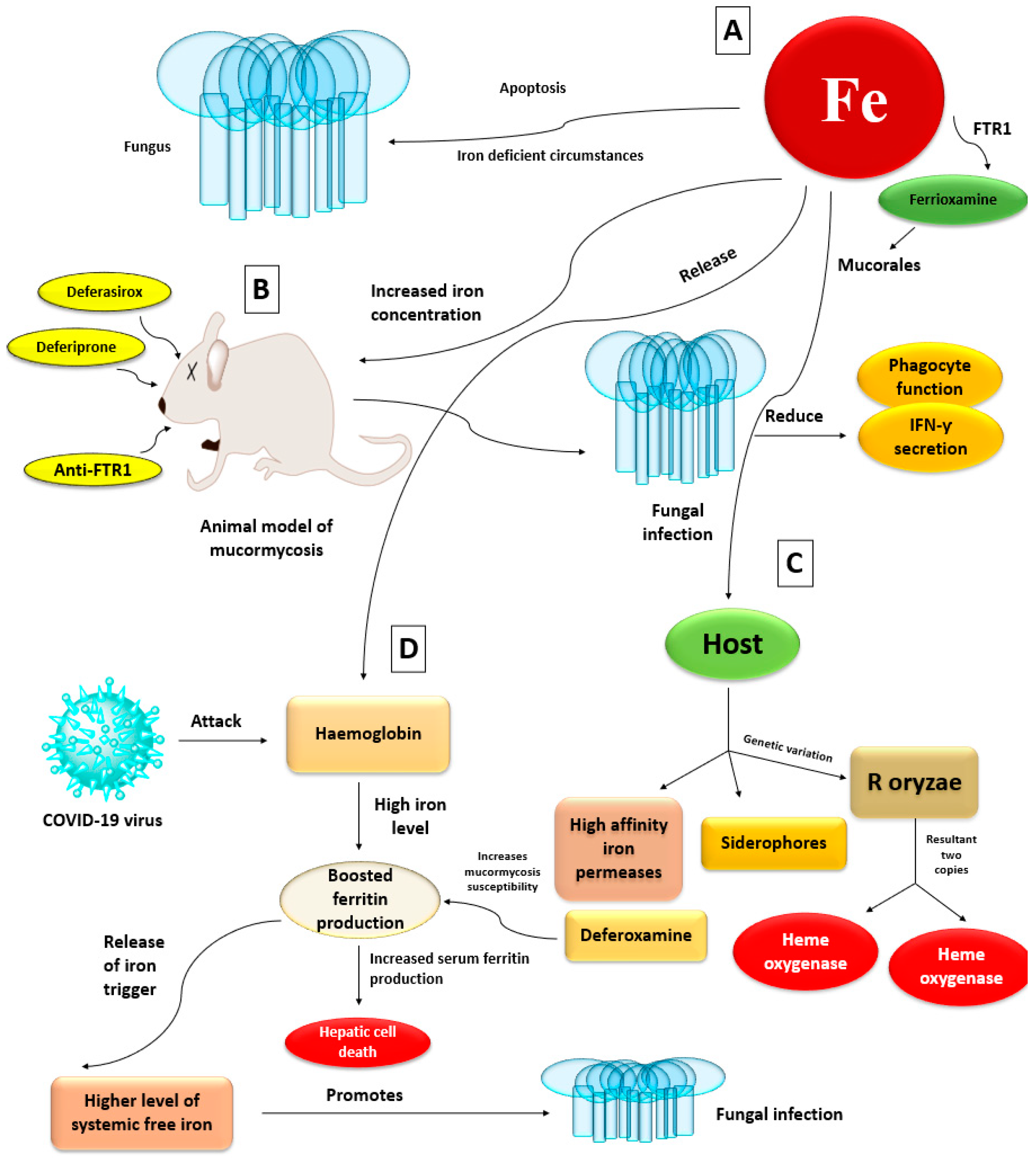
4.4. Iron Uptake
4.5. Interplay with the Endothelium
4.6. Voriconazole Exposure
5. Mucormycosis Outbreak
6. Effects of Black Fungus on COVID-19 Patients
7. Diabetes Patients Are Predisposed to Mucormycosis
8. Implement Control and Preventive Measures
9. Medical Management of Mucormycosis
9.1. Management by Using Antifungal Drug Therapies
9.2. Surgical Management
10. Adjunctive Therapies
11. Preventive Measures
- Good control of sugar level during COVID-19 with or without use of steroids
- Rational use of steroids in the correct dose, with proper timing, and for a suitable duration
- Judicious use of antibiotics/antifungals
- Use of sterile or clean water as humidifiers during oxygen therapy
- Maintaining personal hygiene by thoroughly bathing and scrubbing the body
- Wearing face masks and face shields while visiting dirty or polluted environments
- Wearing concealed shoes, long trousers, long-sleeved shirts, and gloves while handling soil, manure, moss, and the like (especially while gardening)
12. Nanoparticle Formulation of the Drug/Nanoformulation of the Antifungal Drugs
13. Earlier Diagnose Mucormycosis to Overcome the Adverse Effect
14. Mucormycosis Diagnosis Limitations in Patients Infected with Microbial Infection
15. Future Perspective
16. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Bouza, E. Invasive mould infections in the ICU setting: Complexities and solutions. J. Antimicrob. Chemother. 2017, 72, i39–i47. [Google Scholar] [CrossRef]
- Lin, E.; Moua, T.; Limper, A.H. Pulmonary mucormycosis: Clinical features and outcomes. Infection 2017, 45, 443–448. [Google Scholar] [CrossRef]
- Peng, M.; Meng, H.; Sun, Y.; Xiao, Y.; Zhang, H.; Lv, K.; Cai, B. Clinical features of pulmonary mucormycosis in patients with different immune status. J. Thorac. Dis. 2019, 11, 5042. [Google Scholar] [CrossRef]
- Perfect, J.R.; Mourad, A. Management of mucormycoses. In Antifungal Therapy; CRC Press: Boca Raton, FL, USA, 2019; pp. 357–362. [Google Scholar]
- Mohanty, A.; Gupta, P.; Varshney, S.; Kabi, A.; Angral, S. Breaking the mold: A brief review on the diagnostic and treatment approaches of mucormycosis. Int. J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 1. [Google Scholar] [CrossRef]
- Chakrabarti, A. Mucormycosis in Asia. In Clinical Practice of Medical Mycology in Asia; Springer: New York, NY, USA, 2020; pp. 279–292. [Google Scholar]
- Juma, F.; Nagaraj, V.; Darwish, A. Placental Mucormycosis of an IVF-Induced Pregnancy in a Diabetic Patient. Bahrain Med. Bull. 2019, 41, 278–280. [Google Scholar]
- Shamanna, K.; Fathima, A.; Sowjanya, S. Rhino-Orbito-Cerebral Mucormycosis: Our Experience. Headache 2019, 15, 75. [Google Scholar]
- Iqbal, N.; Irfan, M.; Jabeen, K.; Kazmi, M.M.; Tariq, M.U. Chronic pulmonary mucormycosis: An emerging fungal infection in diabetes mellitus. J. Thorac. Dis. 2017, 9, E121. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Chakrabarti, A. Global epidemiology of mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef]
- John, T.M.; Jacob, C.N.; Kontoyiannis, D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: The perfect storm for mucormycosis. J. Fungi 2021, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.; Kong, D.; Chen, S.A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef]
- Ingram, C.W.; Sennesh, J.; Cooper, J.N.; Perfect, J.R. Disseminated Zygomycosis. Report of four cases and review. Rev. Infect. Dis. 1989, 11, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Kubin, C.J.; McConville, T.H.; Dietz, D.; Zucker, J.; May, M.; Nelson, B.; Istorico, E.; Bartram, L.; Small-Saunders, J.; Sobieszczyk, M.E. In Characterization of Bacterial and Fungal Infections in Hospitalized Patients with Coronavirus Disease 2019 and Factors Associated with Health Care-Associated Infections. Open Forum Infect. Dis. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Hoang, K.; Abdo, T.; Reinersman, J.M.; Lu, R.; Higuita, N.I.A. A case of invasive pulmonary mucormycosis resulting from short courses of corticosteroids in a well-controlled diabetic patient. Med. Mycol. Case Rep. 2020, 29, 22–24. [Google Scholar]
- Nishanth, D.G.; Anitha, D.N.; Babu, D.N.A.; Malathi, D.L. Mucormycosis-A Review. Eur. J. Mol. Clin. Med. 2020, 7, 1786–1791. [Google Scholar]
- Kanwar, A.; Jordan, A.; Olewiler, S.; Wehberg, K.; Cortes, M.; Jackson, B.R. A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J. Fungi 2021, 7, 174. [Google Scholar] [CrossRef]
- Baldin, C.; Soliman, S.S.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W. PCR-based approach targeting mucorales-specific gene family for diagnosis of mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef] [PubMed]
- Timesnownews. Available online: https://www.timesnownews.com/india/article/28252-cases-of-mucormycosis-cases-in-the-country-maharashtra-gujarat-lead-the-tally-health-minister/767491 (accessed on 22 August 2021).
- Pal, M. Zygomycosis: A highly infectious emerging opportunistic fungal disease of public health concern. Open Access J. Mycol. Mycol. Sci. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Liberatore, C.; Farina, F.; Greco, R.; Giglio, F.; Clerici, D.; Oltolini, C.; Lupo Stanghellini, M.T.; Barzaghi, F.; Vezzulli, P.; Orsenigo, E. Breakthrough Invasive Fungal Infections in Allogeneic Hematopoietic Stem Cell Transplantation. J. Fungi 2021, 7, 347. [Google Scholar] [CrossRef]
- Choi, J.K.; Cho, S.Y.; Yoon, S.S.; Moon, J.H.; Kim, S.H.; Lee, J.H.; Kim, J.S.; Cheong, J.W.; Jang, J.H.; Seo, B.J. Epidemiology and risk factors for invasive fungal diseases among allogeneic hematopoietic stem cell transplant recipients in Korea: Results of “RISK” study. Biol. Blood Marrow Transplant. 2017, 23, 1773–1779. [Google Scholar] [CrossRef]
- Bavikar, P.; Mehta, V. Rhino-orbital-cerebral mucormycosis: A fatal complication of uncontrolled diabetes mellitus. Cureus 2017, 9, e1841. [Google Scholar] [CrossRef]
- Dayal, D.; Jain, P.; Kumar, R.; Bakshi, J.; Menon, P.; Das, A.; Singhi, S.; Singh, M. Clinical spectrum and outcome of invasive filamentous fungal infections in children with Type 1 diabetes: North Indian experience. Clin. Pediatr. Endocrinol. 2015, 24, 51–57. [Google Scholar] [CrossRef]
- Manesh, A.; Rupali, P.; Sullivan, M.O.; Mohanraj, P.; Rupa, V.; George, B.; Michael, J.S. Mucormycosis—A clinicoepidemiological review of cases over 10 years. Mycoses 2019, 62, 391–398. [Google Scholar] [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiology and Diagnosis of Mucormycosis: An Update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef]
- Das, A.; Oberoi, S.; Trehan, A.; Chakrabarti, A.; Bansal, D.; Saxena, A.K.; Sodhi, K.S.; Kakkar, N.; Srinivasan, R. Invasive fungal disease in pediatric acute leukemia in the nontransplant setting: 8 years’ experience from a tertiary care center in North India. J. Pediatr. Hematol. Oncol. 2018, 40, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Phoompoung, P.; Henry, B.; Daher-Reyes, G.; Sibai, H.; Husain, S. Invasive Mold Infections in FLT3-Mutated Acute Myeloid Leukemia. Clin. Lymph. Myel. Leuk. 2021, 21, e477–e482. [Google Scholar] [CrossRef]
- Patel, M.H.; Patel, R.D.; Vanikar, A.V.; Kanodia, K.V.; Suthar, K.S.; Nigam, L.K.; Patel, H.V.; Patel, A.H.; Kute, V.B.; Trivedi, H.L. Invasive fungal infections in renal transplant patients: A single center study. Ren. Fail. 2017, 39, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Godara, S.; Kute, V.; Goplani, K.; Gumber, M.; Gera, D.; Shah, P.; Vanikar, A.; Trivedi, H. Mucormycosis in renal transplant recipients: Predictors and outcome. Saudi J. Kidney Dis. Transplant. 2011, 22, 751. [Google Scholar]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med. Mycol. 2019, 57, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Singh, G.; Agarwal, R.; Dabas, Y.; Jyotsna, V.P.; Kumar, R.; Xess, I. Emerging Rhizopus microsporus infections in India. J. Clin. Microbiol. 2018, 56, e00433-18. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Kaur, H.; Savio, J.; Rudramurthy, S.M.; Patel, A.; Shastri, P.; Pamidimukkala, U.; Karthik, R.; Bhattacharya, S.; Kindo, A.J. Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study). J. Crit. Care 2019, 51, 64–70. [Google Scholar] [CrossRef]
- Balwan, W.K. Epidemiology of Mucormycosis in India: A Notifiable Disease. Saudi J. Pathol. Microbiol. 2021, 6, 187–191. [Google Scholar]
- Antinori, S.; Nebuloni, M.; Magni, C.; Fasan, M.; Adorni, F.; Viola, A.; Corbellino, M.; Galli, M.; Vago, G.; Parravicini, C. Trends in the postmortem diagnosis of opportunistic invasive fungal infections in patients with AIDS: A retrospective study of 1,630 autopsies performed between 1984 and 2002. Am. J. Clin. Pathol. 2009, 132, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018, 56 (Suppl. 1), S93–S101. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B. Mucormycosis pathogenesis: Beyond rhizopus. Virulence 2017, 8, 1481–1482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, A.S.; Voelz, K. The mucormycete–host interface. Curr. Opin. Microbiol. 2017, 40, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.; Lamaris, G.; Walsh, T.; Kontoyiannis, D. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimcrob. Agents Chemother. 2008, 52, 722–724. [Google Scholar] [CrossRef]
- Petrikkos, G.; Tsioutis, C. Recent advances in the pathogenesis of mucormycoses. Clin. Ther. 2018, 40, 894–902. [Google Scholar] [CrossRef]
- Gebremariam, T.; Lin, L.; Liu, M.; Kontoyiannis, D.P.; French, S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Investig. 2016, 126, 2280–2294. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Kontoyiannis, D.P.; Walsh, T.J. Host defenses against zygomycetes. Clin. Infect. Dis. 2012, 54, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Lanternier, F.; Groll, A.H.; Pagano, L.; Zimmerli, S.; Herbrecht, R.; Lortholary, O.; Petrikkos, G.L. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia(ECIL 3). Haematologica 2013, 98, 492. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Van Der Meer, J.W.; Kullberg, B.J.; Van De Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef]
- Putri, I.H.; Tunjungputri, R.N.; De Groot, P.G.; van der Ven, A.J.; de Mast, Q. Thrombocytopenia and Platelet Dysfunction in Acute Tropical Infectious Diseases, Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: New York, NY, USA, 2018; pp. 683–690. [Google Scholar]
- Bongiovanni, D.; Santamaria, G.; Klug, M.; Santovito, D.; Felicetta, A.; Hristov, M.; von Scheidt, M.; Aslani, M.; Cibella, J.; Weber, C. Transcriptome analysis of reticulated platelets reveals a prothrombotic profile. Thromb. Haemost. 2019, 119, 1795–1806. [Google Scholar] [CrossRef]
- Schulze, B.; Rambach, G.; Schwartze, V.U.; Voigt, K.; Schubert, K.; Speth, C.; Jacobsen, I.D. Ketoacidosis alone does not predispose to mucormycosis by Lichtheimia in a murine pulmonary infection model. Virulence 2017, 8, 1657–1667. [Google Scholar] [CrossRef]
- Perkhofer, S.; Kainzner, B.; Kehrel, B.E.; Dierich, M.P.; Nussbaumer, W.; Lass-Flörl, C. Potential antifungal effects of human platelets against zygomycetes in vitro. J. Infect. Dis. 2009, 200, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Olson, J.A.; Negrin, R.S. Natural killer cells in allogeneic transplantation: Effect on engraftment, graft-versus-tumor, and graft-versus-host responses. Biol. Blood Marrow Transplant. 2009, 15, 765–776. [Google Scholar] [CrossRef]
- Kuldanek, S.; Pasko, B.; DomBourian, M.; Annen, K. Cellular Therapy in Pediatric Hematologic Malignancies. Clin. Lab. Med. 2021, 41, 121–132. [Google Scholar] [CrossRef]
- Bachiller, M.; Battram, A.M.; Perez-Amill, L.; Martín-Antonio, B. Natural Killer Cells in Immunotherapy: Are We Nearly There? Cancers 2020, 12, 3139. [Google Scholar] [CrossRef] [PubMed]
- Lax, C.; Pérez-Arques, C.; Navarro-Mendoza, M.I.; Cánovas-Márquez, J.T.; Tahiri, G.; Pérez-Ruiz, J.A.; Osorio-Concepción, M.; Murcia-Flores, L.; Navarro, E.; Garre, V. Genes, pathways, and mechanisms involved in the virulence of mucorales. Genes 2020, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Jegaskanda, S.; Vanderven, H.A.; Tan, H.-X.; Alcantara, S.; Wragg, K.M.; Parsons, M.S.; Chung, A.W.; Juno, J.A.; Kent, S.J. Influenza virus infection enhances antibody-mediated NK cell functions via type I interferon-dependent pathways. J. Virol. 2019, 93, e02090-18. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Jaber, B.; Lee, S.; Perianayagam, M.; King, A.; Pereira, B.; Balakrishnan, V. Impact of iron dextran on polymorphonuclear cell function among hemodialysis patients. Clin. Nephrol. 2002, 58, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Ibrahim, A.S.; Skory, C.; Grabherr, M.G.; Burger, G.; Butler, M.; Elias, M.; Idnurm, A.; Lang, B.F.; Sone, T. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009, 5, e1000549. [Google Scholar] [CrossRef]
- Roilides, E.; Antachopoulos, C.; Simitsopoulou, M. Pathogenesis and host defence against Mucorales: The role of cytokines and interaction with antifungal drugs. Mycoses 2014, 57, 40–47. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar]
- Liu, M.; Lin, L.; Gebremariam, T.; Luo, G.; Skory, C.D.; French, S.W.; Chou, T.F.; Edwards, J.E., Jr.; Ibrahim, A.S. Fob1 and Fob2 proteins are virulence determinants of Rhizopus oryzae via facilitating iron uptake from ferrioxamine. PLoS Pathog. 2015, 11, e1004842. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Edwards, J.E., Jr.; Fu, Y.; Spellberg, B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J. Antimicrob. Chemother. 2006, 58, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Gebermariam, T.; Fu, Y.; Lin, L.; Husseiny, M.I.; French, S.W.; Schwartz, J.; Skory, C.D.; Edwards, J.E.; Spellberg, B.J. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig. 2007, 117, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Andes, D.; Perez, M.; Anglim, A.; Bonilla, H.; Mathisen, G.E.; Walsh, T.J.; Ibrahim, A.S. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. J. Antimicrob. Chemother. 2009, 53, 3122–3125. [Google Scholar] [CrossRef]
- Spellberg, B.; Ibrahim, A.S.; Chin-Hong, P.V.; Kontoyiannis, D.P.; Morris, M.I.; Perfect, J.R.; Fredricks, D.; Brass, E.P. The Deferasirox–AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: A randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2012, 67, 715–722. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, H.; Collins, M.; Tsai, H.F.; Spellberg, B.; Edwards, J.E., Jr.; Kwon-Chung, K.J.; Ibrahim, A.S. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol. Lett. 2004, 235, 169–176. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Lin, L.; Luo, G.; Husseiny, M.I.; Skory, C.D.; Fu, Y.; French, S.W.; Edwards, J.; John, E.; et al. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010, 77, 587–604. [Google Scholar] [CrossRef]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Pongas, G.; Lewis, R.; Samonis, G.; Kontoyiannis, D. Voriconazole-associated zygomycosis: A significant consequence of evolving antifungal prophylaxis and immunosuppression practices? Clin. Microbiol. Infect. 2009, 15, 93–97. [Google Scholar] [CrossRef]
- Lamaris, G.A.; Ben-Ami, R.; Lewis, R.E.; Chamilos, G.; Samonis, G.; Kontoyiannis, D.P. Increased virulence of Zygomycetes organisms following exposure to Voriconazole: A study involving fly and murine models of zygomycosis. J. Infect. Dis. 2009, 199, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Lewis, R.E.; Liao, G.; Wang, W.; Prince, R.A.; Kontoyiannis, D.P. Voriconazole pre-exposure selects for breakthrough mucormycosis in a mixed model of Aspergillus fumigatus-Rhizopus oryzae pulmonary infection. Virulence 2011, 2, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, A. Outbreaks of zygomycosis in hospitals Definition of a Hospital Outbreak. Clin. Microbiol. Infect. 2009, 15, 55–59. [Google Scholar] [CrossRef]
- Kato, H.; Foster, C.M.; Karri, K. Incidence, predisposing conditions and outcomes of cutaneous mucormycosis: A national database study. Mycoses 2021, 64, 569–572. [Google Scholar] [CrossRef]
- Bonifaz, A.; Vázquez-González, D.; Tirado-Sánchez, A.; Ponce-Olivera, R.M. Cutaneous zygomycosis. Clin. Dermatol. 2012, 30, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ema, N.T.; Rakhi, N.N.; Saha, O.; Rahaman, M.M. Parallel Outbreaks of Deadly Pathogens(SARS-CoV-2, H5N8, EVD, Black fungi) around the World in 2021: Priorities for Achieving Control with Socio-Economic and Public Health Impact. COVID 2021, 1, 203–217. [Google Scholar] [CrossRef]
- Rocha, I.C.N.; Hasan, M.M.; Goyal, S.; Patel, T.; Jain, S.; Ghosh, A.; Denise, T.; Cedeño, T.D.D. COVID-19 and mucormycosis syndemic: Double health threat to a collapsing healthcare system in India. Trop. Med. Int. Health 2021, 26, 1016–1018. [Google Scholar] [CrossRef]
- Selarka, L.; Sharma, S.; Saini, D.; Sarma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, in press. [CrossRef]
- Ibrahim, A.; Edwards, J.; Filler, S. Zygomycosis. In Clinical Mycology; Dismukes, W.E., Pappas, P.G., Sobel, J.D., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 241–251. [Google Scholar]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2020, 13, 236–301. [Google Scholar] [CrossRef] [PubMed]
- Shinde, Y.B.; Kore, S. A Review on Mucormycosis with recent pharmacological treatment. J. Drug Deliv. Ther. 2021, 11, 145–149. [Google Scholar] [CrossRef]
- Alqarihi, A.; Gebremariam, T.; Gu, Y.; Swidergall, M.; Alkhazraji, S.; Soliman, S.S.; Bruno, V.M.; Edwards, J.E., Jr.; Filler, S.G.; Uppuluri, P. GRP78 and integrins play different roles in host cell invasion during mucormycosis. mBio 2020, 11, e01087-20. [Google Scholar] [CrossRef] [PubMed]
- Omara, F.; Blakley, B.; Huang, H.S. Effect of iron status on endotoxin-induced mortality, phagocytosis and interleukin-1 alpha and tumor necrosis factor-alpha production. Vet. Hum. Toxicol. 1994, 36, 423–428. [Google Scholar]
- Rauthan, P.; Sharma, D.C. Mucormycosis: Pathogenesis, Diagnosis, and Management. Asian J. Pharm. Res. Dev. 2021, 9, 144–153. [Google Scholar]
- Baldin, C.; Ibrahim, A.S. Molecular mechanisms of mucormycosis-the bitter and the sweet. PLoS Pathog. 2017, 13, e1006408. [Google Scholar] [CrossRef]
- Deigendesch, N.; Nunez, J.C.; Stenzel, W. Parasitic and fungal infections. Handb. Clin. Neurol. 2018, 145, 245–262. [Google Scholar]
- Bonifaz, A.; Tirado-Sánchez, A.; Hernández-Medel, M.L.; Araiza, J.; Kassack, J.J.; del Angel-Arenas, T.; Moisés-Hernández, J.F.; Paredes-Farrera, F.; Gómez-Apo, E.; Treviño-Rangel, R.D.J. Mucormycosis at a tertiary-care center in Mexico. A 35-year retrospective study of 214 cases. Mycoses 2021, 64, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Waqar Elahi, M.; Ullah, W.; Abdullah, H.M.A.; Ahmad, E.; Al Mohajer, M.; Majeed, A. Invasive mucormycosis induced pneumopericardium: A rare cause of pneumopericardium in an immunocompromised patient. Case Rep. Infect. Dis. 2017, 2017, 1424618. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Sipsas, N.V.; Gamaletsou, M.N.; Anastasopoulou, A.; Kontoyiannis, D.P. Therapy of mucormycosis. J. Fungi 2018, 4, 90. [Google Scholar] [CrossRef]
- Adler-Moore, J. AmBisome targeting to fungal infections. Bone Marrow Transplant. 1994, 14, S3–S7. [Google Scholar]
- Lestner, J.M.; Howard, S.J.; Goodwin, J.; Gregson, L.; Majithiya, J.; Walsh, T.J.; Jensen, G.M.; Hope, W.W. Pharmacokinetics and pharmacodynamics of amphotericin B deoxycholate, liposomal amphotericin B, and amphotericin B lipid complex in an in vitro model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2010, 54, 3432–3441. [Google Scholar] [CrossRef]
- Takemoto, K.; Yamamoto, Y.; Ueda, Y. Evaluation of antifungal pharmacodynamic characteristics of AmBisome against Candida albicans. Microbiol. Immunol. 2006, 50, 579–586. [Google Scholar] [CrossRef]
- Readio, J.D.; Bittman, R. Equilibrium binding of amphotericin B and its methyl ester and borate complex to sterols. Biochim. Biophys. Acta 1982, 685, 219–224. [Google Scholar] [CrossRef]
- Fujimoto, K.; Takemoto, K. Efficacy of liposomal amphotericin B against four species of Candida biofilms in an experimental mouse model of intravascular catheter infection. J. Infect. Chemother. 2018, 24, 958–964. [Google Scholar] [CrossRef]
- Kawai, A.; Yamagishi, Y.; Mikamo, H. In vitro efficacy of liposomal amphotericin B, micafungin and fluconazole against non-albicans Candida species biofilms. J. Infect. Chemother. 2015, 21, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.K.; Chandrasekar, P.H. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: Current evidence, safety, efficacy, and clinical recommendations. Infect. Drug Resist. 2016, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, M.; Ostrosky-Zeichner, L. Isavuconazole: Mechanism of action, clinical efficacy, and resistance. J. Fungi 2020, 6, 324. [Google Scholar] [CrossRef]
- Song, J.; Zhai, P.; Zhang, Y.; Zhang, C.; Sang, H.; Han, G.; Keller, N.P.; Lu, L. The Aspergillus fumigatus damage resistance protein family coordinately regulates ergosterol biosynthesis and azole susceptibility. mBio 2016, 7, e01919-15. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J. Fusco-Almeida AM. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front. Microbiol. 2017, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433. [Google Scholar] [CrossRef]
- Shoham, S.; Magill, S.S.; Merz, W.G.; Gonzalez, C.; Seibel, N.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Walsh, T.J. Primary treatment of zygomycosis with liposomal amphotericin B: Analysis of 28 cases. Med. Mycol. 2010, 48, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.M.; Serena, C.; Mariné, M.; Pastor, F.J.; Guarro, J. Posaconazole combined with amphotericin B, an effective therapy for a murine disseminated infection caused by Rhizopus oryzae. Antimicrob. Agents Chemother. 2008, 52, 3786–3788. [Google Scholar] [CrossRef]
- Pitman, S.K.; Drew, R.H.; Perfect, J.R. Addressing current medical needs in invasive fungal infection prevention and treatment with new antifungal agents, strategies and formulations. Expert Opin. Emerg. Drugs 2011, 16, 559–586. [Google Scholar] [CrossRef]
- Groll, A.H.; Desai, A.; Han, D.; Howieson, C.; Kato, K.; Akhtar, S.; Kowalski, D.; Lademacher, C.; Lewis, W.; Pearlman, H. Pharmacokinetic assessment of drug-drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin. Pharmacol. Drug Dev. 2017, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ananda-Rajah, M.R.; Kontoyiannis, D. Isavuconazole: A new extended spectrum triazole for invasive mold diseases. Future Microbiol. 2015, 10, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A new broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef]
- Trang, T.P.; Hanretty, A.M.; Langelier, C.; Yang, K. Use of isavuconazole in a patient with voriconazole-induced QT c prolongation. Transpl. Infect. Dis. 2017, 19, e12712. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Rafati, H.; Ilkit, M.; Tolooe, A.; Hedayati, M.T.; Verweij, P. Systemic antifungal agents: Current status and projected future developments. In Human Fungal Pathogen Identification; Springer: New York, NY, USA, 2017; pp. 107–139. [Google Scholar]
- Miyazaki, M.; Horii, T.; Hata, K.; Watanabe, N.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; Inoue, S.; Matsukura, M. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 2011, 55, 4652–4658. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Wiederhold, N.P.; Shaw, K.J.; Patterson, T.F.; Filler, S.; Ibrahim, A. In APX001A Protects Immunosuppressed Mice from Rhizopus delemar Infection. Open Forum Infect. Dis. 2017, 4 (Suppl. 1), S475. [Google Scholar] [CrossRef]
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards, J., Jr.; Filler, S.G.; Goldberg, R.; Spellberg, B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis. 2008, 47, 364–371. [Google Scholar] [CrossRef]
- Spellberg, B.; Fu, Y.; Edwards, J.E., Jr.; Ibrahim, A.S. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob. Agents Chemother. 2005, 49, 830–832. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Fu, Y.; Edwards, J.E., Jr.; Spellberg, B. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob. Agents Chemother. 2008, 52, 1556–1558. [Google Scholar] [CrossRef]
- Reed, C.; Ibrahim, A.; Edwards, J.E., Jr.; Walot, I.; Spellberg, B. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob. Agents Chemother. 2006, 50, 3968–3969. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Valentini, C.; Posteraro, B.; Girmenia, C.; Ossi, C.; Pan, A.; Candoni, A.; Nosari, A.; Riva, M.; Cattaneo, C. Zygomycosis in Italy: A survey of FIMUA-ECMM (Federazione Italiana di Micopatologia Umana ed Animale and European Confederation of Medical Mycology). J. Chemother. 2009, 21, 322–329. [Google Scholar] [CrossRef]
- Pulle, M.V.; Puri, H.V.; Asaf, B.B.; Bishnoi, S.; Sharma, S.; Kumar, A. Outcomes of early anti-fungal therapy with aggressive surgical resection in pulmonary mucormycosis. Lung India Off. Organ Indian Chest Soc. 2021, 38, 314. [Google Scholar]
- Becker, B.; Schuster, F.; Ganster, B.; Seidl, H.; Schmid, I. Cutaneous mucormycosis in an immunocompromised patient. Lancet Infect. Dis. 2006, 6, 536. [Google Scholar] [CrossRef]
- Jenks, J.D.; Gangneux, J.P.; Schwartz, I.S.; Alastruey-Izquierdo, A.; Lagrou, K.; Thompson, G.R., III; Lass-Flörl, C.; Hoenigl, M. Diagnosis of breakthrough fungal infections in the clinical mycology laboratory: An ecmm consensus statement. J. Fungi 2020, 6, 216. [Google Scholar] [CrossRef]
- Carvalho, B.; Monteiro, P.; Camacho, Ó.; Vaz, R. Hyperbaric oxygen treatment: Results in seven patients with severe bacterial postoperative central nervous system infections and refractory mucormycosis. Diving Hyperb. Med. 2021, 51, 86–93. [Google Scholar]
- Bennett, M.; Kaide, C.G.; Matheson, E.; Bari, V. Hyperbaric Oxygen Therapy and Utilization in Infectious Disease. Curr. Emerg. Hosp. Med. Rep. 2018, 6, 101–109. [Google Scholar] [CrossRef]
- Ademe, M. Immunomodulation for the Treatment of Fungal Infections: Opportunities and Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Singh, S. Management of Mucormycosis. Curr. Fungal Infect. Rep. 2020, 14, 348–360. [Google Scholar] [CrossRef]
- Chatterjee, P.; Nagi, N.; Agarwal, A.; Das, B.; Banerjee, S.; Sarkar, S.; Gupta, N.; Gangakhedkar, R.R. The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J. Med. Res. 2020, 151, 147. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Ram, S.; Levitz, S.M. The “Black Fungus” in India: The Emerging Syndemic of COVID-19–Associated Mucormycosis. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Chaurasia, J.; Khan, R.; Dhand, C.; Verma, S. Role of Medicinal plants of Traditional Use in Recuperating Devastating COVID-19 Situation. Med. Aromat. Plants 2020, 9, 359. [Google Scholar]
- Voltan, A.R.; Quindós, G.; Alarcón, K.P.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715. [Google Scholar] [CrossRef] [PubMed]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Barratt, G.; Bretagne, S. Optimizing efficacy of Amphotericin B through nanomodification. Int. J. Nanomed. 2007, 2, 301. [Google Scholar]
- Faustino, C.; Pinheiro, L. Lipid systems for the delivery of amphotericin b in antifungal therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying amphotericin B–based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Kontoyiannis, D.P.; Fredricks, D.; Morris, M.I.; Perfect, J.R.; Chin-Hong, P.V.; Ibrahim, A.S.; Brass, E.P. Risk factors for mortality in patients with mucormycosis. Med. Mycol. 2012, 50, 611–618. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J., Jr.; Ibrahim, A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C. Zygomycosis: Conventional laboratory diagnosis. Clin. Microbiol. Infect. 2009, 15, 60–65. [Google Scholar] [CrossRef]
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.S.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 2015, 21, 684.e11–684.e18. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Petraitiene, R.; Walsh, T.J. Nucleic acid amplification methodologies for the detection of pulmonary mold infections. Expert Rev. Mol. Diagn. 2017, 17, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Yaman, G.; Akyar, I.; Can, S. Evaluation of the MALDI TOF-MS method for identification of Candida strains isolated from blood cultures. Diagn. Microbiol. Infect. Dis. 2012, 73, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) working group on zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Gamaletsou, M.N.; McGinnis, M.R.; Hayden, R.T.; Kontoyiannis, D.P. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin. Infect. Dis. 2012, 54, S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Offidani, M.; Fianchi, L.; Nosari, A.; Candoni, A.; Picardi, M.; Corvatta, L.; D’Antonio, D.; Girmenia, C.; Martino, P.; et al. Mucormycosis in hematologic patients. Haematologica 2004, 89, 207–214. [Google Scholar]
- Gomes, M.Z.; Lewis, R.E.; Kontoyiannis, D.P. Mucormycosis caused by unusual mucormycetes, non-rhizopus, -mucor, and -lichtheimia species. Clin. Microbiol. Rev. 2011, 24, 411–445. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Antachopoulos, C.; Hughes, J.E.; Cotton, M.P.; Kasai, M.; Harrington, S.; Gamaletsou, M.N.; Bacher, J.D.; Kontoyiannis, D.P.; et al. Increased virulence of Cunninghamella bertholletiae in experimental pulmonary mucormycosis: Correlation with circulating molecular biomarkers, sporangiospore germination and hyphal metabolism. Med. Mycol. 2013, 51, 72–82. [Google Scholar] [CrossRef]
- Monheit, J.E.; Cowan, D.F.; Moore, D.G. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch. Pathol. Lab. Med. 1984, 108, 616–618. [Google Scholar]

| Treatment Protocol | Mode of Action | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| Therapeutic Intervention | ||||
| Lipid formulation of Amphotericin B (Polyenes derivatives) | The cellular membrane is substantially targeted by Amphotericin B loaded liposomes, which induce fungicidal activity by binding to ergosterol in the fungal cell membrane |
|
| [89,102,103] |
| Posaconazole with or without lipid polyenes (Triazoles derivative) | Inhibits fungal lanosterol 14 α-demethylase enzyme synthesis |
|
| [104,105] |
| Isavuconazole (A new broad-spectrum triazoles derivatives) | Cytochrome P450-dependent lanosterol 14-demethylase enzyme, which is required for the production of ergosterol, a component of the fungal membrane, is inhibited by isavuconazole |
|
| [106,107,108,109] |
| VT-1161 | Potent inhibitor of CYP51 and possess in-vitro activity against Mucorales, including Cunninghamella, Lichtheimia, and Rhizopus oryzae |
|
| [110] |
| APX001A | The inositol acyltransferase suppressed by APX001A, which limits the development of Glycosylphosphatidylinositol-anchored proteins and producing antifungal effect |
|
| [111,112] |
| Caspofungin plus lipid polyene | Inhibit the enzyme β-1,3-d-glucan synthase |
|
| [113,114] |
| Anidulafungin plus lipid polyene | Inhibit the enzyme β-1,3-d-glucan synthase. |
|
| [115] |
| Deferasirox plus lipid polyenes | Reducing the available iron load and thus inhibiting the fungal growth and lack siderophore capability |
|
| [62,116] |
| Surgical Intervention | ||||
| Rhino-orbito- cerebral infection; Soft tissue infection; and localized pulmonary lesion | A critical component of effective therapy is prompt surgical debridement, which should be repeated if required. When surgery is required and feasible, it must be robust. Because the Mucorales hyphae may spread infection swiftly, it is important to remove not just necrotic tissues but also infected healthy-looking tissues in the surrounding area. |
|
| [117,118,119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, R.K.; Salem-Bekhit, M.M.; Bhattacharjee, B.; Almoshari, Y.; Ikbal, A.M.A.; Alshamrani, M.; Bharali, A.; Salawi, A.; Widyowati, R.; Alshammari, A.; et al. Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies. Antibiotics 2021, 10, 1079. https://doi.org/10.3390/antibiotics10091079
Sahu RK, Salem-Bekhit MM, Bhattacharjee B, Almoshari Y, Ikbal AMA, Alshamrani M, Bharali A, Salawi A, Widyowati R, Alshammari A, et al. Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies. Antibiotics. 2021; 10(9):1079. https://doi.org/10.3390/antibiotics10091079
Chicago/Turabian StyleSahu, Ram Kumar, Mounir M. Salem-Bekhit, Bedanta Bhattacharjee, Yosif Almoshari, Abu Md Ashif Ikbal, Meshal Alshamrani, Alakesh Bharali, Ahmad Salawi, Retno Widyowati, Abdulrahman Alshammari, and et al. 2021. "Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies" Antibiotics 10, no. 9: 1079. https://doi.org/10.3390/antibiotics10091079
APA StyleSahu, R. K., Salem-Bekhit, M. M., Bhattacharjee, B., Almoshari, Y., Ikbal, A. M. A., Alshamrani, M., Bharali, A., Salawi, A., Widyowati, R., Alshammari, A., & Elbagory, I. (2021). Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies. Antibiotics, 10(9), 1079. https://doi.org/10.3390/antibiotics10091079







