Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria
Abstract
1. Introduction
2. Isolation, Characterization, and Bioassays of Phytochemicals
2.1. Microfluidic Technology
2.2. Host-Pathogen Co-Culture Assay
2.3. Colorimetric Assay of pH
2.4. In Silico Screening
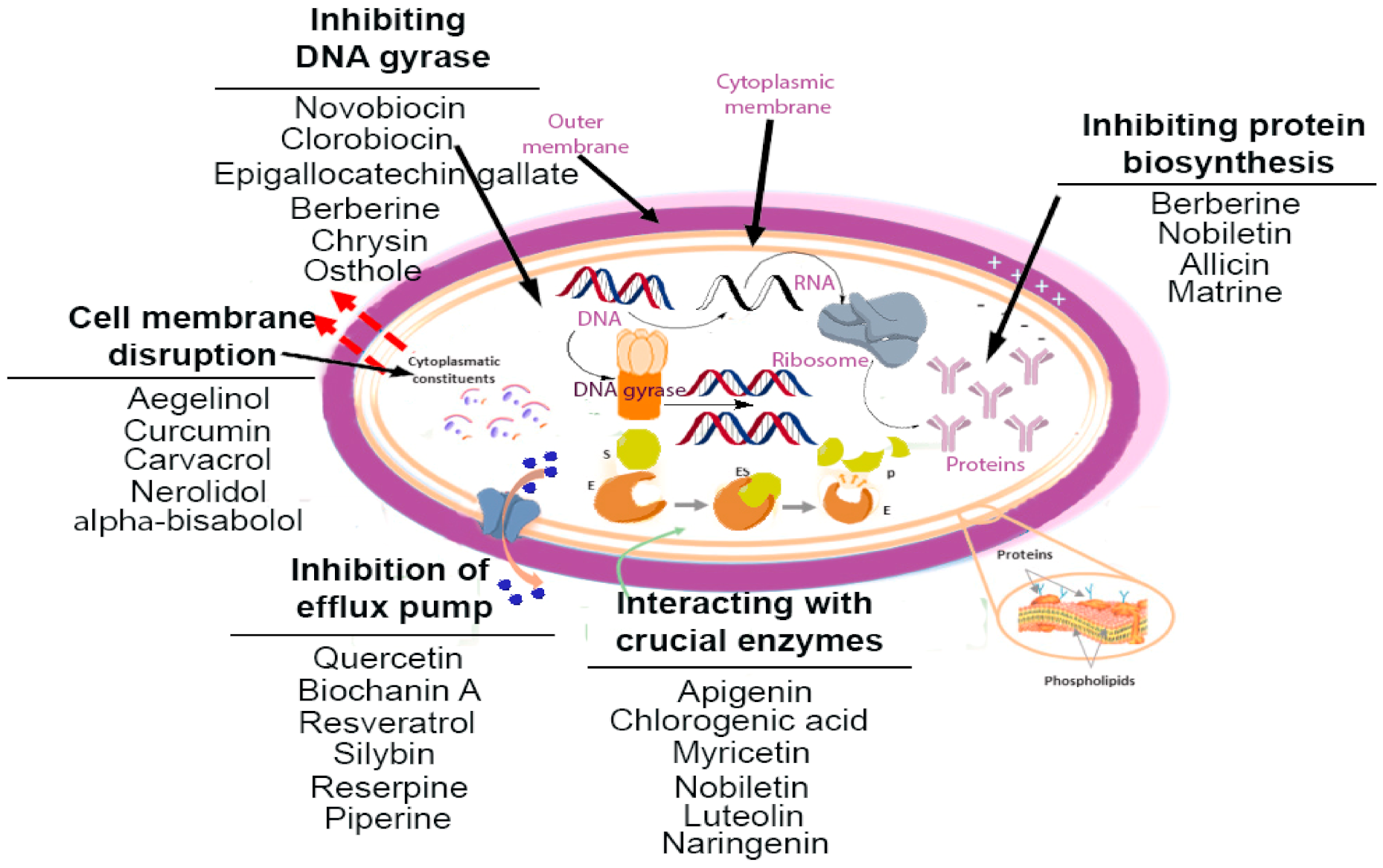
3. Mechanistic Insights on Phytochemicals
| Compound | Chemical Structure | Active against (MIC/MBC Values) | Class of Phytochemicals | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Piperine | 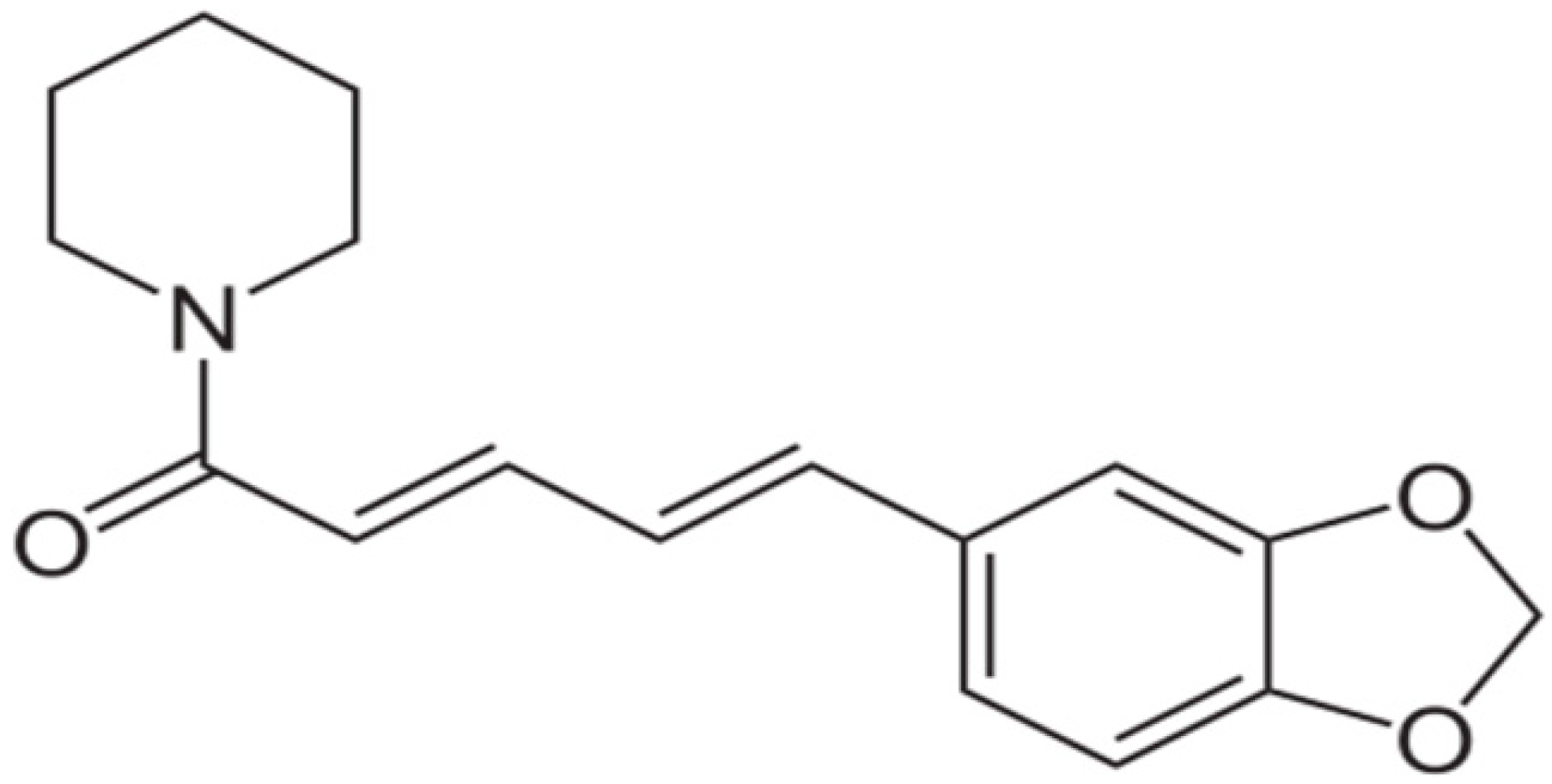 | Staphylococcus aureus and Bacillus subtilis (MIC values of 225 µg/mL) | Alkaloids | Inhibition of efflux pump | [73,74] |
| Berberine |  | Candida albicans (MIC values ranging from 125 to 500 μg/ml) | DNA intercalation; inhibiting RNA polymerase, DNA gyrase, and topoisomerase IV, and IA; inhibiting protein biosynthesis, Inhibition of cell division | [75,76,77] | |
| Dictamnine | 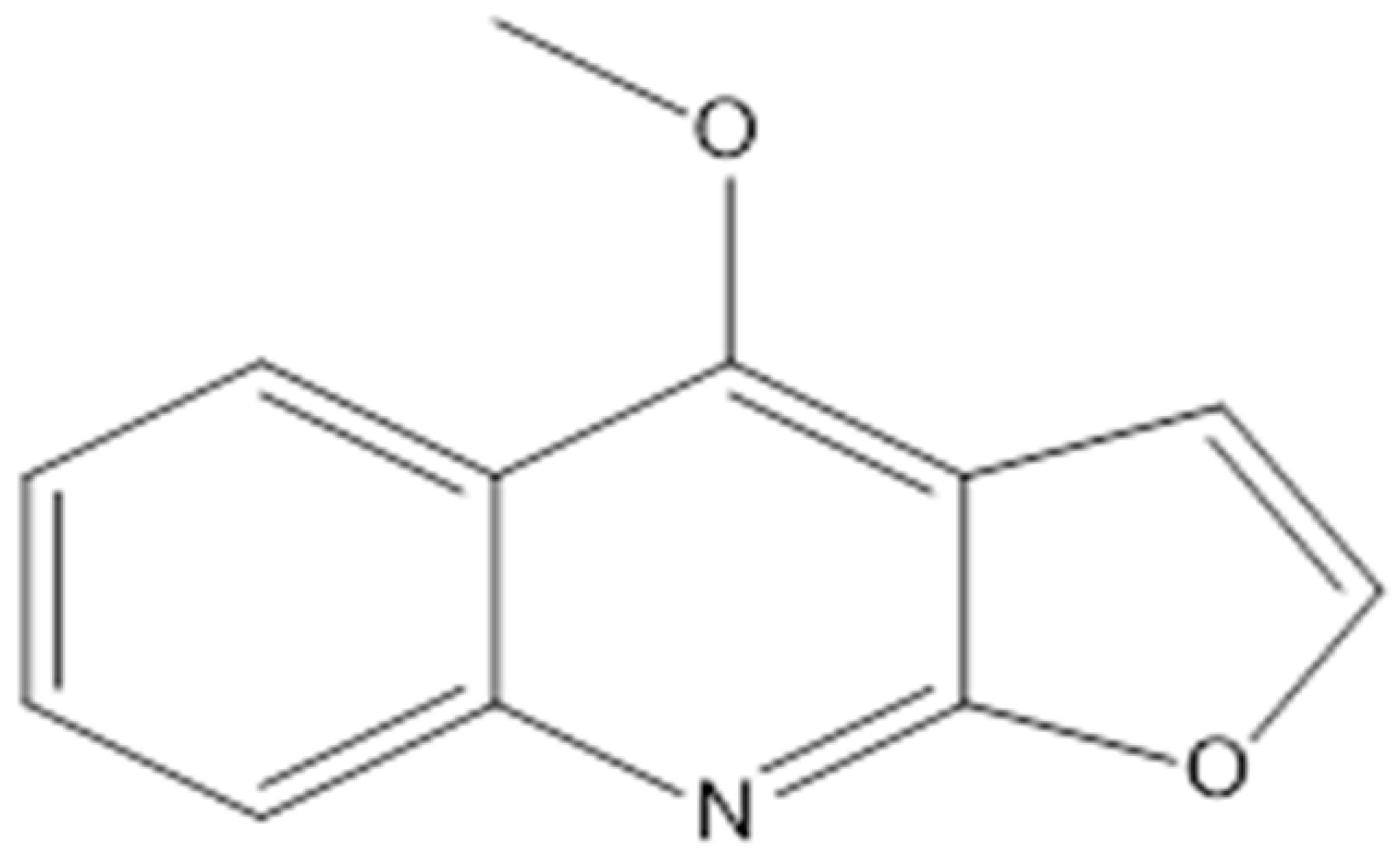 | Saccharomyces cerevisiae (MIC value of 64 μg/ml) | Inhibiting type II topoisomerase | [78,79] | |
| Reserpine | 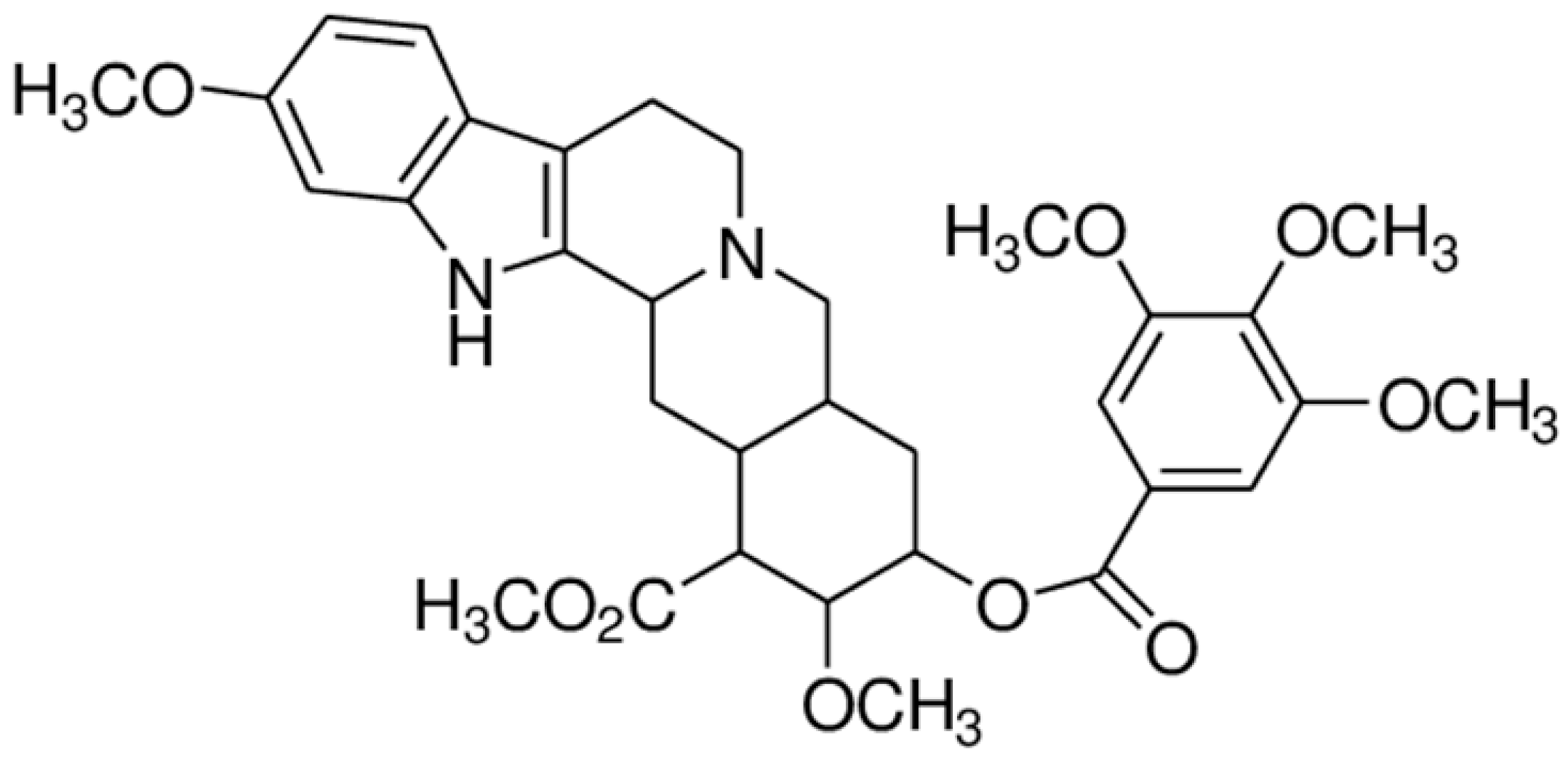 | Inhibition of efflux pump | [80] | ||
| Sanguinarine | 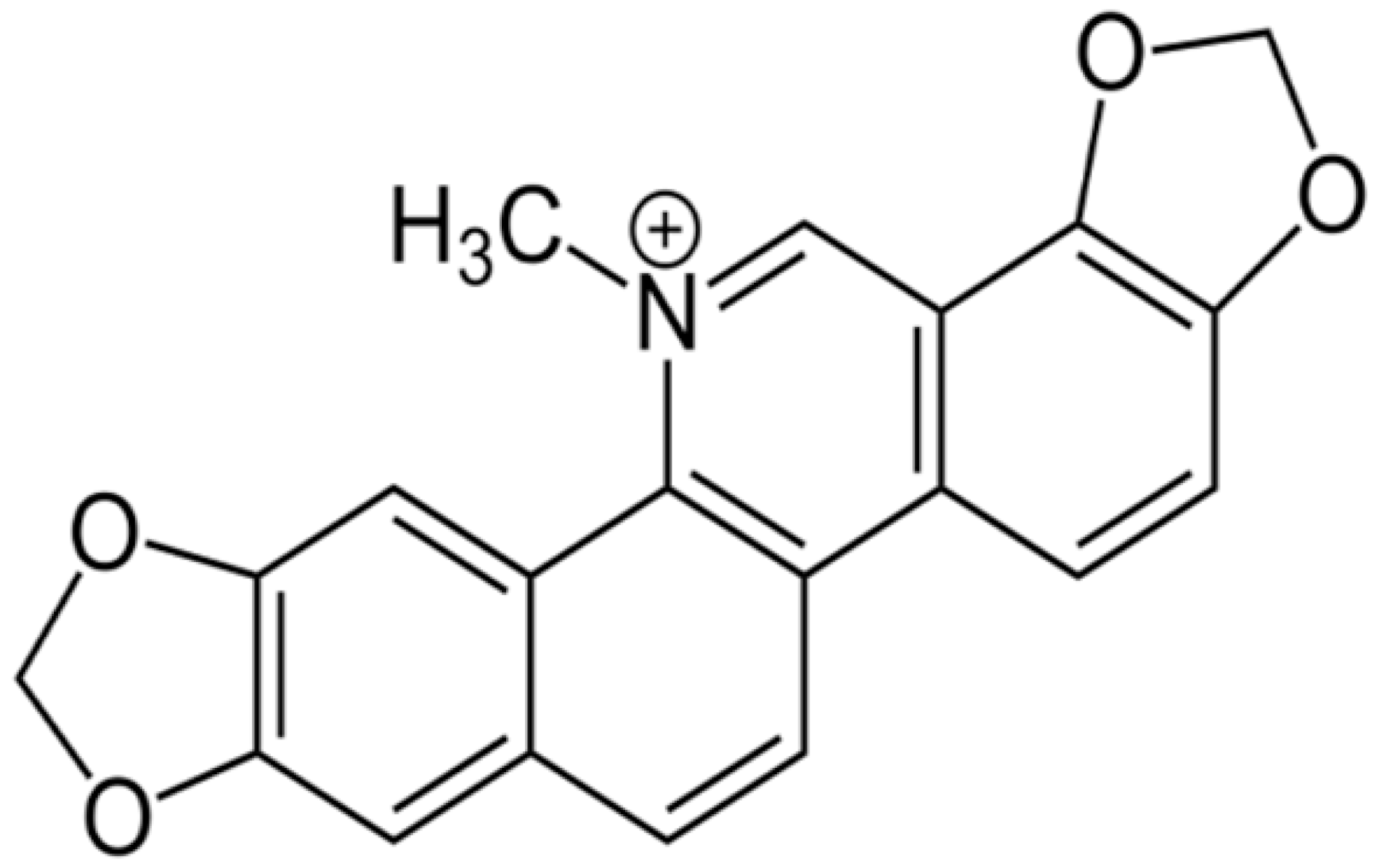 | carbapenem-resistant Serratia marcescens (MIC90 of 32 μg/ml) | Inhibiting replication and transcription | [81,82] | |
| Chanoclavine | 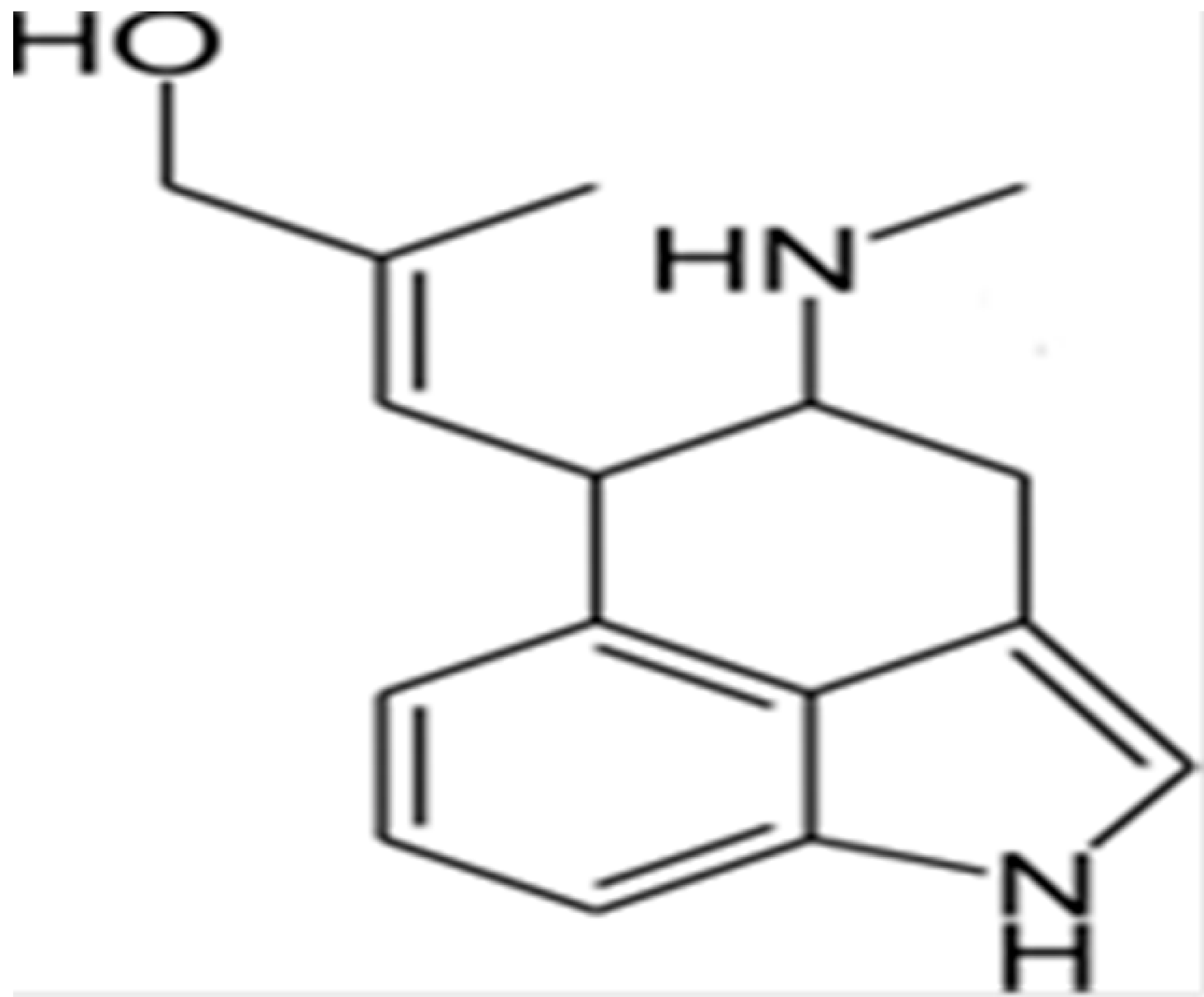 | Inhibition of efflux pump | [83] | ||
| Conessine | 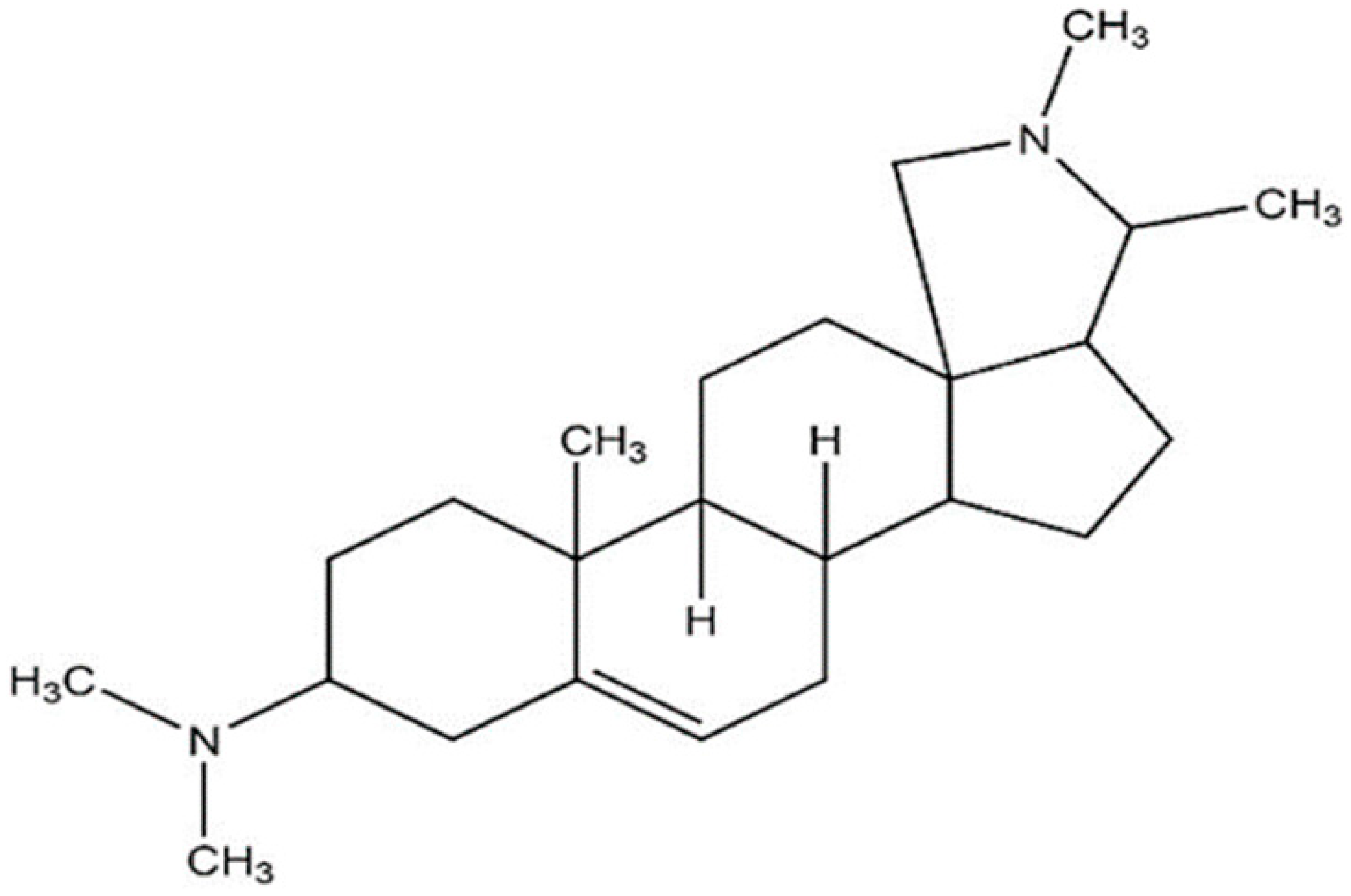 | Micrococcus luteus ATCC 9341 (MIC value of 15.6 μg per disc) | Inhibition of efflux pump | [84,85] | |
| Chelerythrine |  | MRSA (MIC values ranged from 2 to 4 μg/mL) and extended-spectrum β-lactamases Escherichia coli (MIC values varied from 16 to 256 μg/mL) | Damaging the bacterial cells | [86,87] | |
| Matrine | 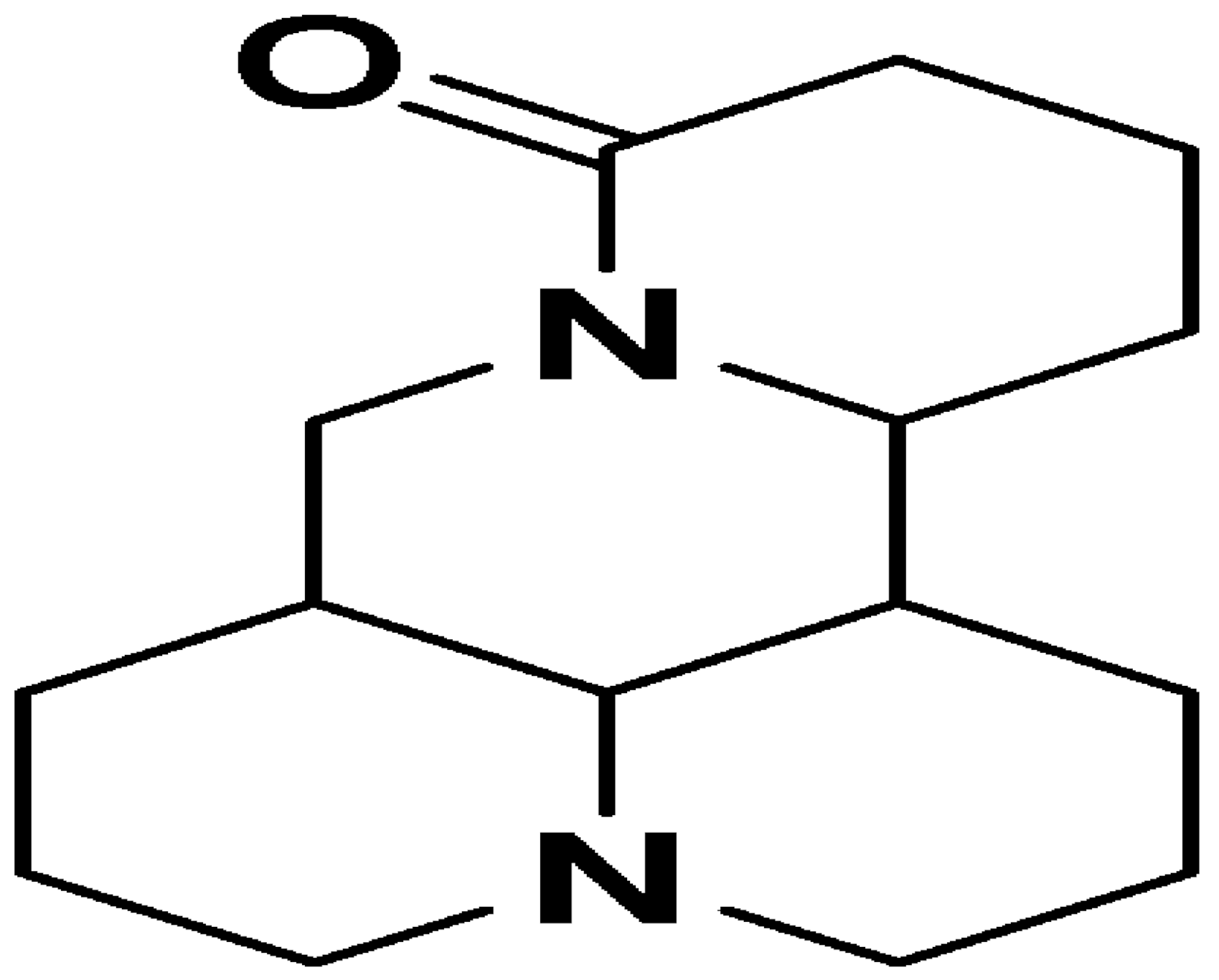 | E. coli and Bacillus subtilis (12.5 μg/mL) | Inhibiting the synthesis of proteins | [88,89] | |
| Camptothecin | 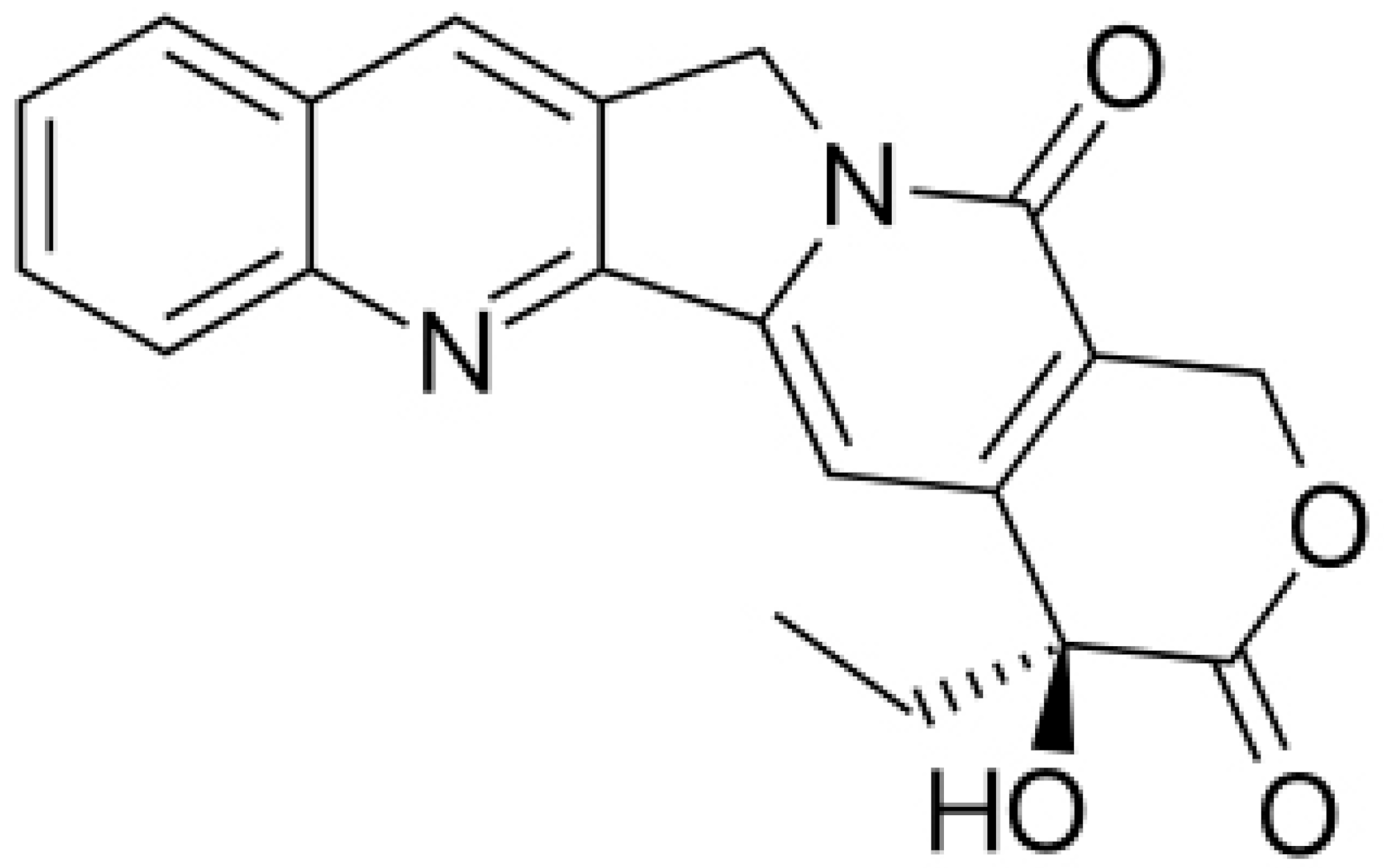 | Cleaving the intermediate complex of DNA topoisomerase I | [90] | ||
| Caffeine | 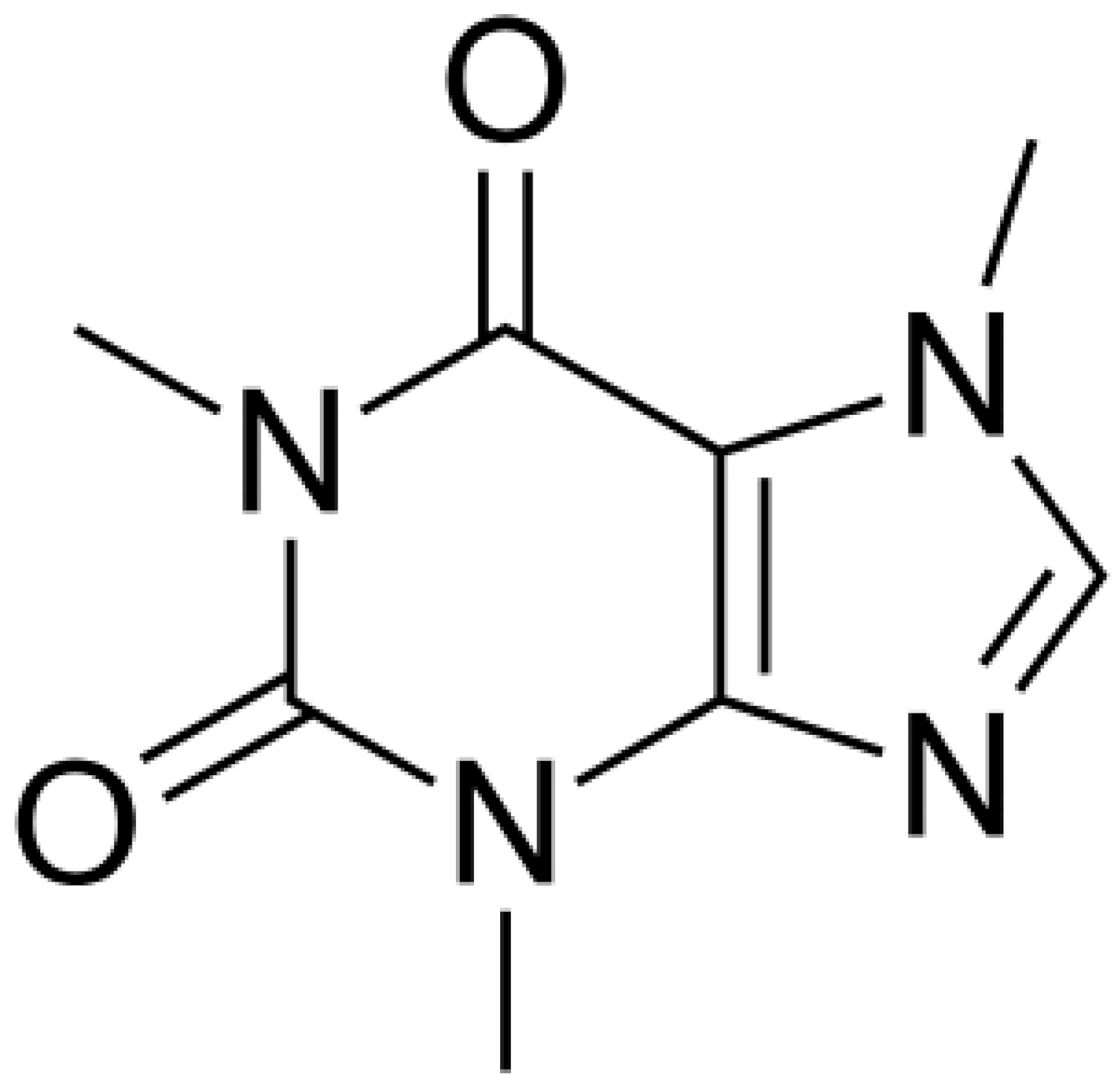 | P. aeruginosa (MIC value of 200 μg/mL) | Interaction with the quorum sensing proteins and inhibiting biofilm formation | [91,92] | |
| Allicin | 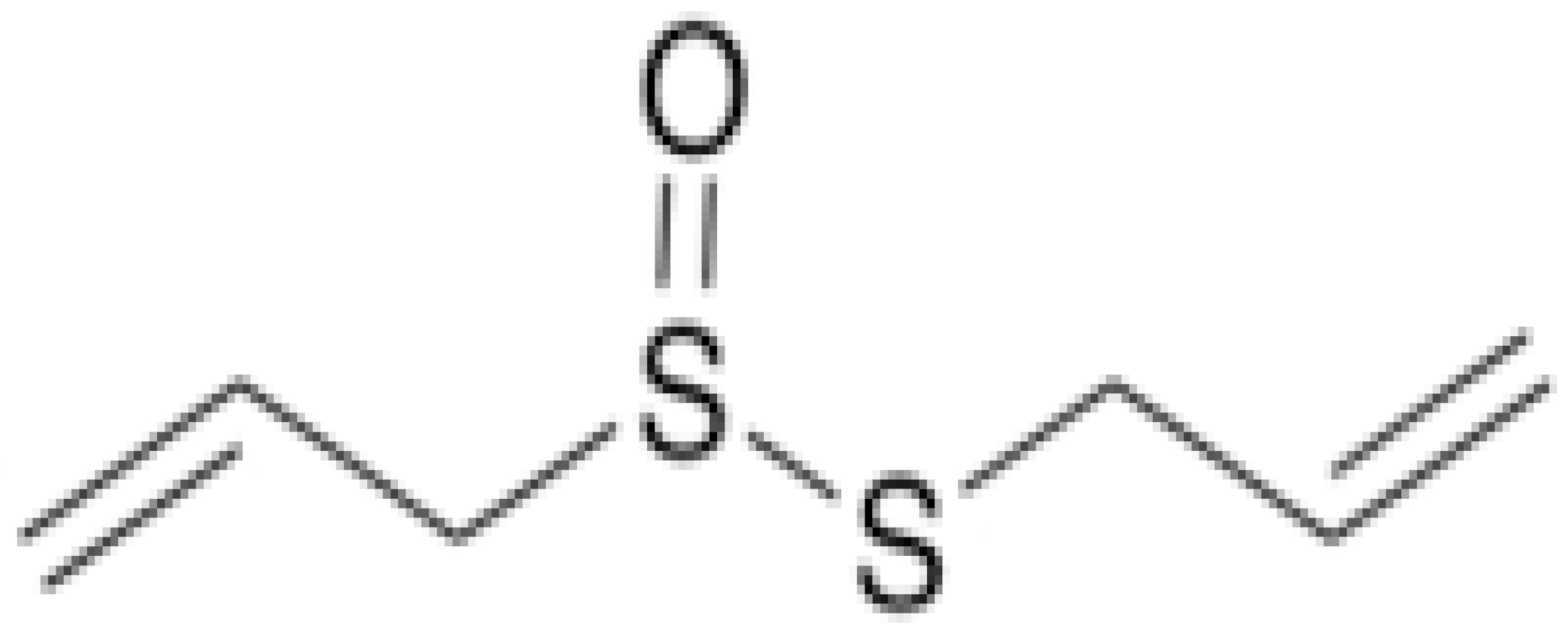 | C. albicans (MIC value of 8 µg/ml) | Organosulfur | Inhibiting sulfhydryl-dependent enzymes, inhibiting the DNA and protein synthesis | [93,94] |
| Ajoene | 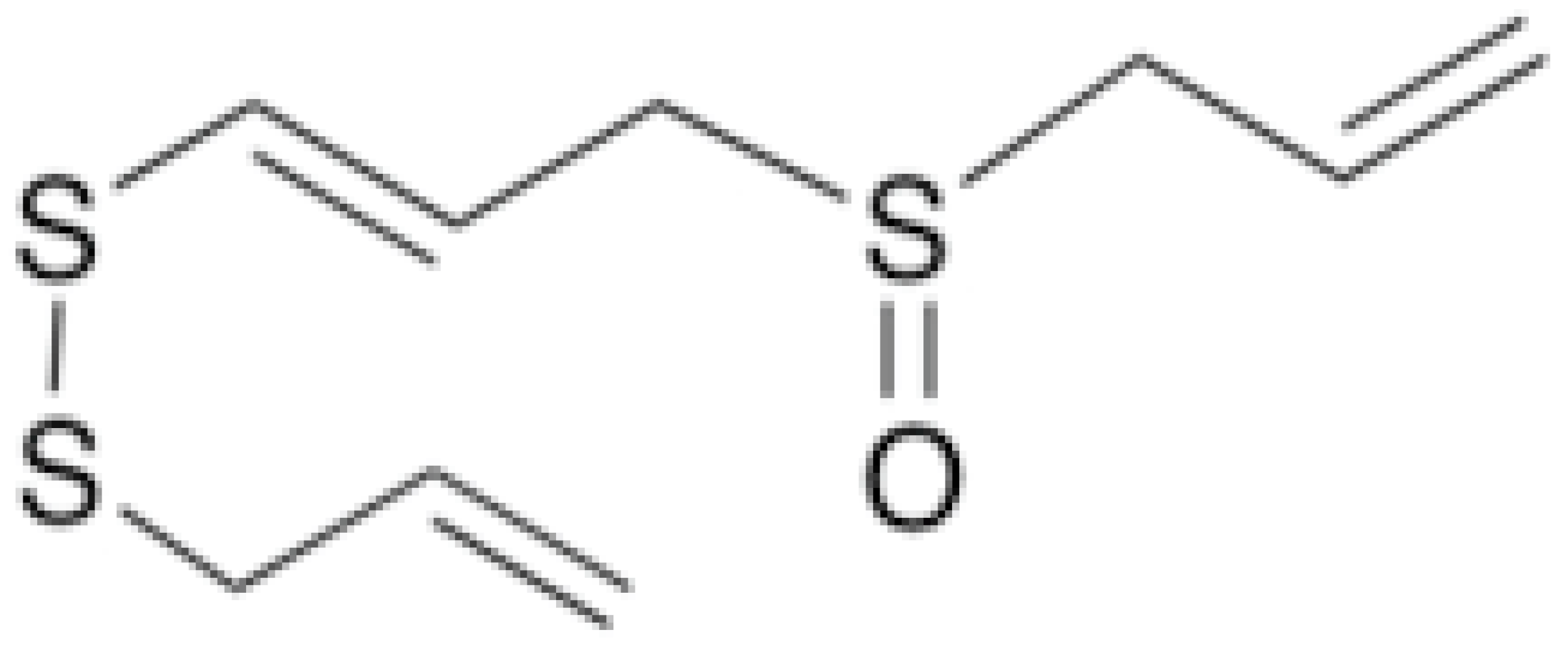 | Histoplasma capsulatum (MIC values varied from 2.5 to 5 μg/mL) | Inhibiting sulfhydryl-dependent enzymes | [93,95] | |
| Isothiocyanates |  | Attacking the sulfhydryl groups of enzymes, damaging the cell wall integrity, and leakage of cellular metabolites | [96] | ||
| Diallyl Sulfides | 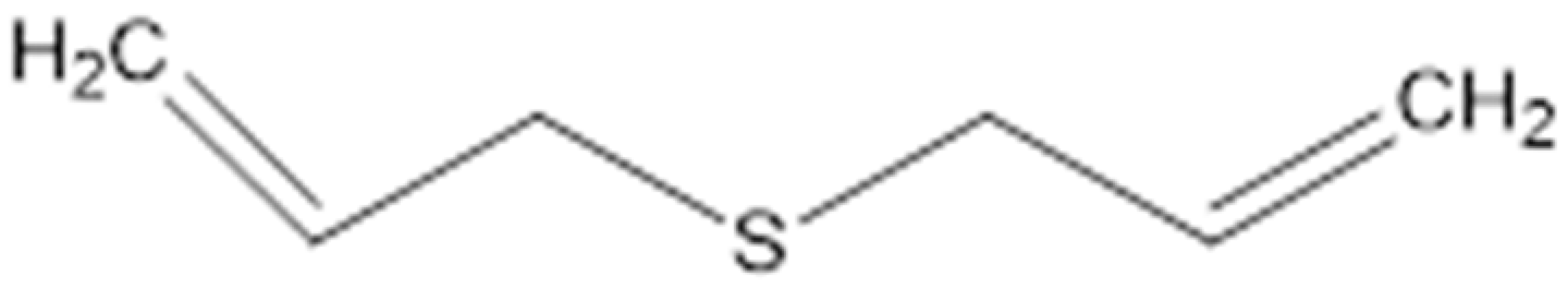 | C. albicans (MIC value of 500 µg/ml) | Inhibiting glutathione (GSH) S-transferase (GST) activity. Interaction with the quorum sensing proteins and inhibiting biofilm formation | [97,98] | |
| Diallyl trisulfide (Allitridin) | 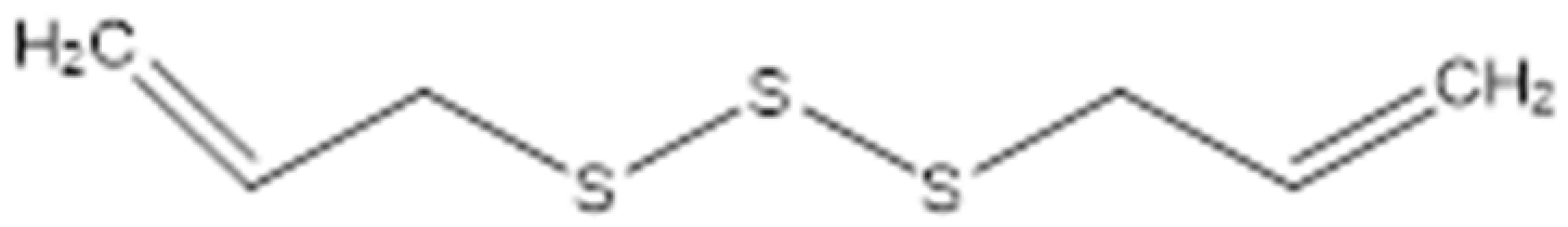 | Destructing the bacterial cell membrane. Decreasing the activity of the bacterial membrane transporter system. | [99] | ||
| Resveratrol |  | Multidrug resistant (MDR) Gram-negative (MICs ranging from 32 μg/mL to 128 μg/mL) | Polyphenolic compounds | Inhibition of efflux pump | [50,51] |
| Baicalein |  | S. typhimurium (MIC value of 64 µg/ml) | Inhibition of efflux pump | [54,100] | |
| Biochanin A | 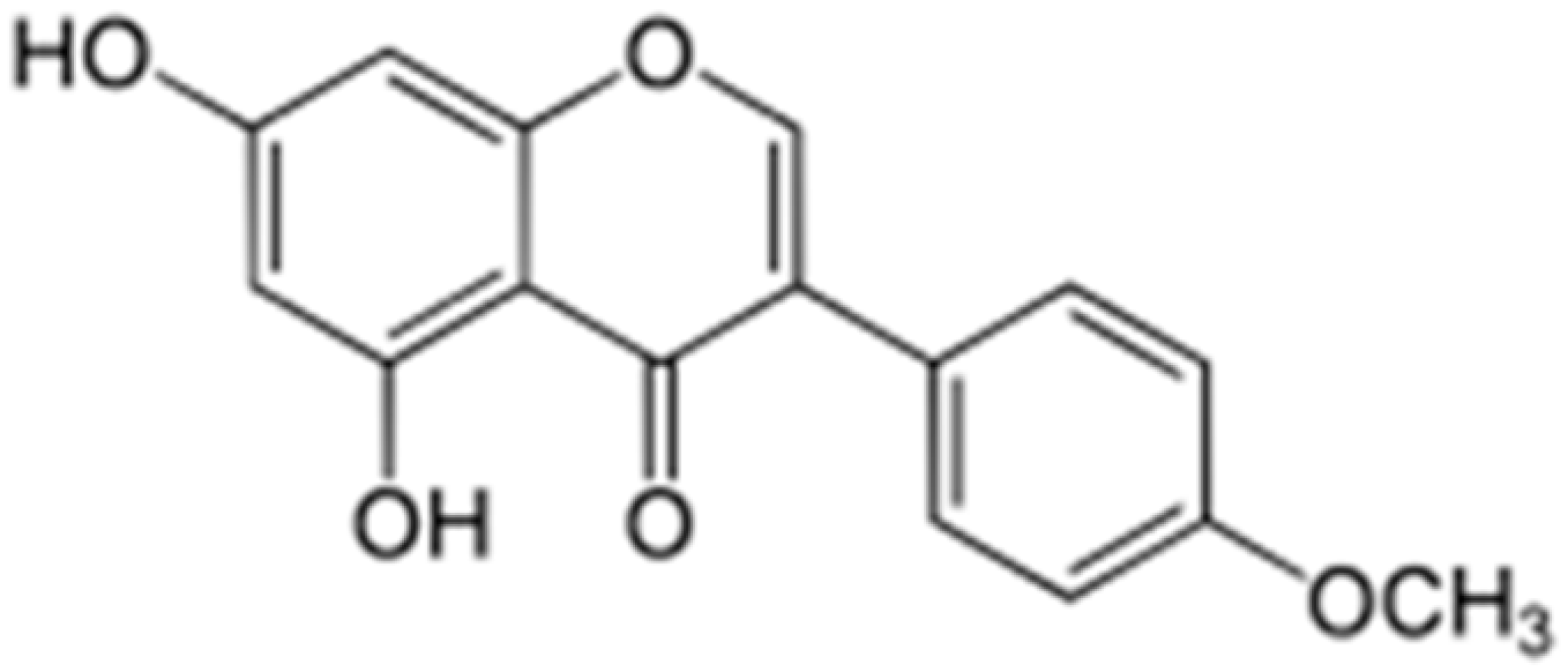 | S. aureus (MIC values varied from 64 to 512 μg/mL) | Inhibition of efflux pump | [100,101] | |
| Chrysosplenol-D |  | Inhibition of efflux pump | [102] | ||
| Chrysoplenetin |  | Inhibition of efflux pump | [102] | ||
| Silybin | 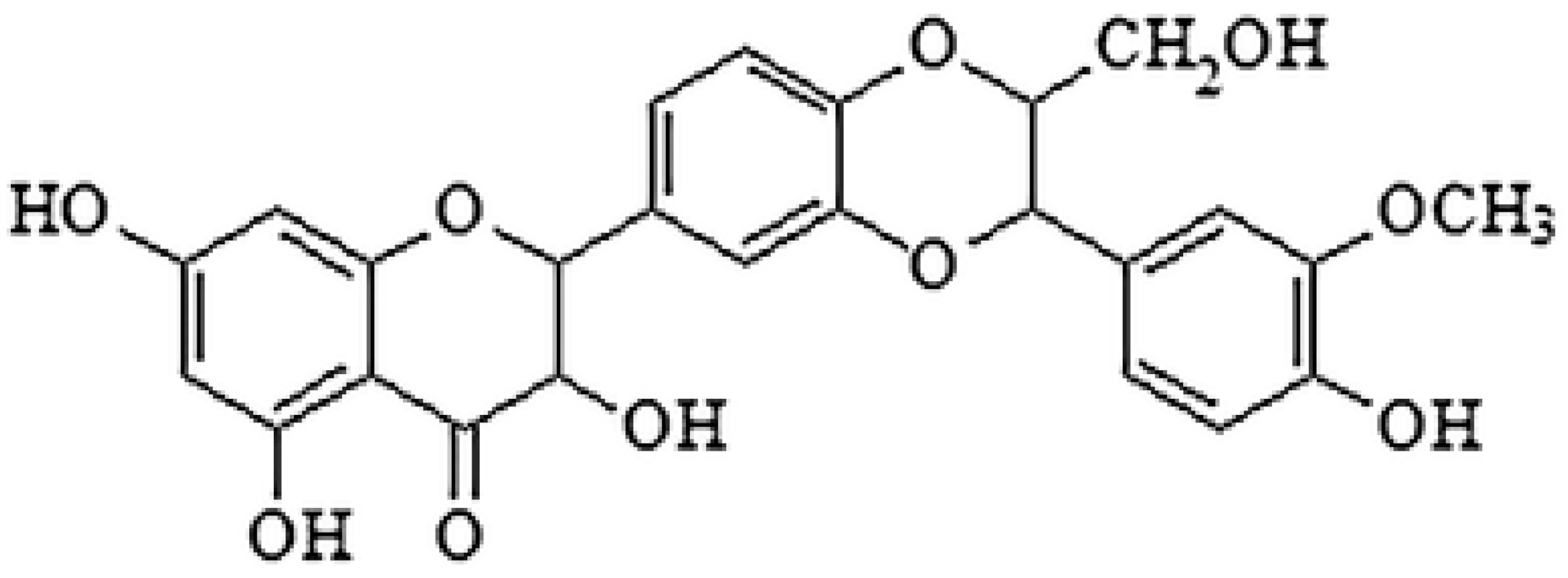 | Inhibition of efflux pump | [103] | ||
| Kaempferol |  | Inhibition of efflux pump | [104] | ||
| Quercetin | 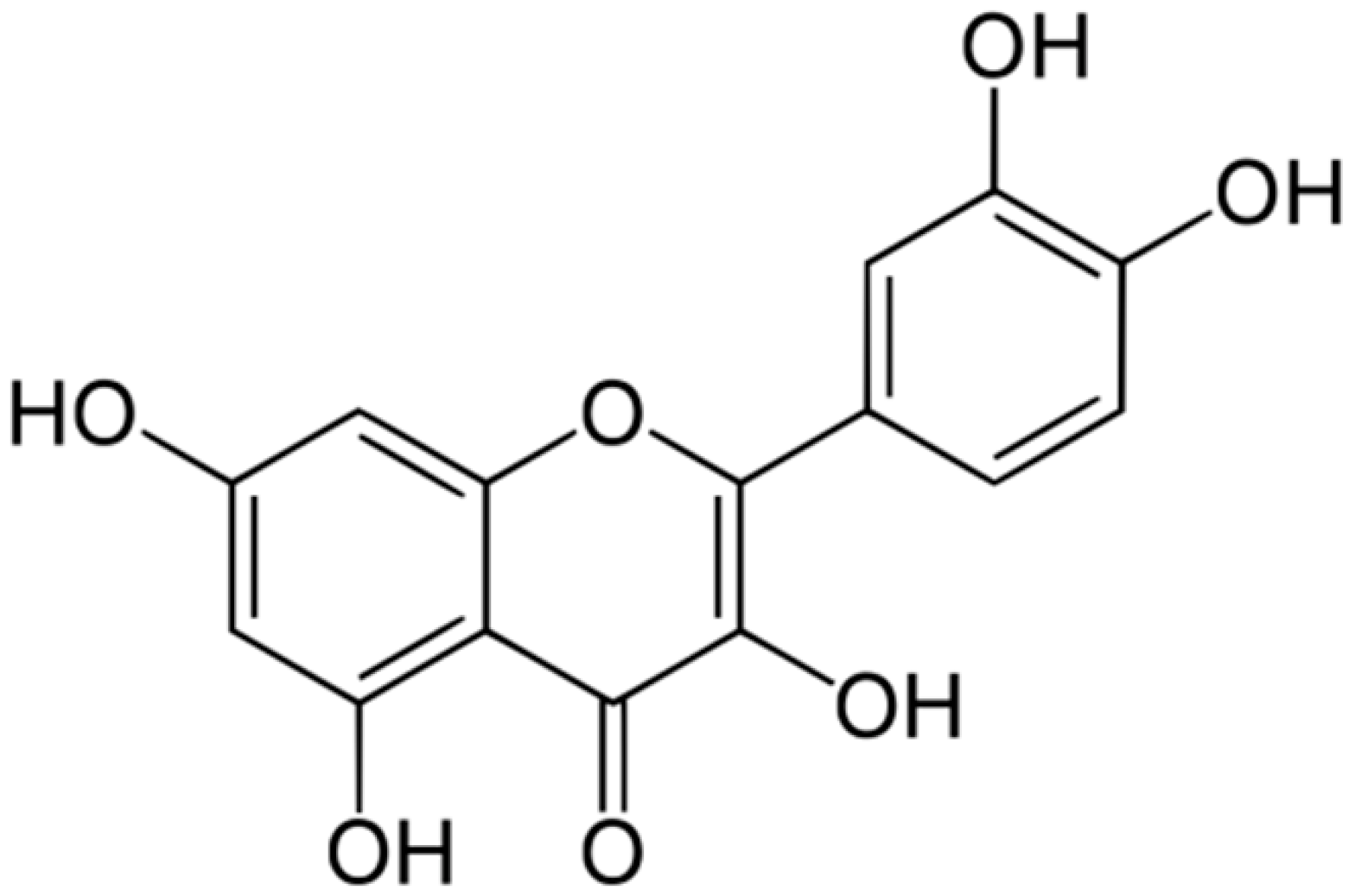 | Aspergillus fumigatus (MIC values of 16–64 μM) | Inhibition of efflux pump, Interacting with some crucial enzymes such as β-lactamase, and cell membrane disruption | [45,105] | |
| Guttiferone-A | 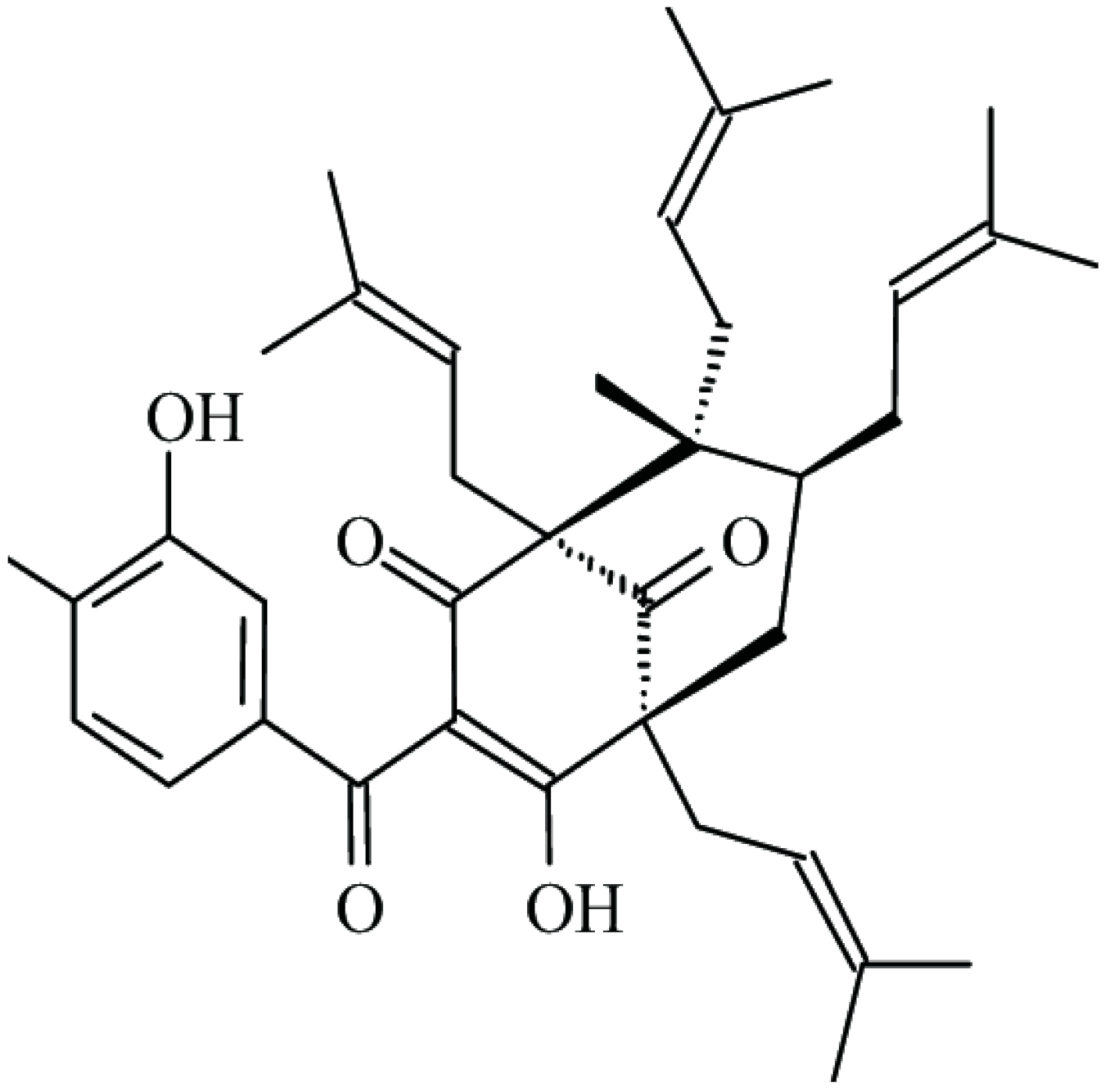 | β-lactamase inhibition | [48] | ||
| 4-Butanylanisole | β-lactamase inhibition | [49] | |||
| Gallic acid | 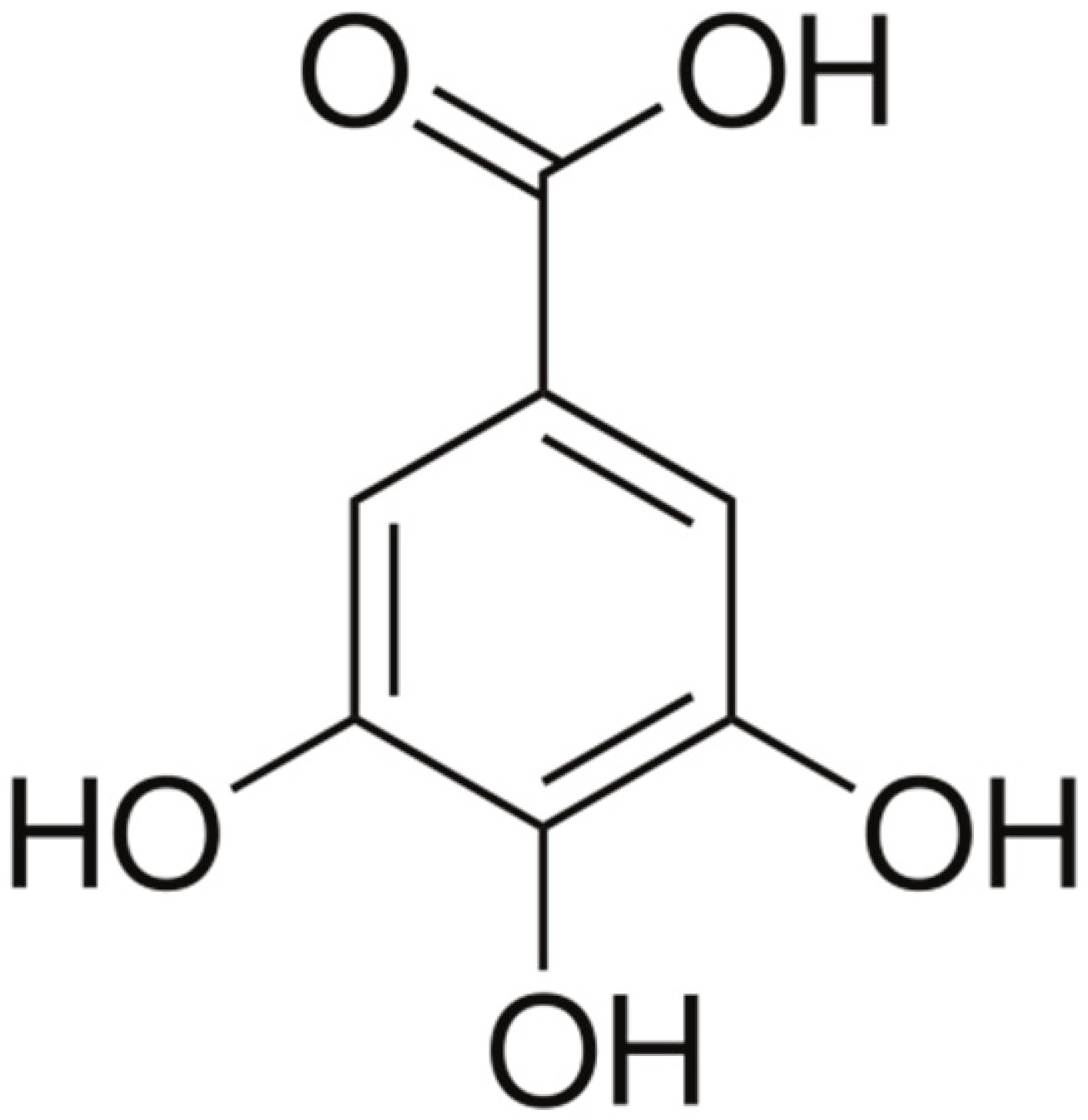 | Cell membrane disruption, and Mg2+ Chelation | [106] | ||
| Epigallocatechin gallate | 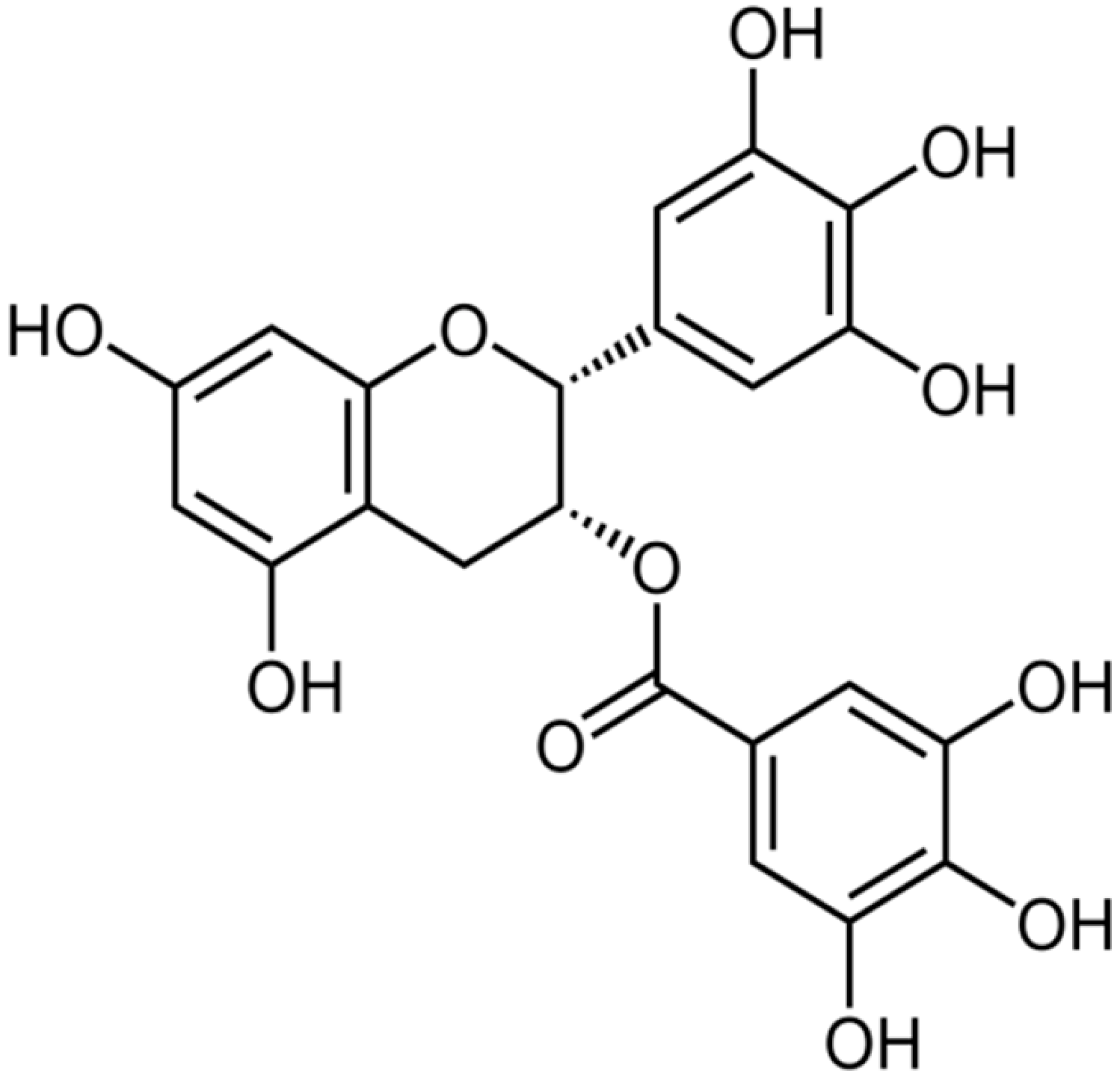 | S. aureus (MIC values of 7.81–62.5 μg/mL) | Inhibiting the B subunit of DNA gyrase, penicillinase, and β-lactamase | [41,42,43,107,108] | |
| 3-p-trans-Coumaroyl-2-hydroxyquinic acid |  | Damaging the cytoplasmic membrane | [109] | ||
| Hydroxycinnamic acids (p-Coumaric, Caffeic, and Ferulic acids) |  | Interfering with membrane integrity | [110] | ||
| Naringenin | 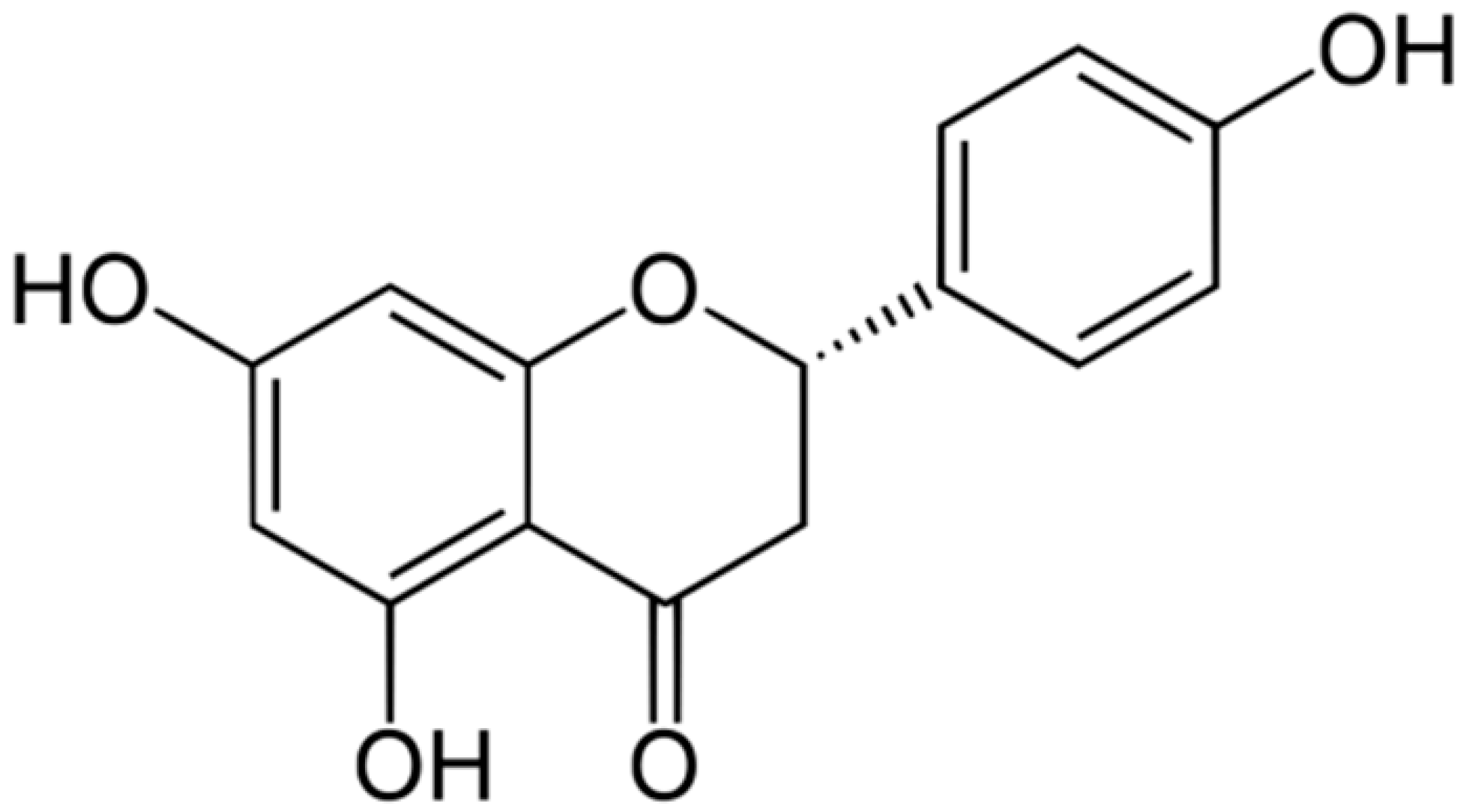 | Interacting with some crucial enzymes | [111,112,113] | ||
| Eriodictyol |  | Streptococcus mutans and P. aeruginosa (MIC values of 1 mg/mL) | Interacting with some crucial enzymes | ||
| Taxifolin | 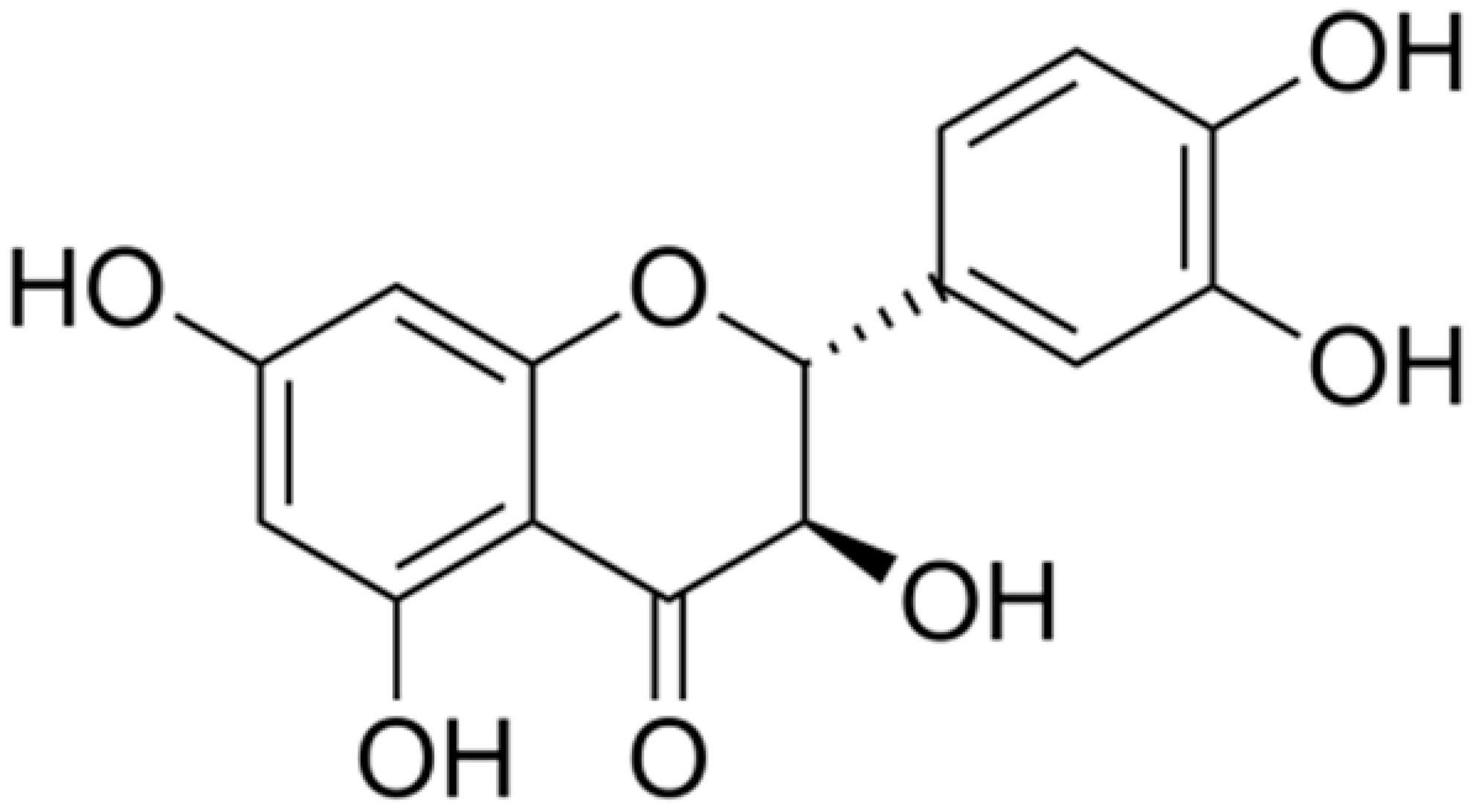 | Helicobacter pylori (MIC = 625 μg/mL) | Interacting with some crucial enzymes | ||
| Curcumin |  | Shigella dysenteriae and Campylobacter jejuni (MIC values of 256 μg/mL) | Damaging the cell membranes | [114,115] | |
| Apigenin | 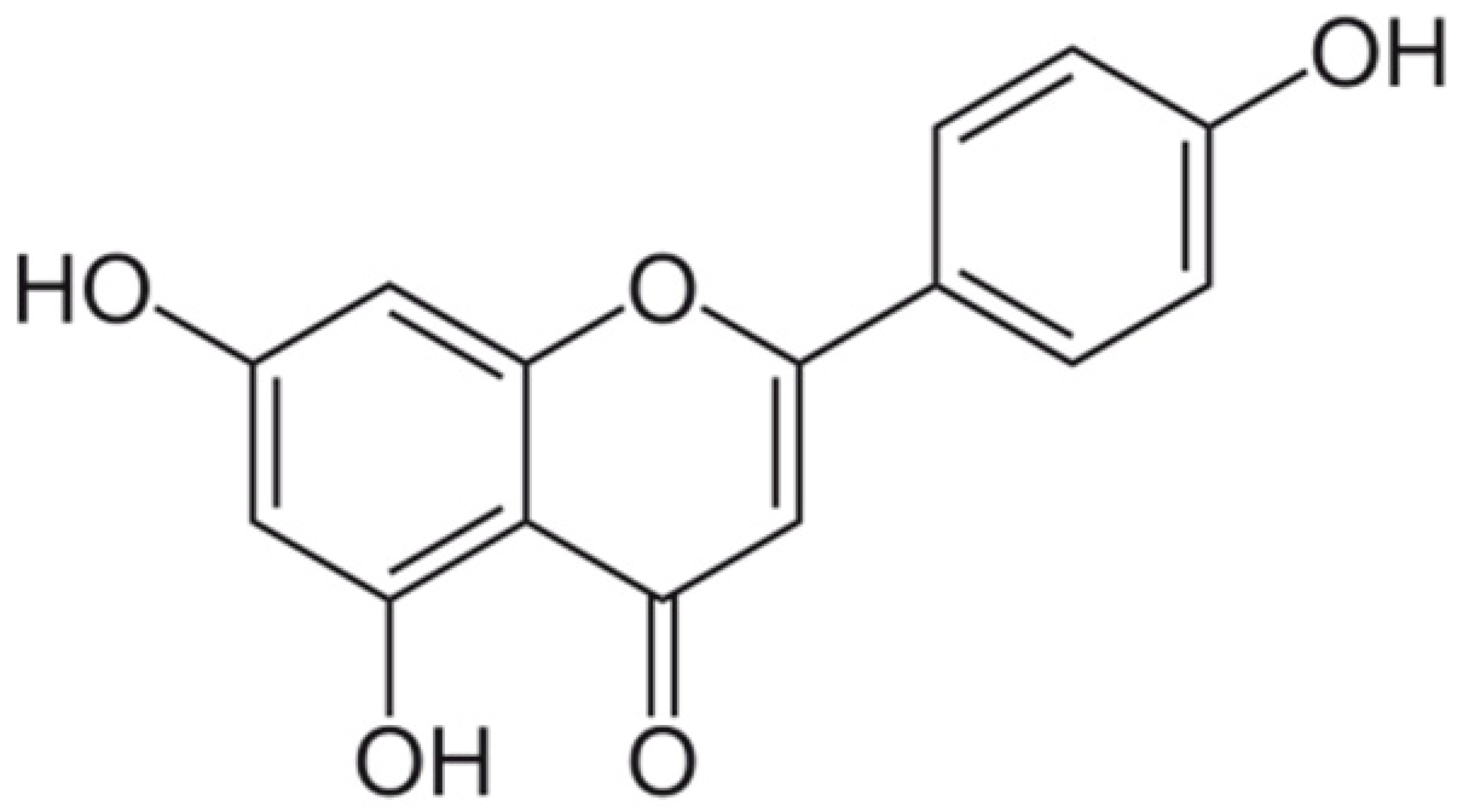 | Interacting with some crucial enzymes | [116] | ||
| Sophoraflavanone G | 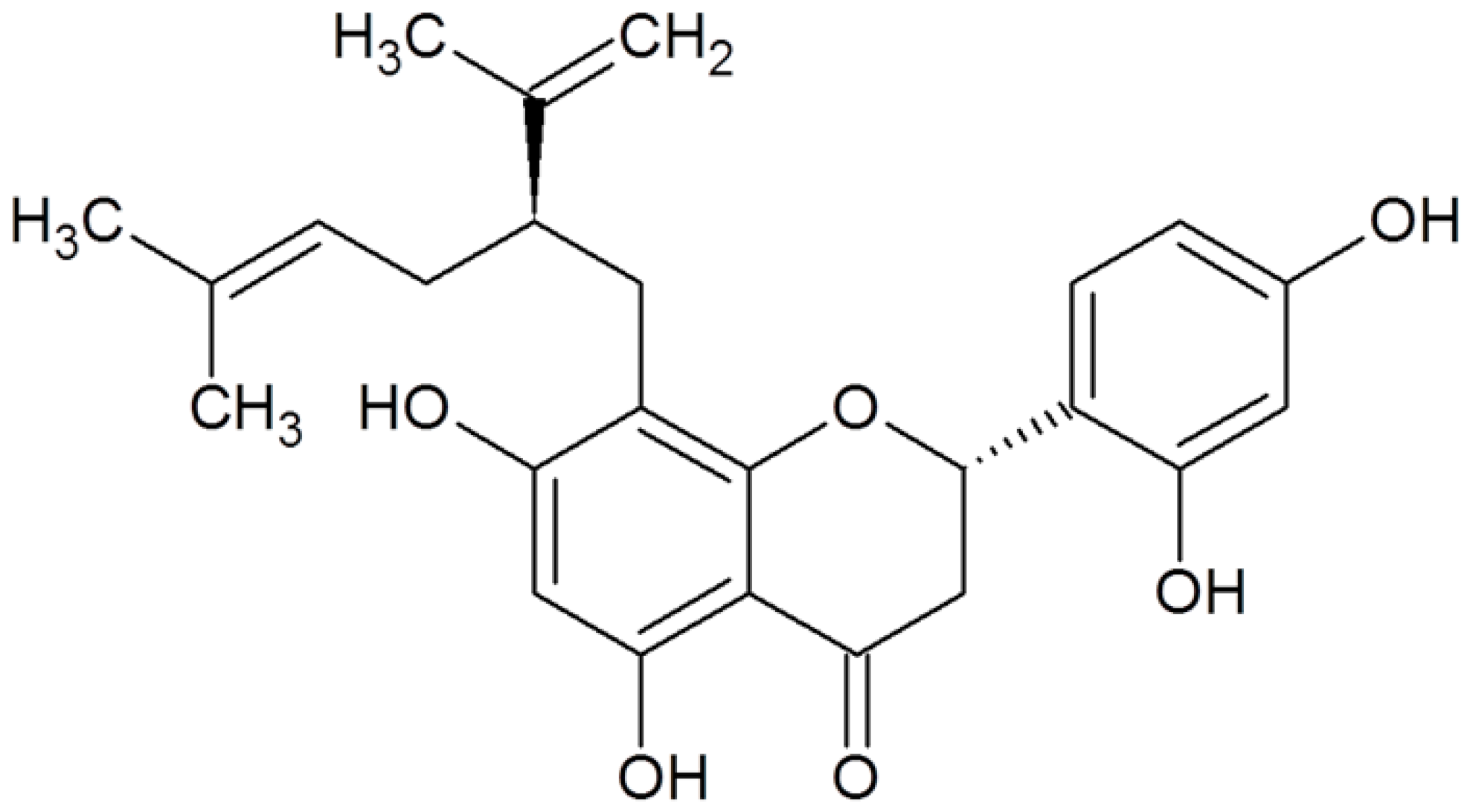 | MRSA (MIC values of 0.5–8 μg/mL) | Interacting with peptidoglycan and inhibiting cell wall biosynthesis | [52,53] | |
| Acetosyringone | 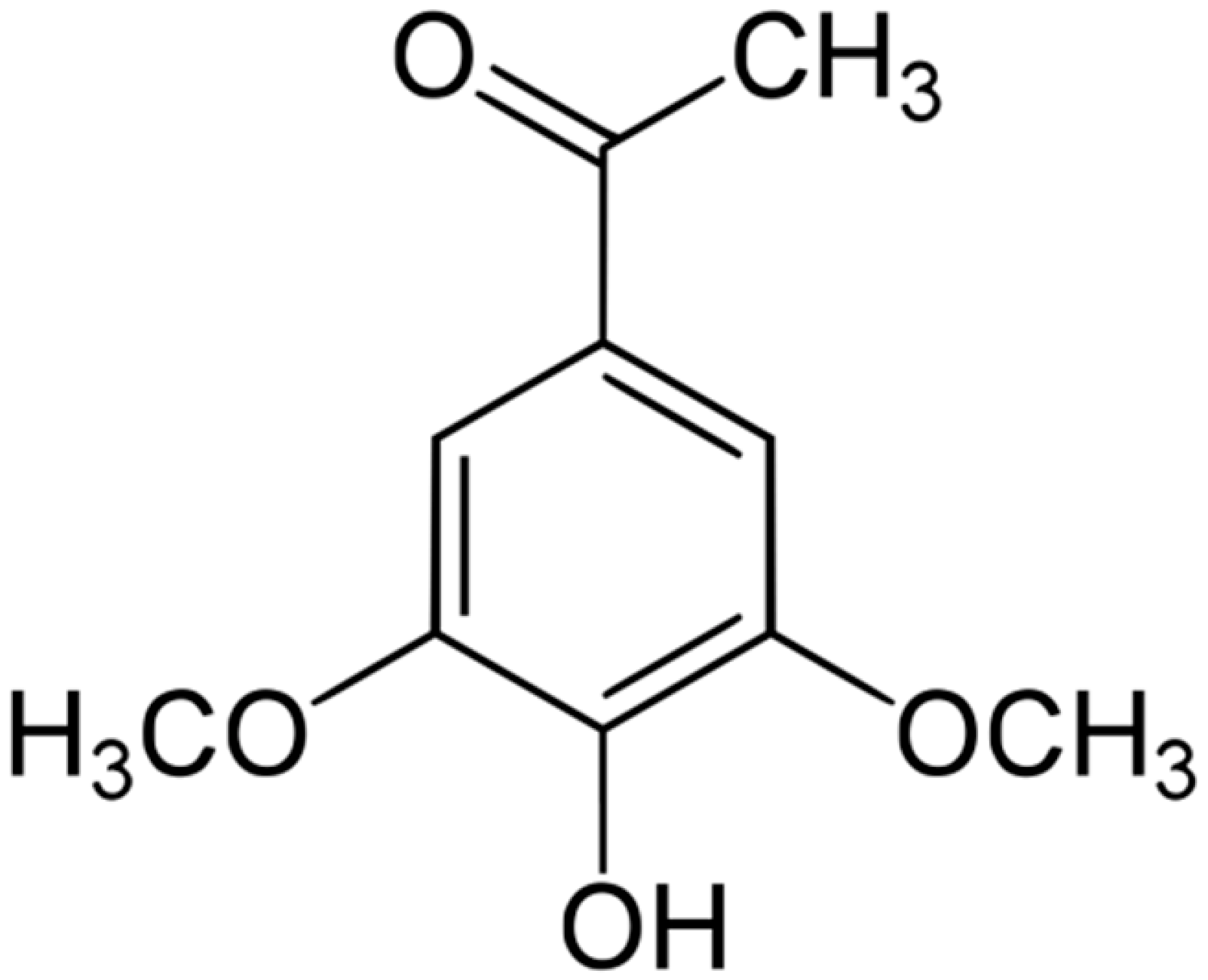 | S. cerevisiae (MIC = 24 mM) | Depolarization of the bacterial cell membrane | [117,118] | |
| Chlorogenic acid | 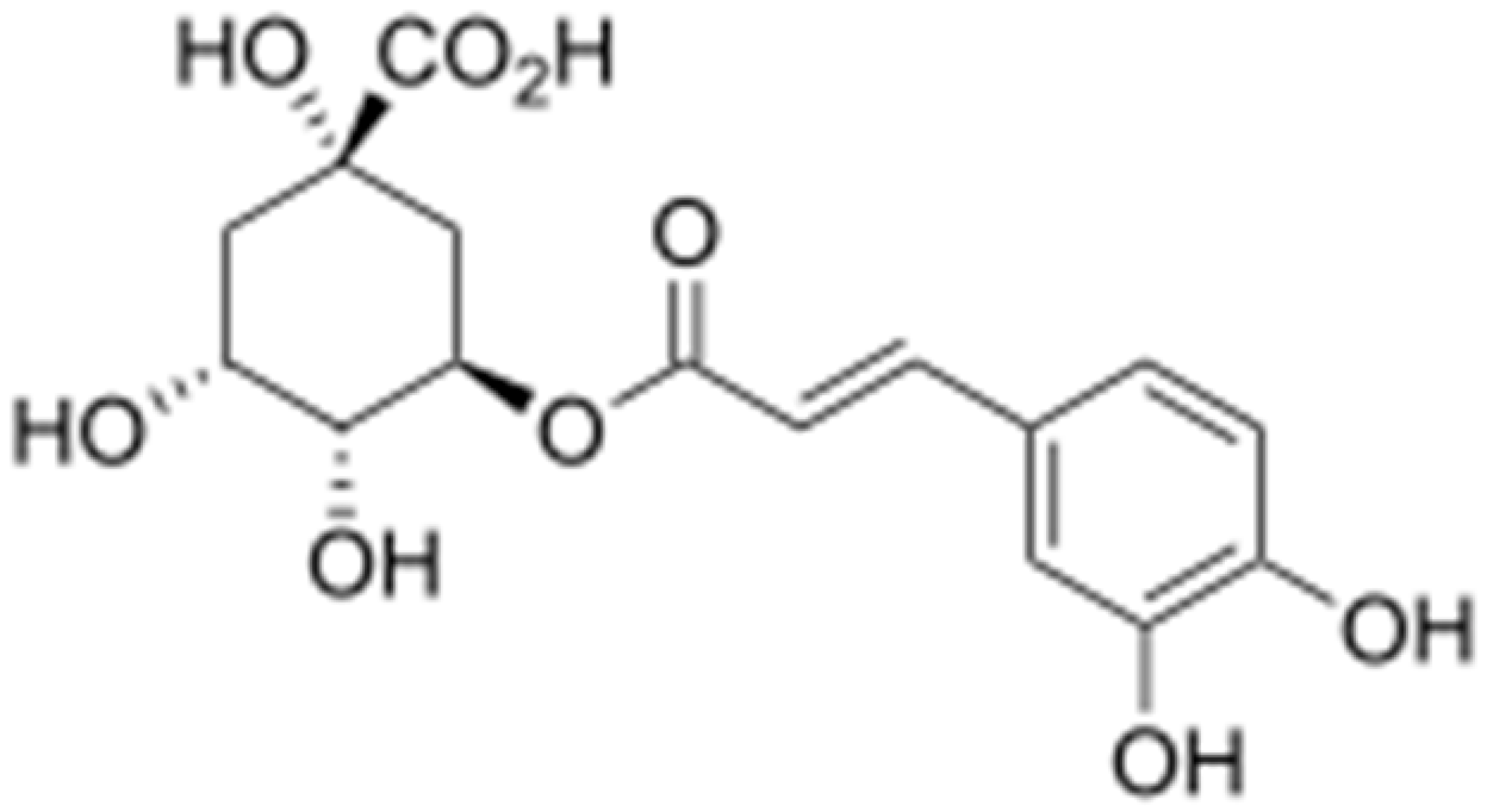 | Providencia alcalifaciens, Moraxella catarrhalis, S. aureus, and E. coli ( MIC values of 60 to 100 μM) | Interacting with some crucial enzymes | [119] | |
| Galangin | 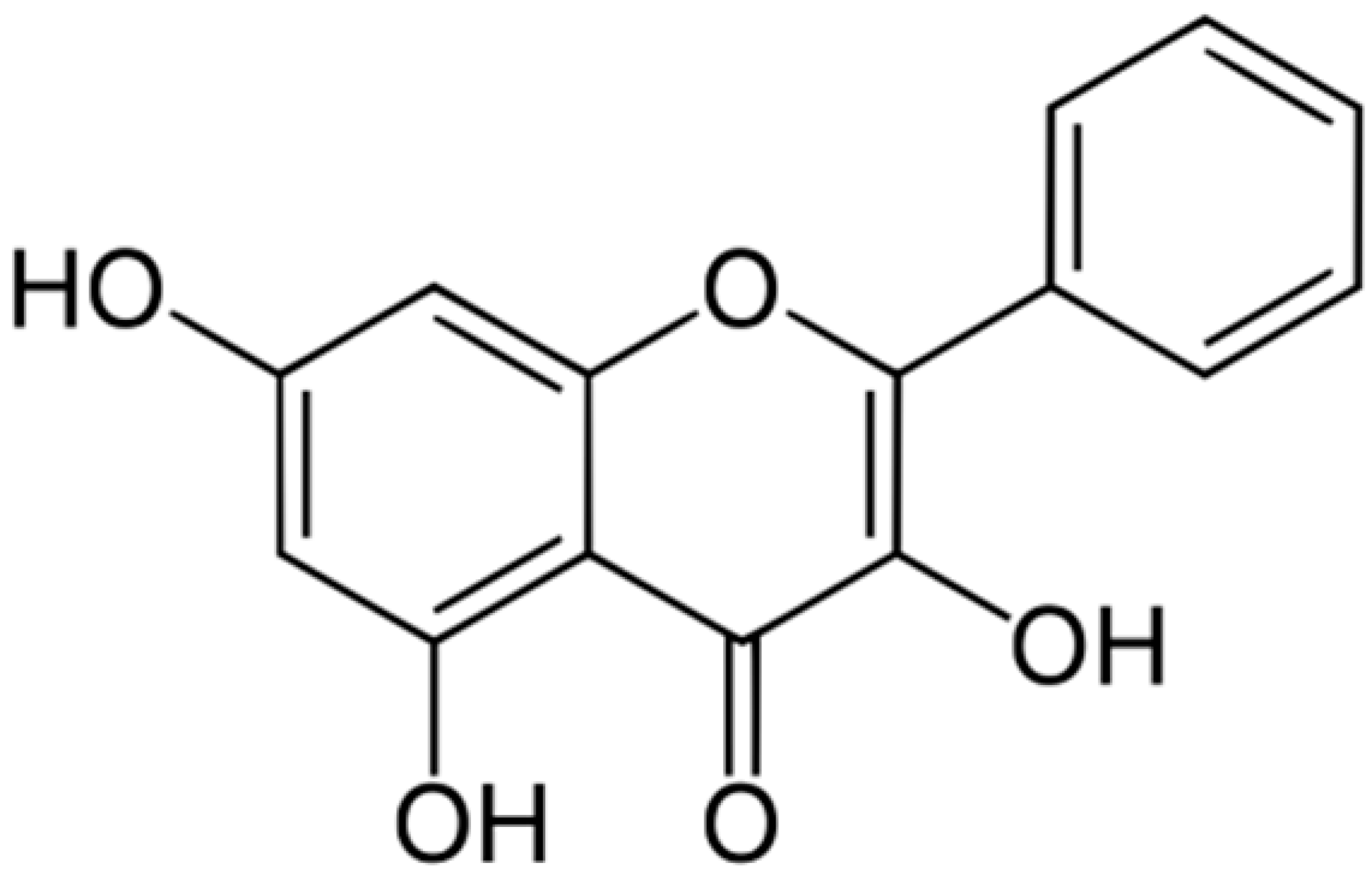 | S. aureus (MIC = 32 μg/mL) | Damaging of the cytoplasmic membrane and inhibition of β-lactamase | [44,120] | |
| Genistein | 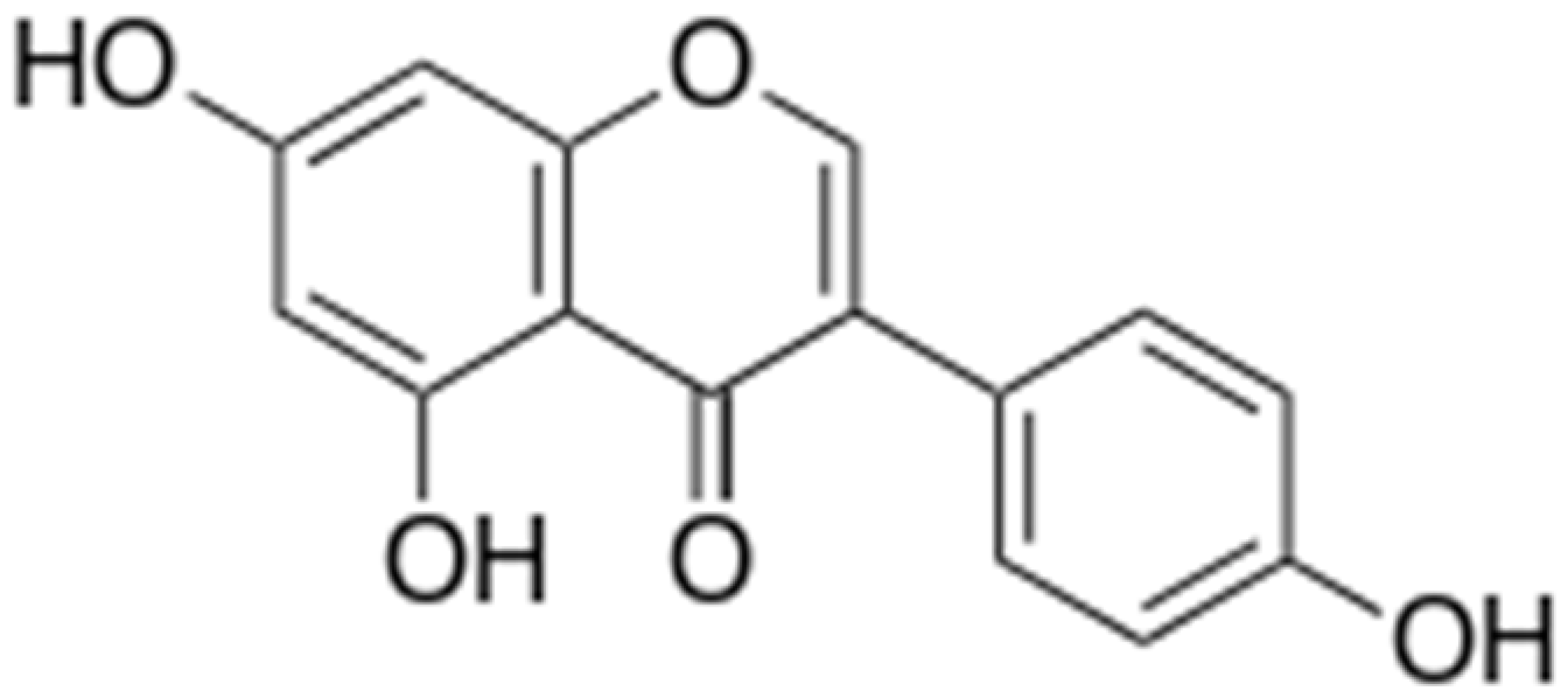 | Inhibition of efflux pump | [121] | ||
| Ononin | 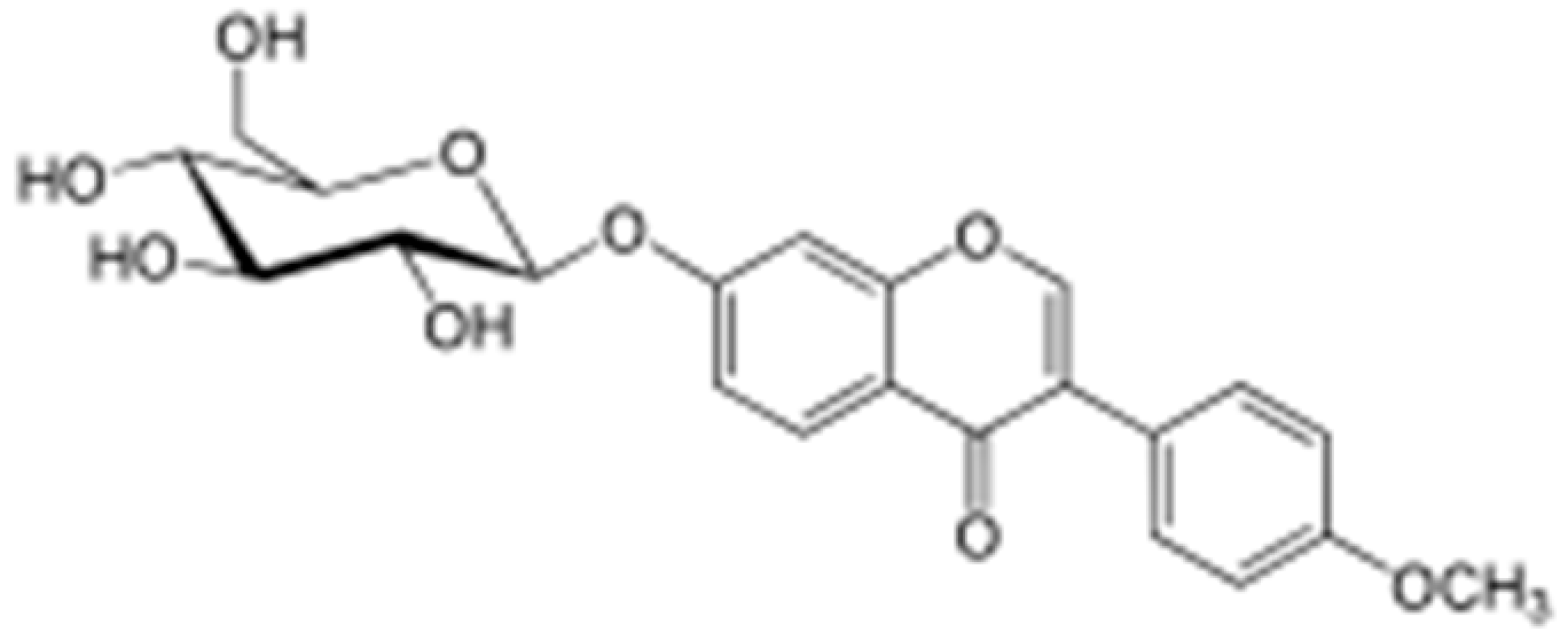 | ||||
| Tangeritin |  | Cell membrane disruption, DNA gyrase inhibition, Reduced protein synthesis, Interacting with some crucial enzymes | [122] | ||
| 5,6,7,4’- Tetramethoxyflavone | 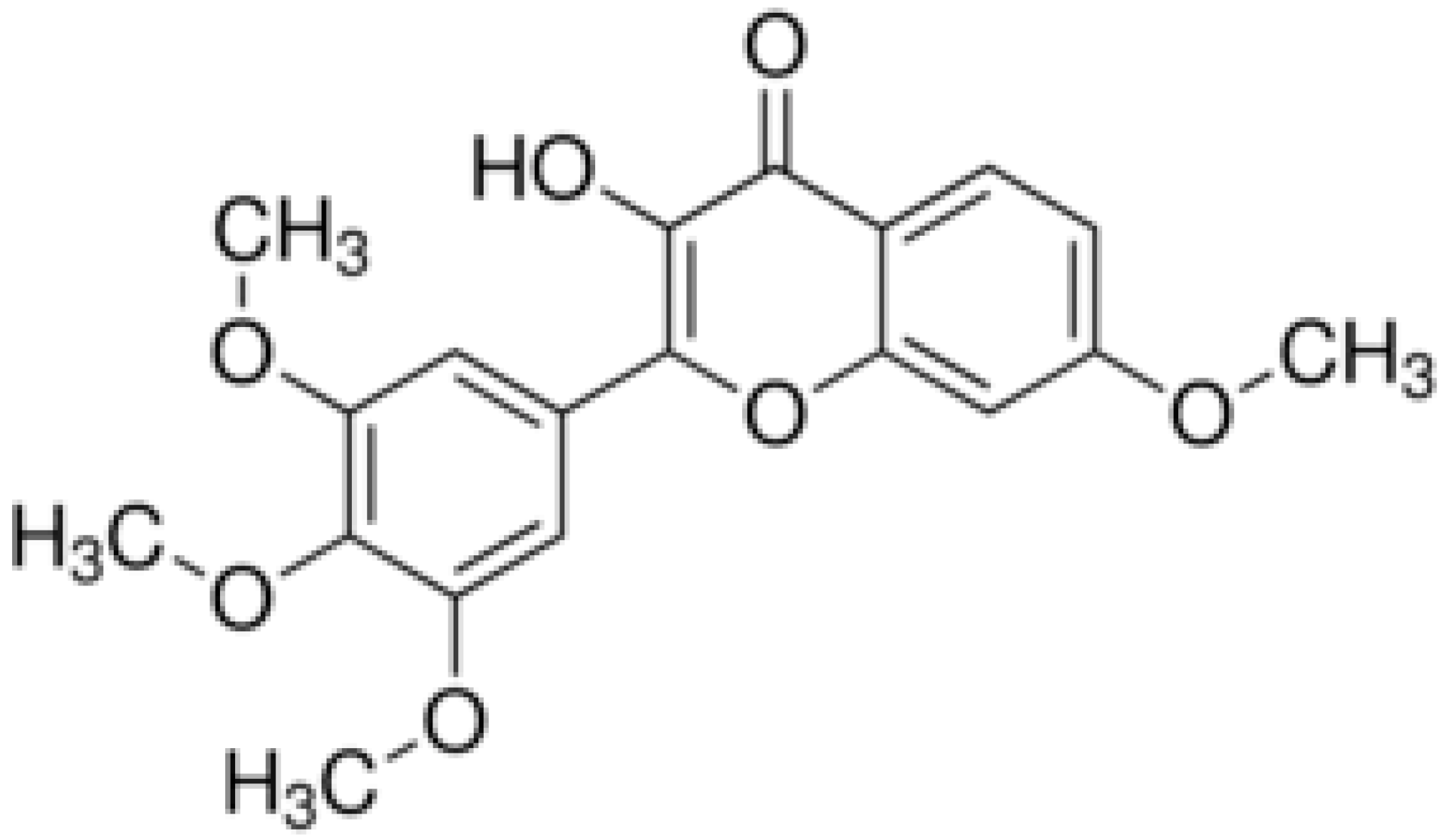 | Cell membrane disruption, DNA gyrase inhibition | |||
| Chrysin | 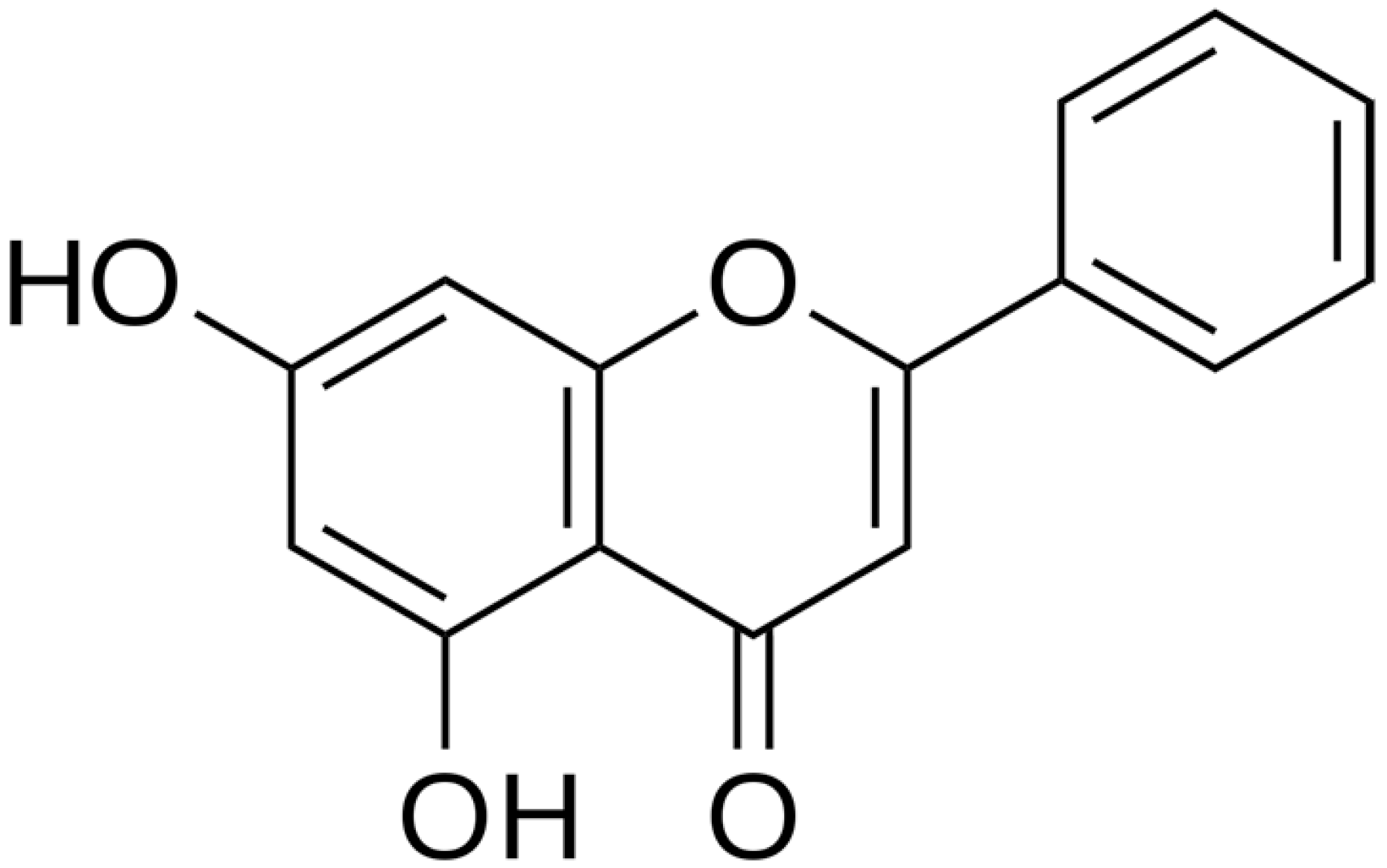 | H. pylori (MIC = 6.25 μg/mL) | Cell membrane disruption, DNA gyrase inhibition | [123,124] | |
| Luteolin | 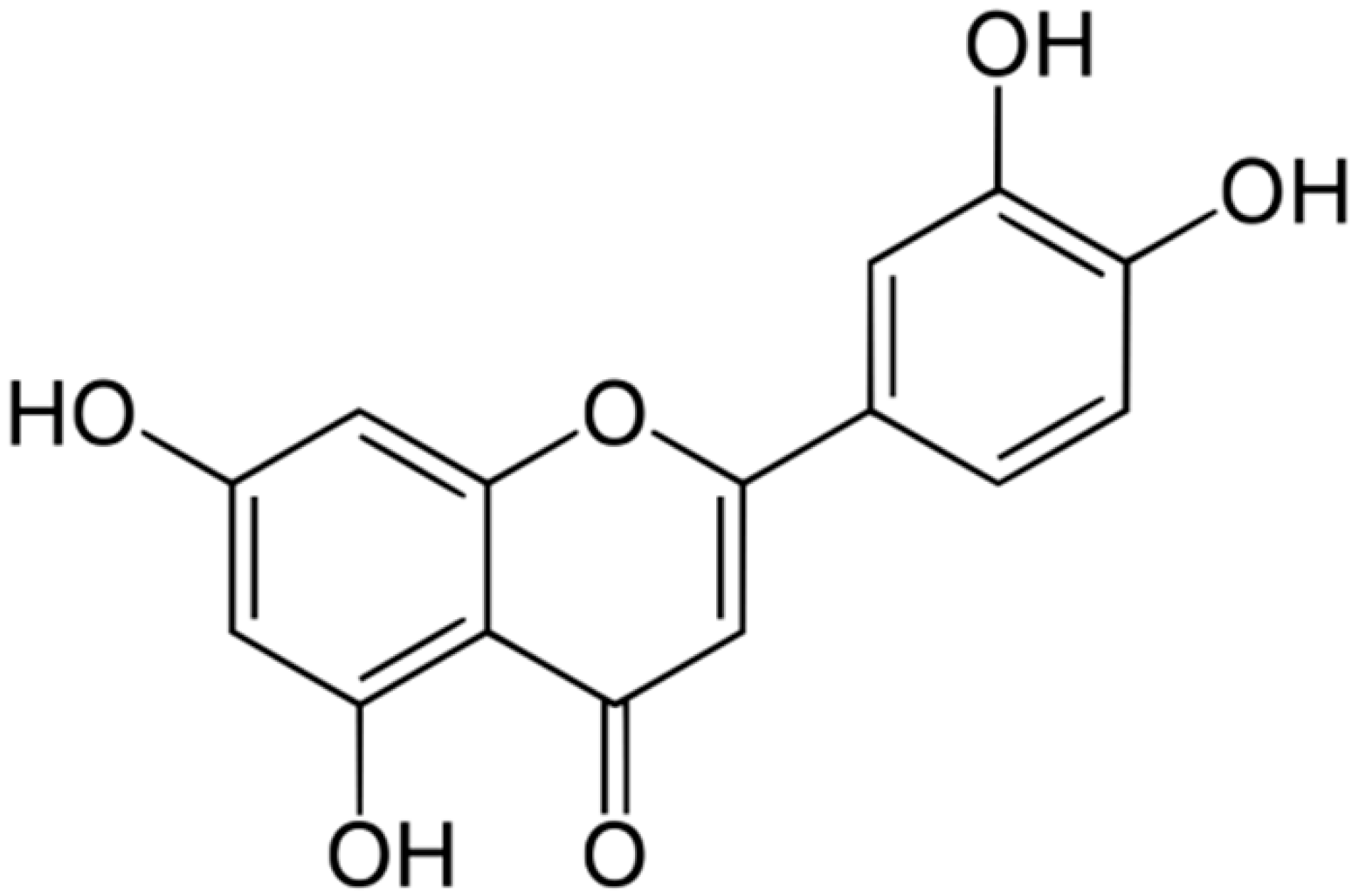 | S. aureus (MIC = 16–32 μg/mL) and Listeria monocytogenes (MIC = 32–64 μg/mL) | Cell membrane disruption, DNA gyrase inhibition, Type III secretion inactivation, Interacting with some crucial enzymes | [125,126,127] | |
| Myricetin |  | S. aureus (MIC = 256 μg/mL) | DNA gyrase inhibition, Type III secretion inactivation, Interacting with some crucial enzymes | ||
| Nobiletin |  | Cell membrane disruption, DNA gyrase inhibition, Reduced protein synthesis, Interacting with some crucial enzymes | |||
| Totaral | 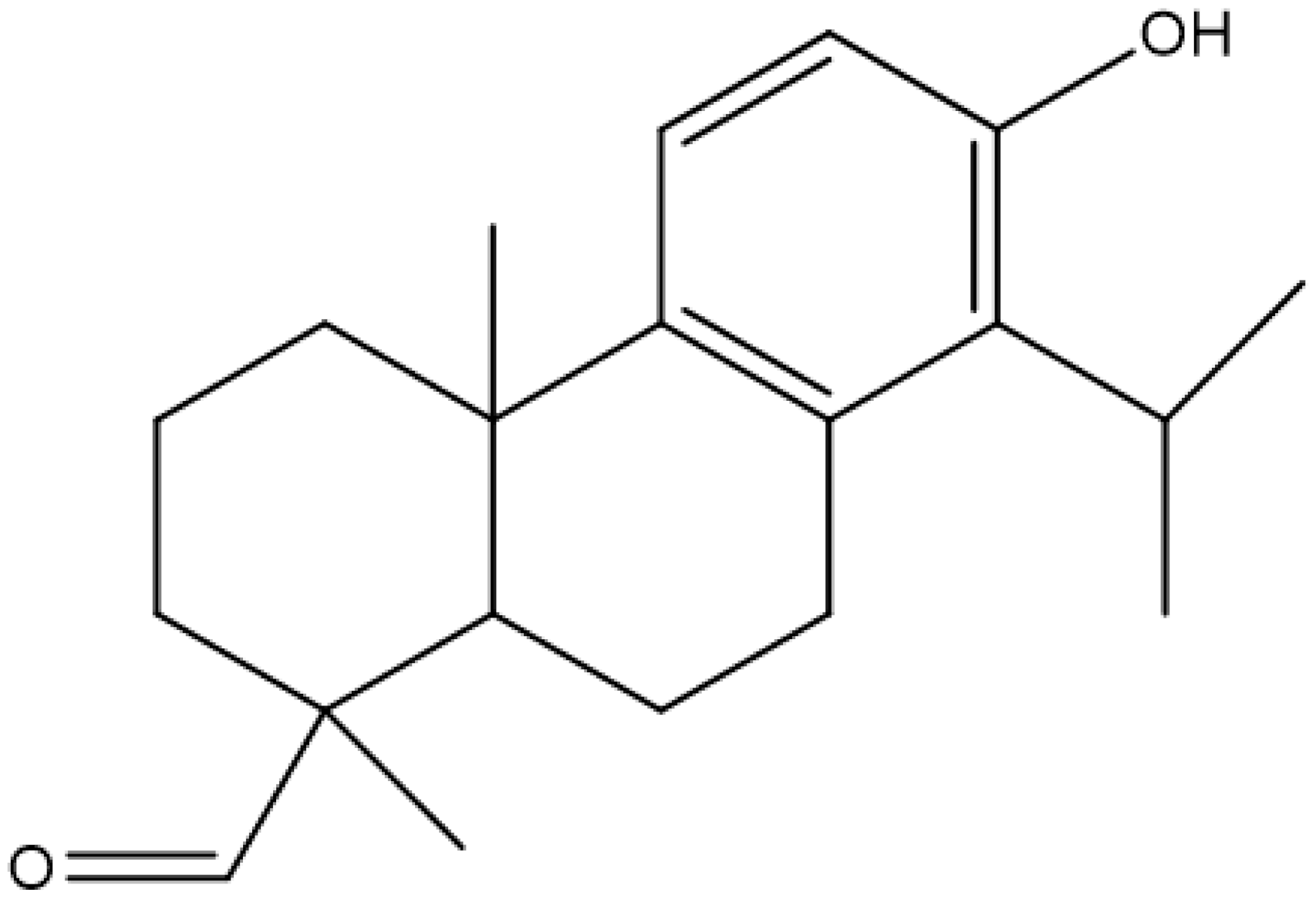 | Reduced expression of enterotoxins, multi-drug efflux pump inhibitor | [128] | ||
| Tannic acid |  | S. aureus (MIC = 512 μg/mL) | Ion binding | [129,130] | |
| (+)-Catechin |  | MRSA (MIC = 78.1–156.2 μg/ml) | Inhibition of bacterial gene expression | [131,132] | |
| Aegelinol | 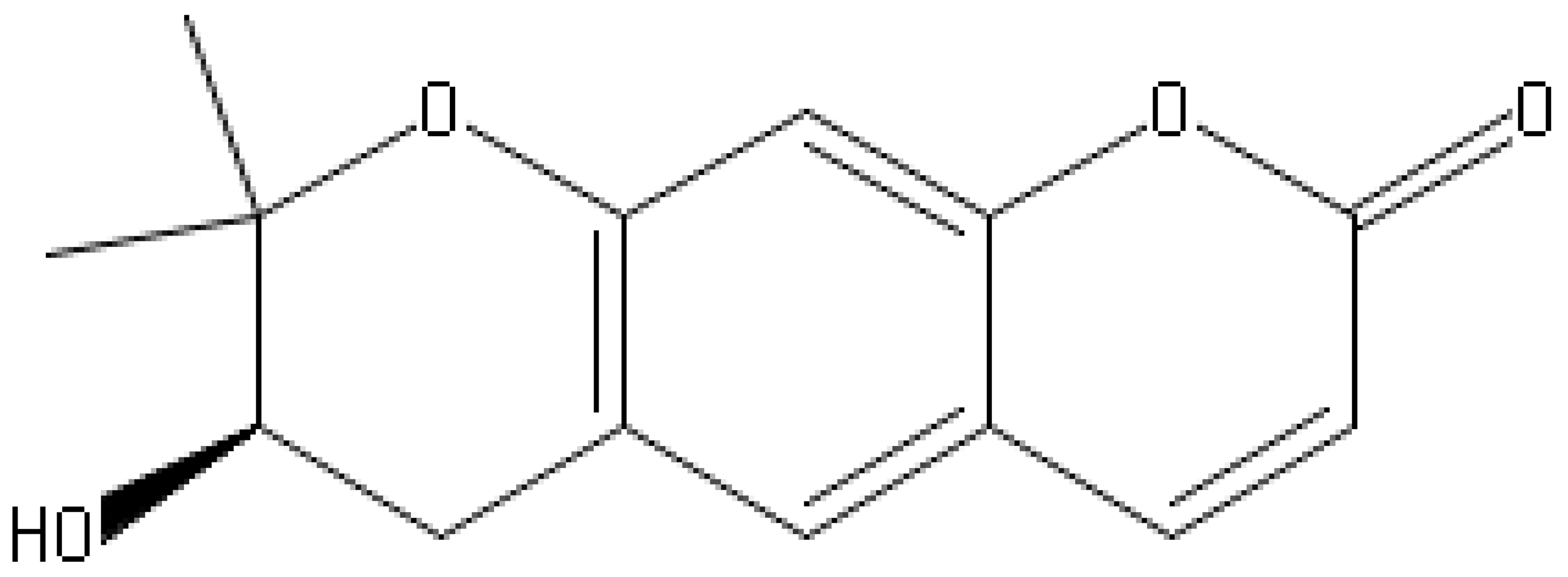 | S. aureus, S. thypii, Enterobacter cloacae and E. earogenes (MIC = 16 μg/mL) | Coumarins | Cell membrane Disruption | [68,133] |
| Agasyllin | 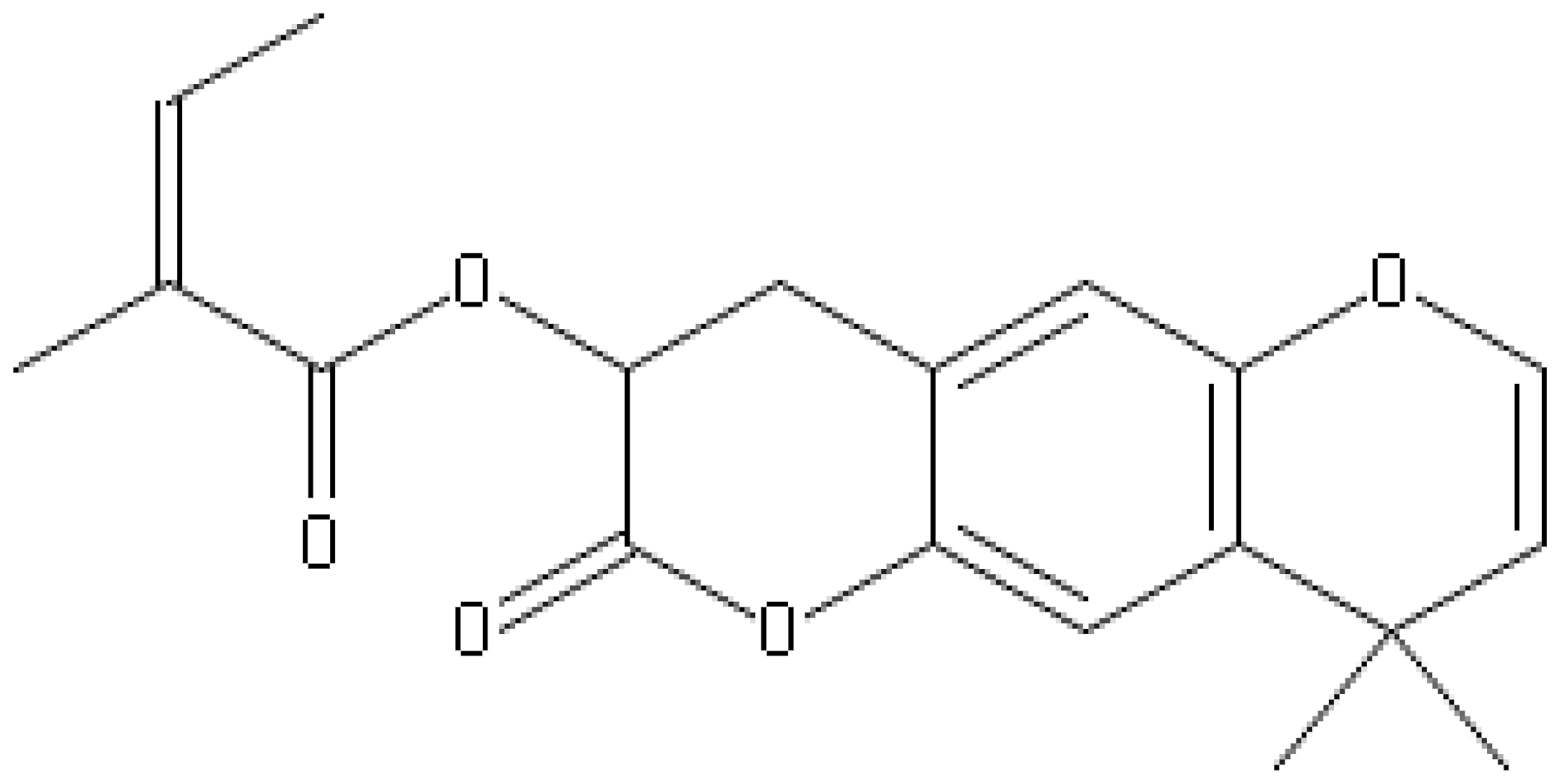 | S. aureus, S. thypii, Enterobacter cloacae and E. earogenes (MIC = 32 μg/mL) | Cell membrane Disruption | ||
| Osthole |  | DNA gyrase inhibitor | [134] | ||
| Clorobiocin | 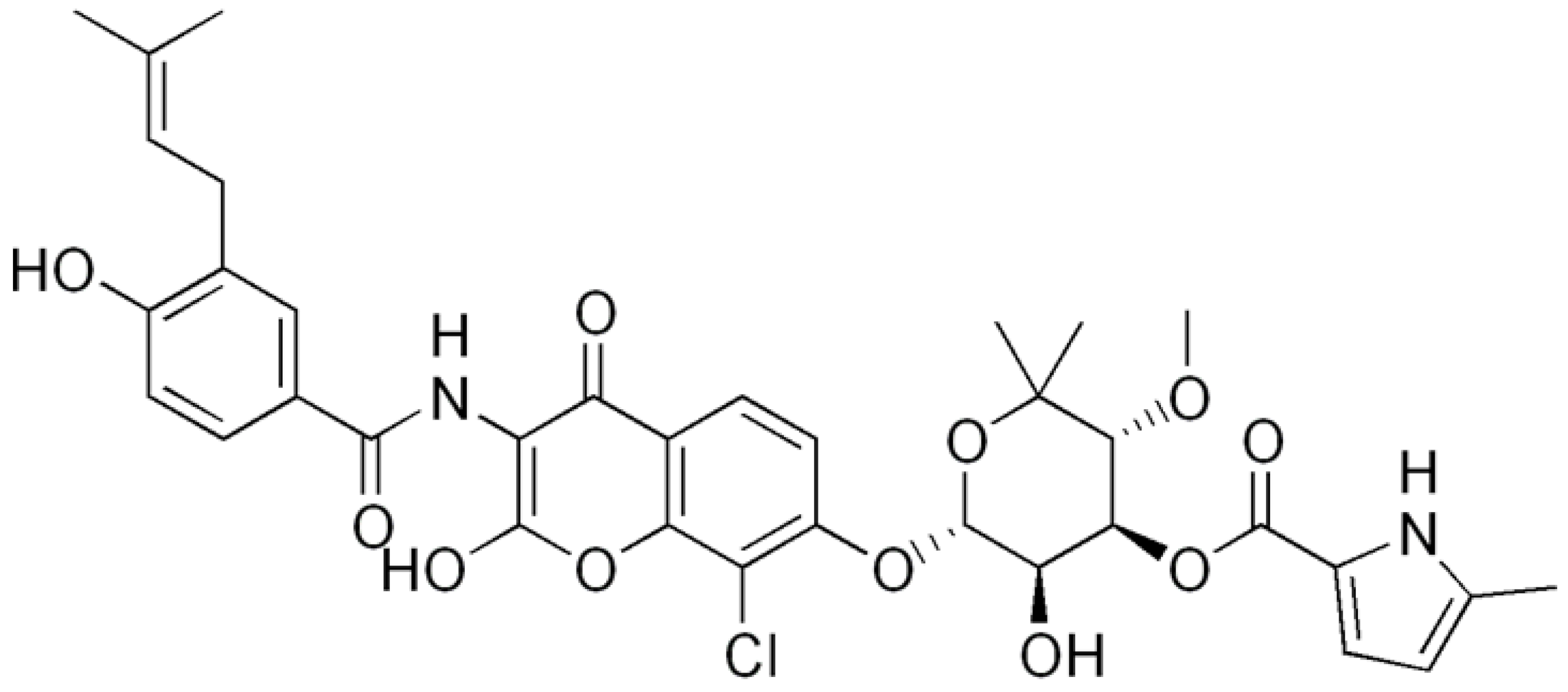 | Inhibiting of DNA topoisomerase type II (DNA gyrase) | [135,136,137] | ||
| Novobiocin | 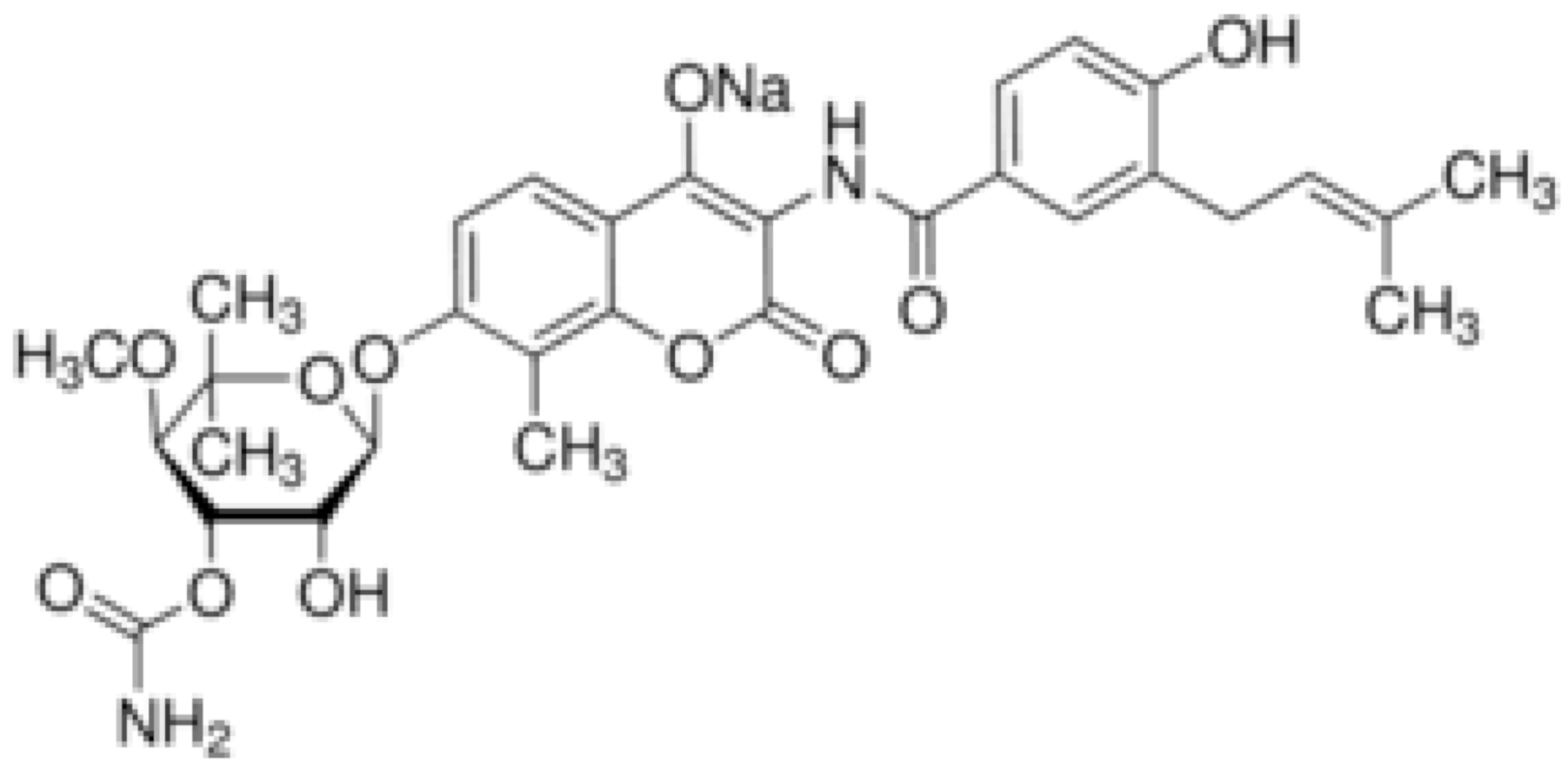 | S. aureus and S. gallinarum (MIC = 2 and 0.25 mg/L) | |||
| Coumermycin A1 | 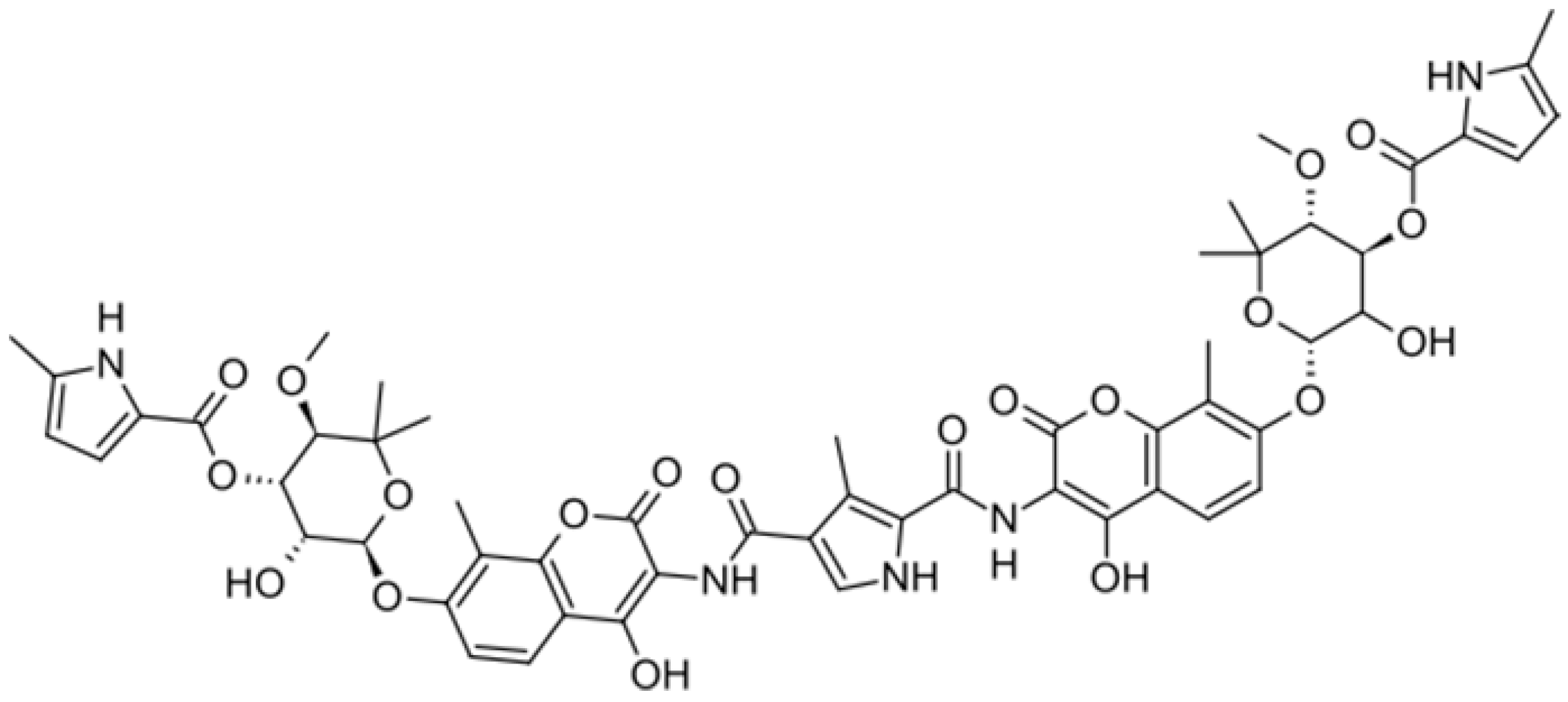 | ||||
| Bergamottin | 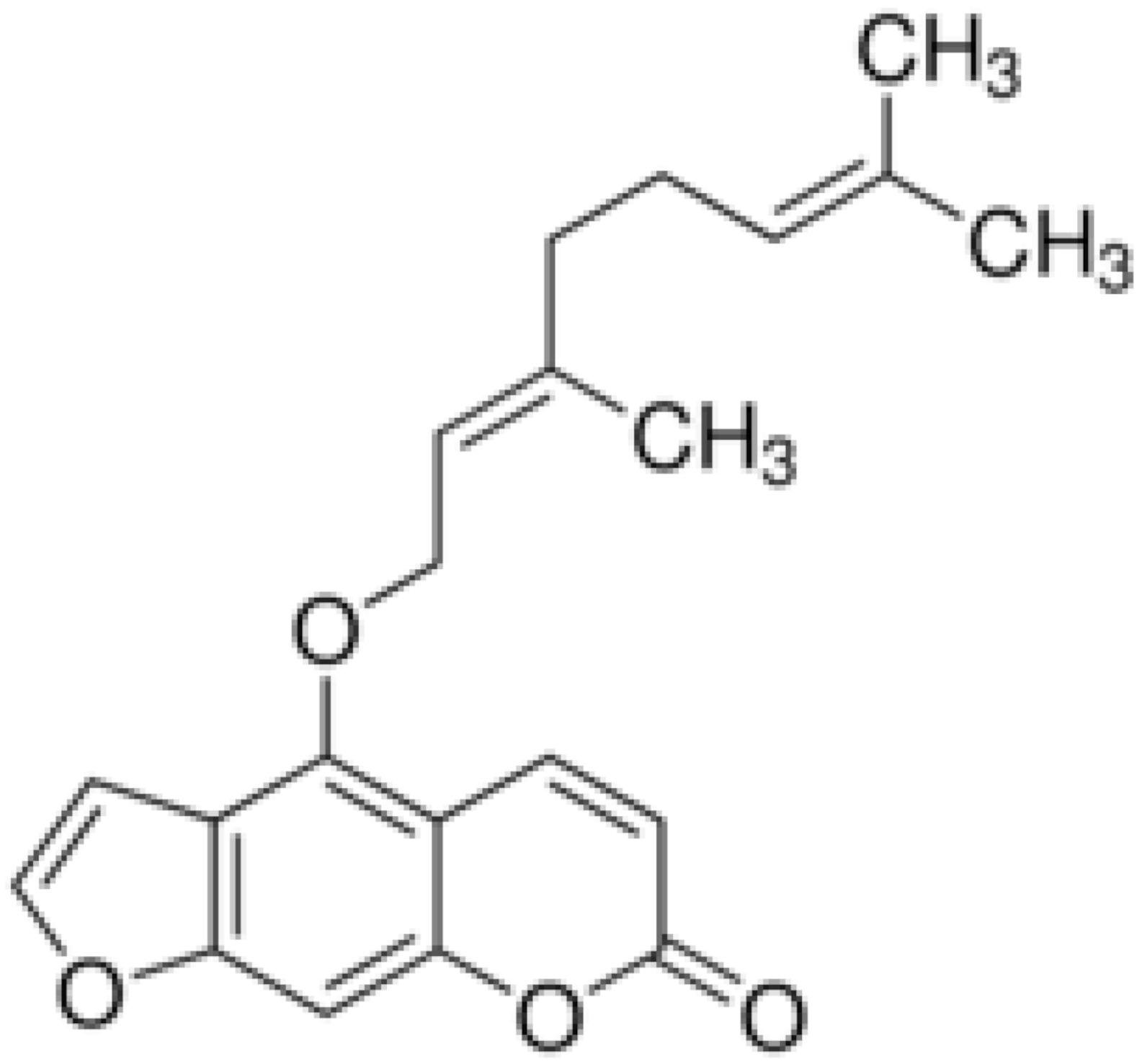 | Inhibition of efflux pump | [138,139] | ||
| 6-Geranyl coumarin | 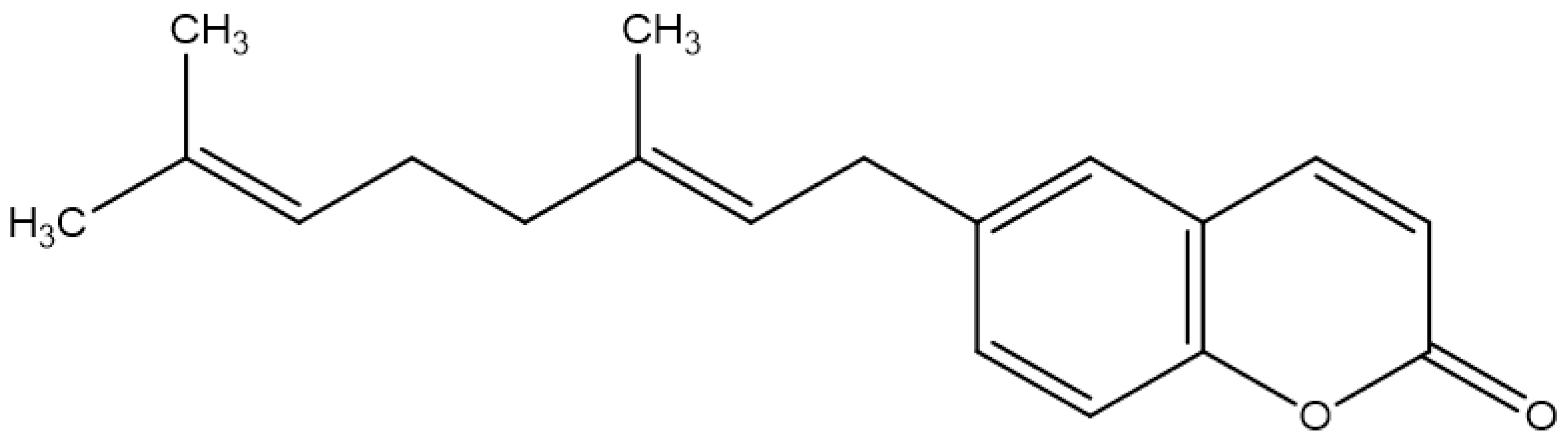 | ||||
| Gallbanic acid | 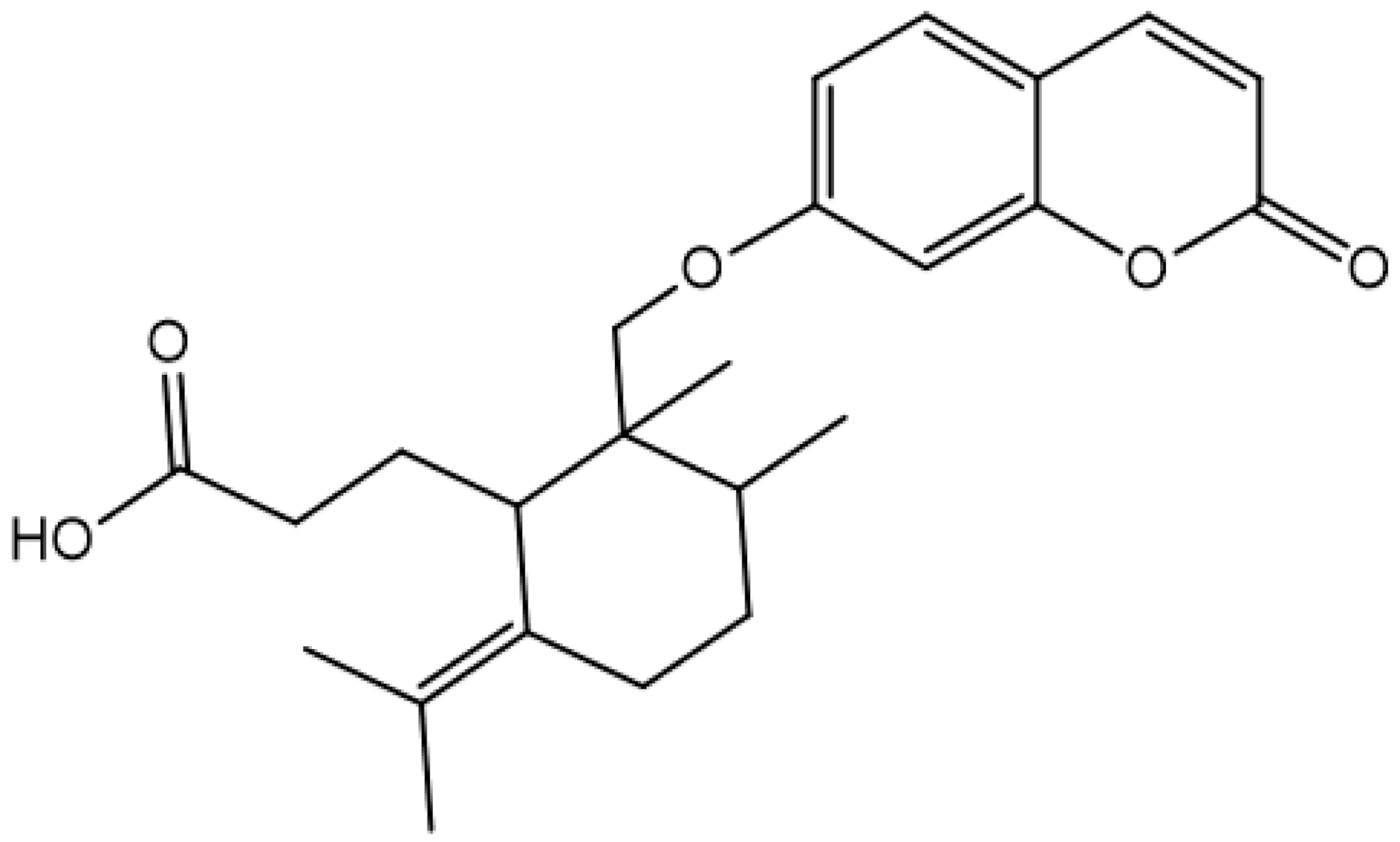 | ||||
| Daphnetin | 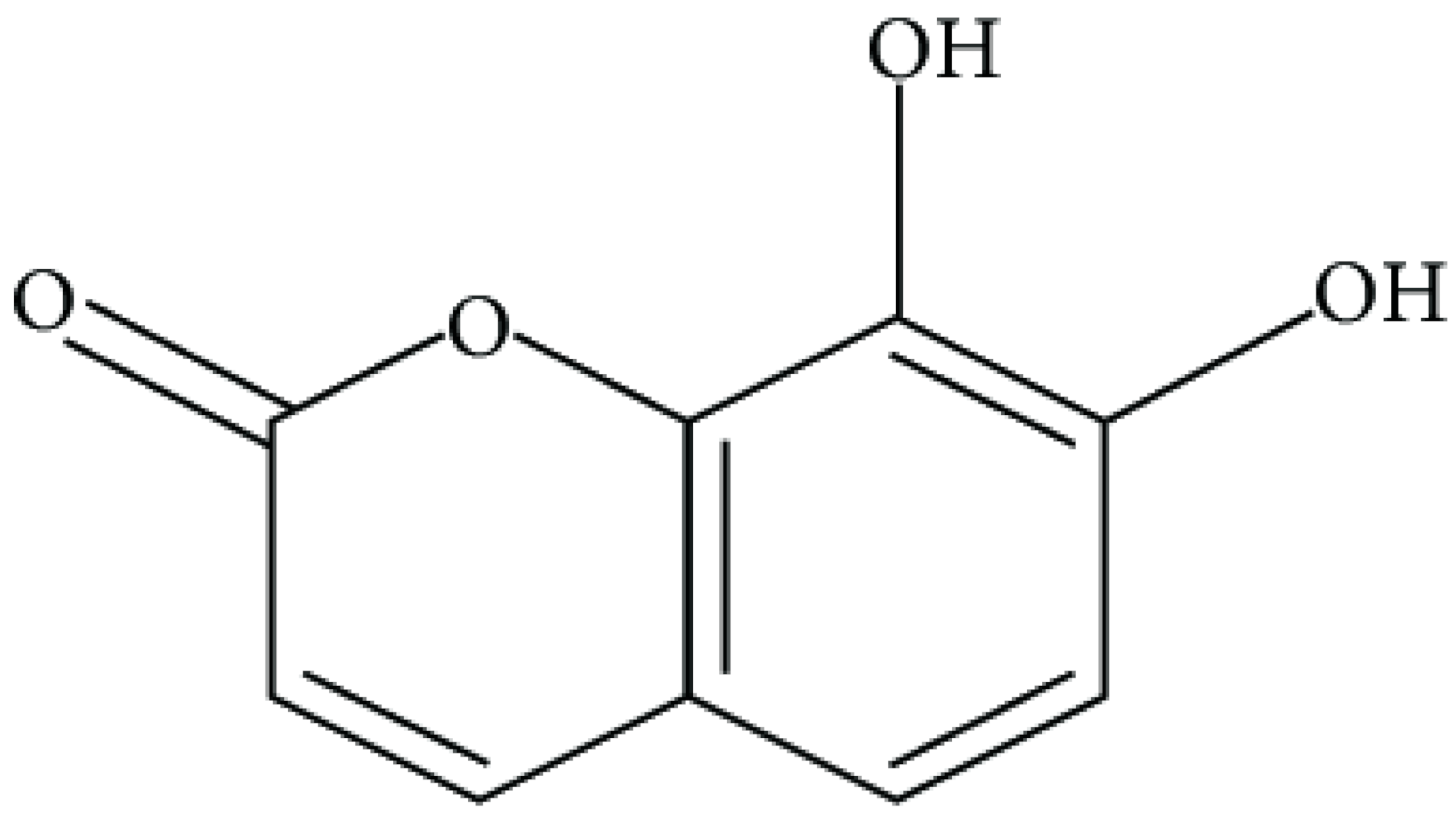 | P. fluorescens and Shewanella putrefaciens (MIC values were 0.16 and 0.08 mg/mL, respectively) | Cell membrane Disruption, Type III secretion inactivation | [140,141,142] | |
| Esculetin | 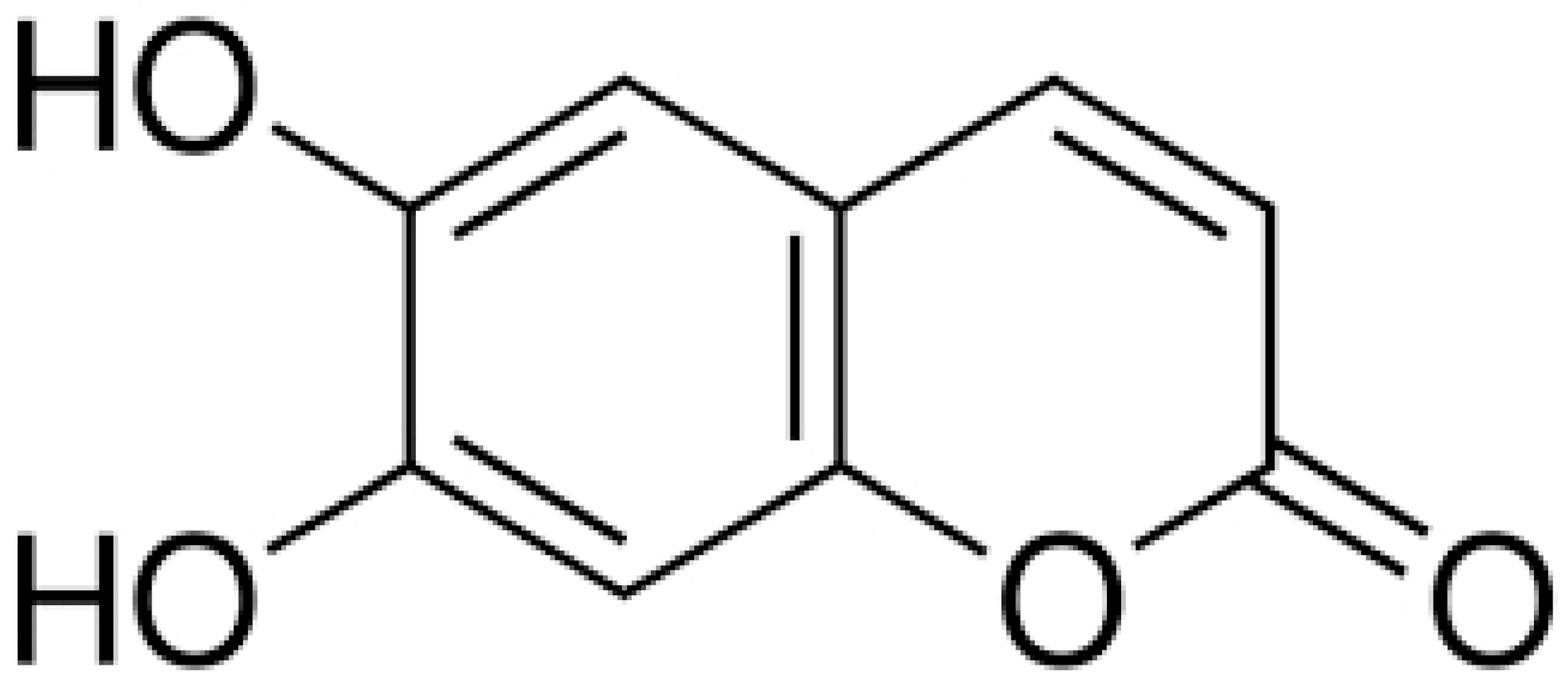 | Ralstonia pseudosolanacearum (MIC = 125 mg/mL) | |||
| Umbelliferone | 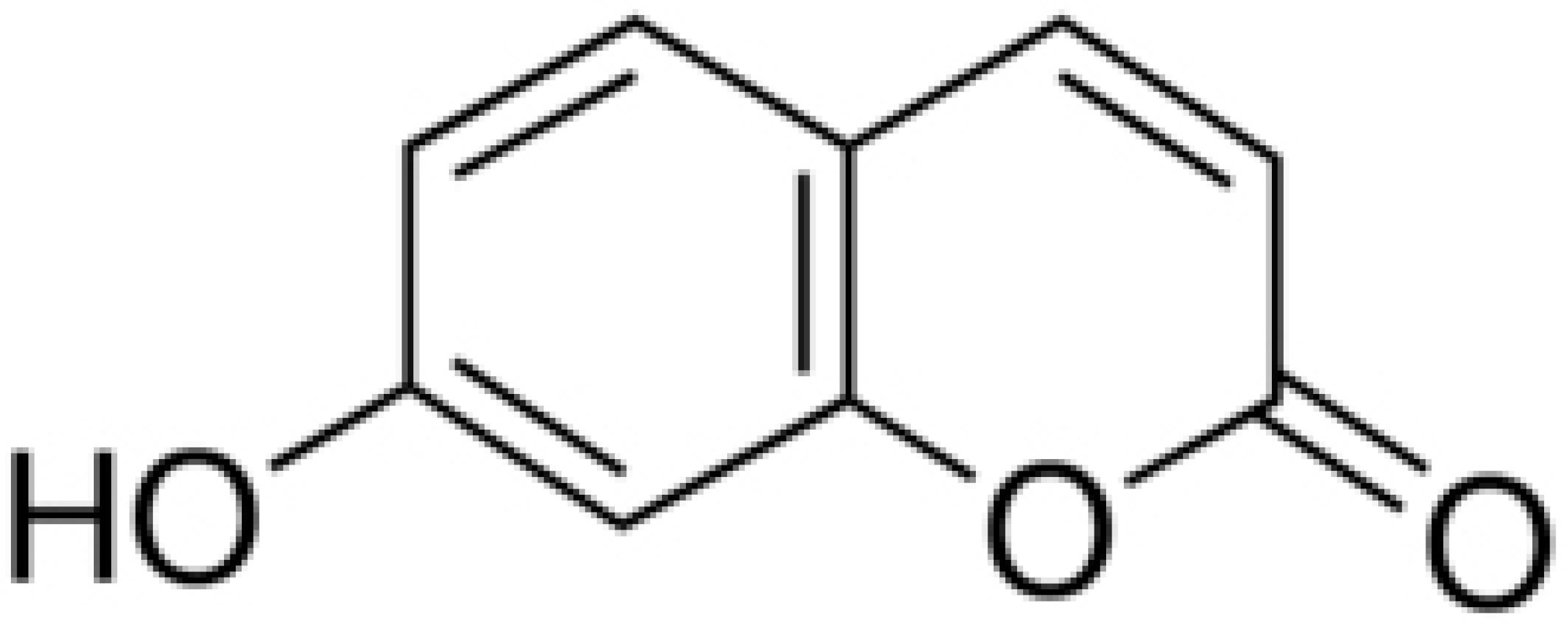 | R. pseudosolanacearum (MIC = 325 mg/mL) | |||
| Carvacrol | 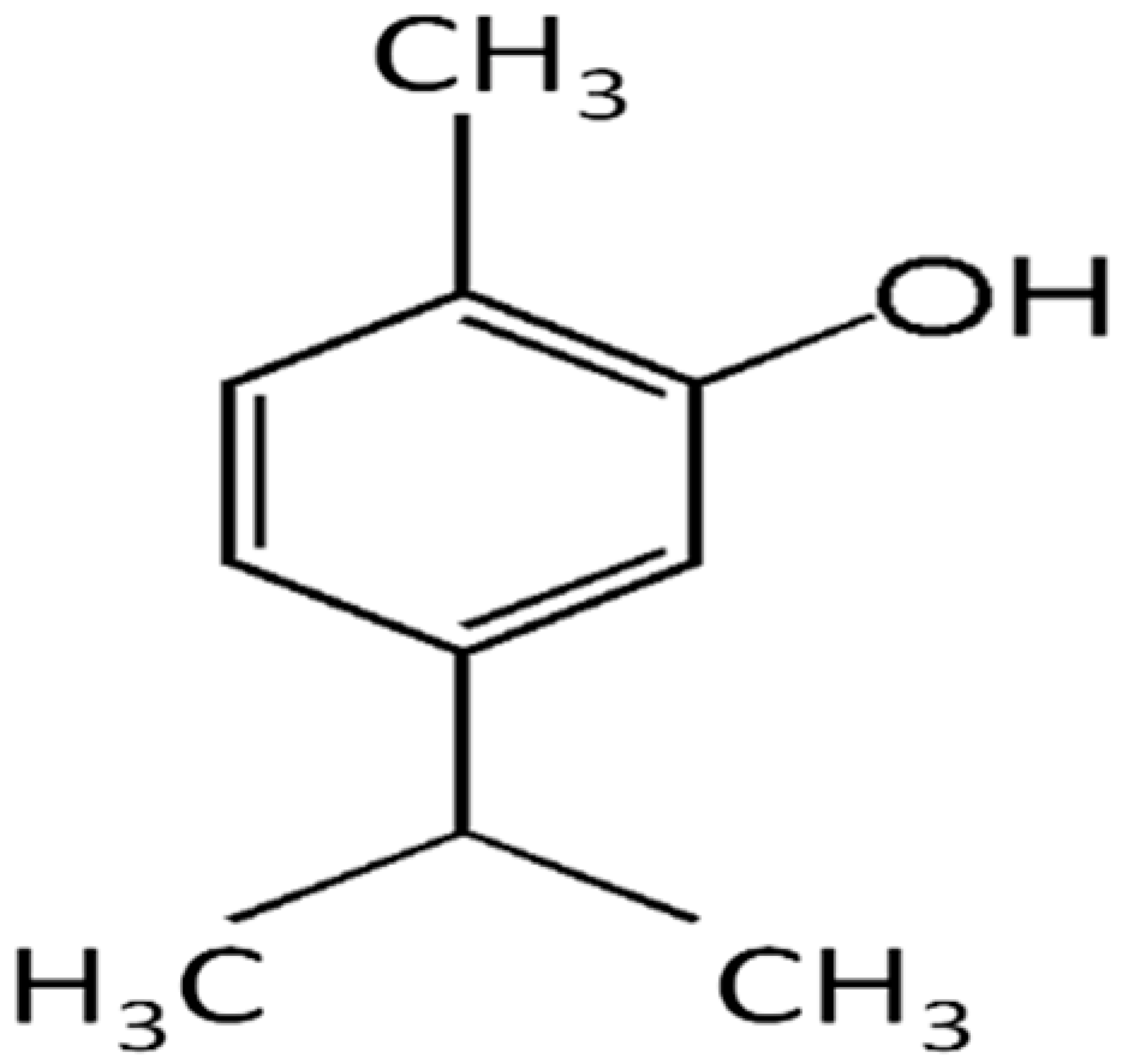 | Streptococcus pyogenes (MIC = 125 μg/mL) | Terpenes | Disrupting cell membrane integrity, Inhibition of efflux pump | [143,144,145,146,147] |
| Thymol |  | B. cereus (MIC = 0.625 mg/mL) | |||
| Soyasaponin V | 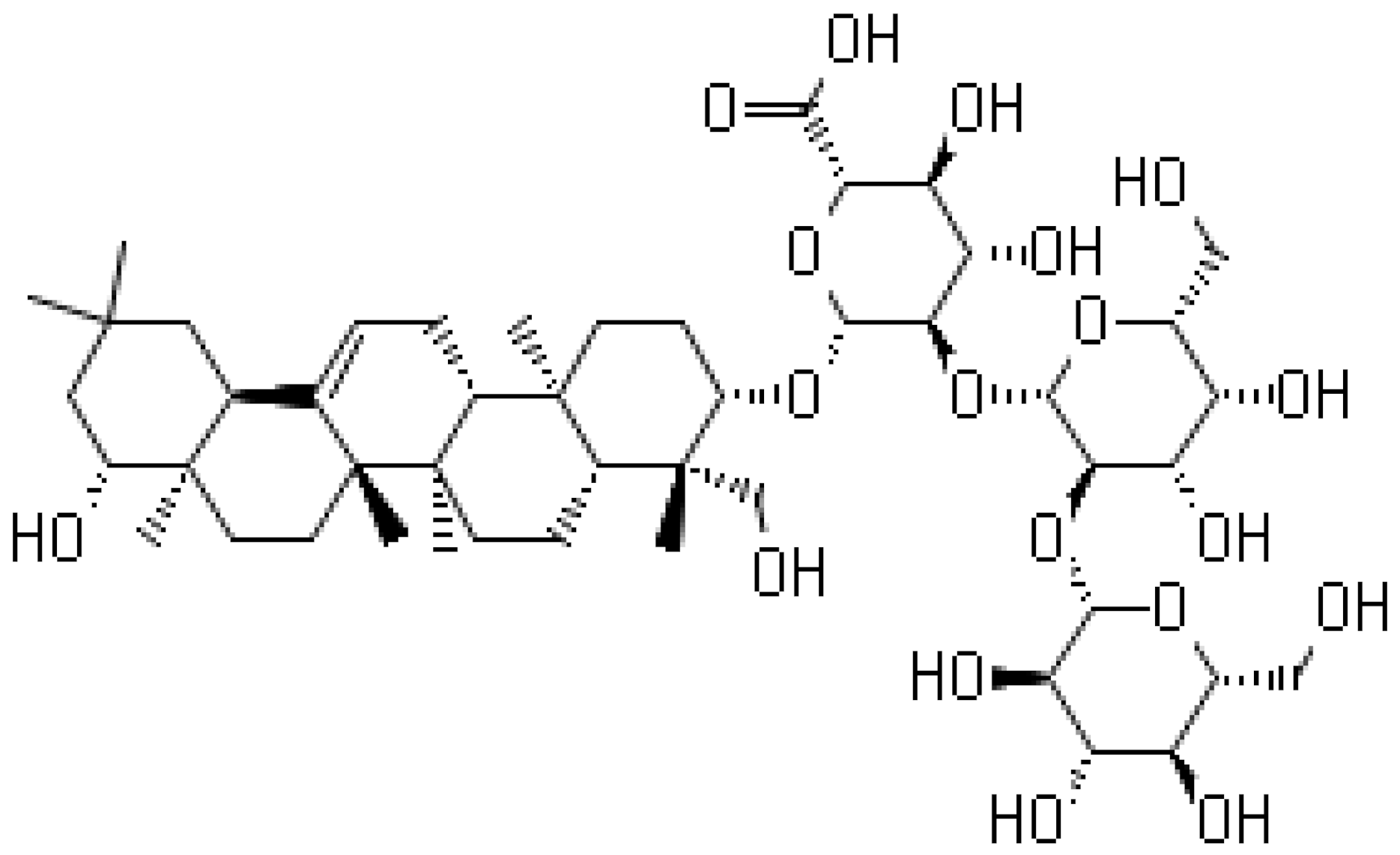 | Inhibition of the New Dehli Metallo-β-lactamase 1 | |||
| Eugenol |  | E. coli (MIC ranging from 0.0312 to 8 μg/mL) | Disrupting cell membrane integrity | ||
| α-Pinene | 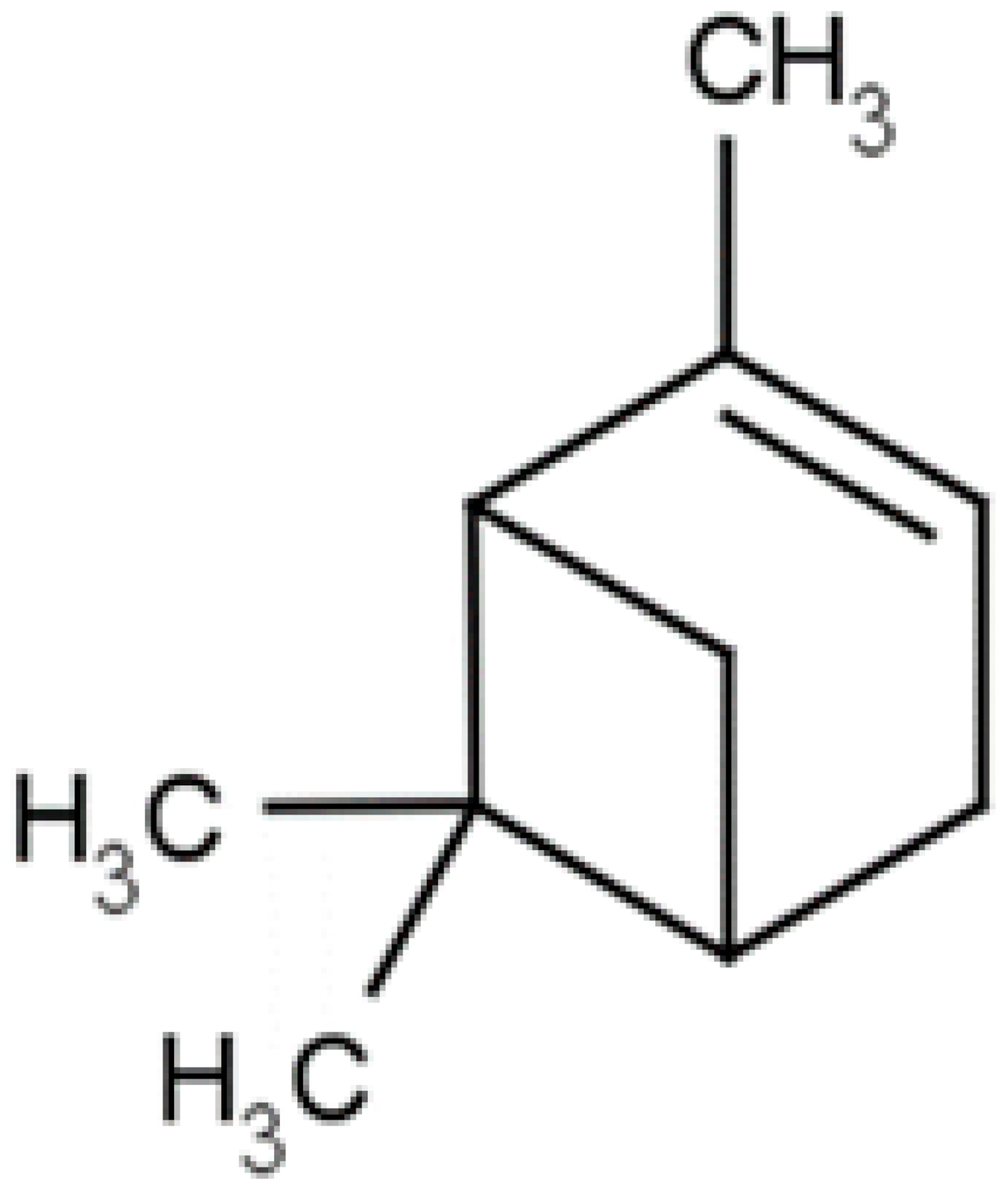 | H. pylori ( MIC ranged from 275 to 1100 μg/mL) | |||
| Limonene | 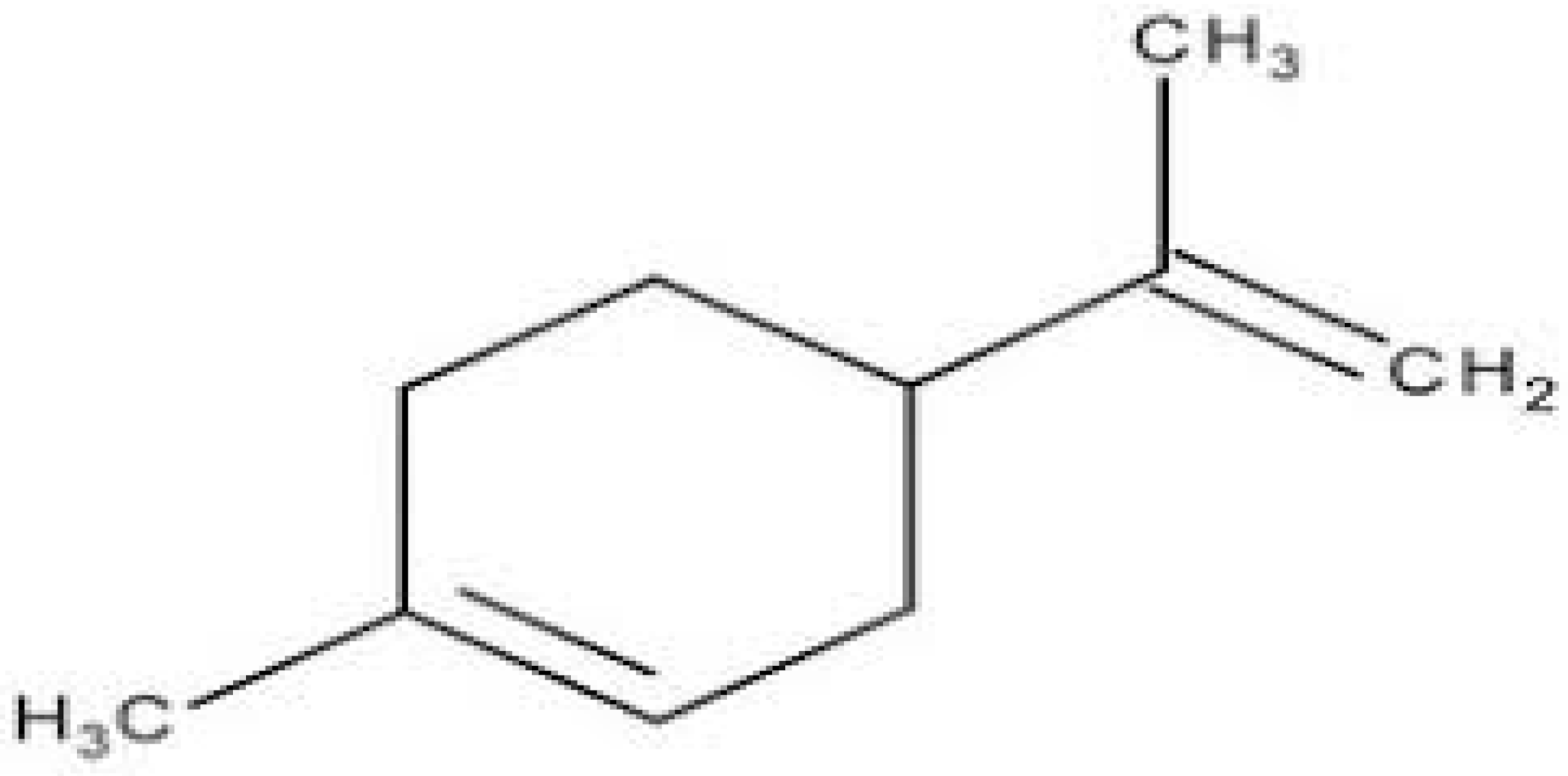 | Standard S. aureus (MIC = 256 μg/mL) and resistant P. aeruginosa (MIC = 512 μg/mL) | |||
| Menthol | 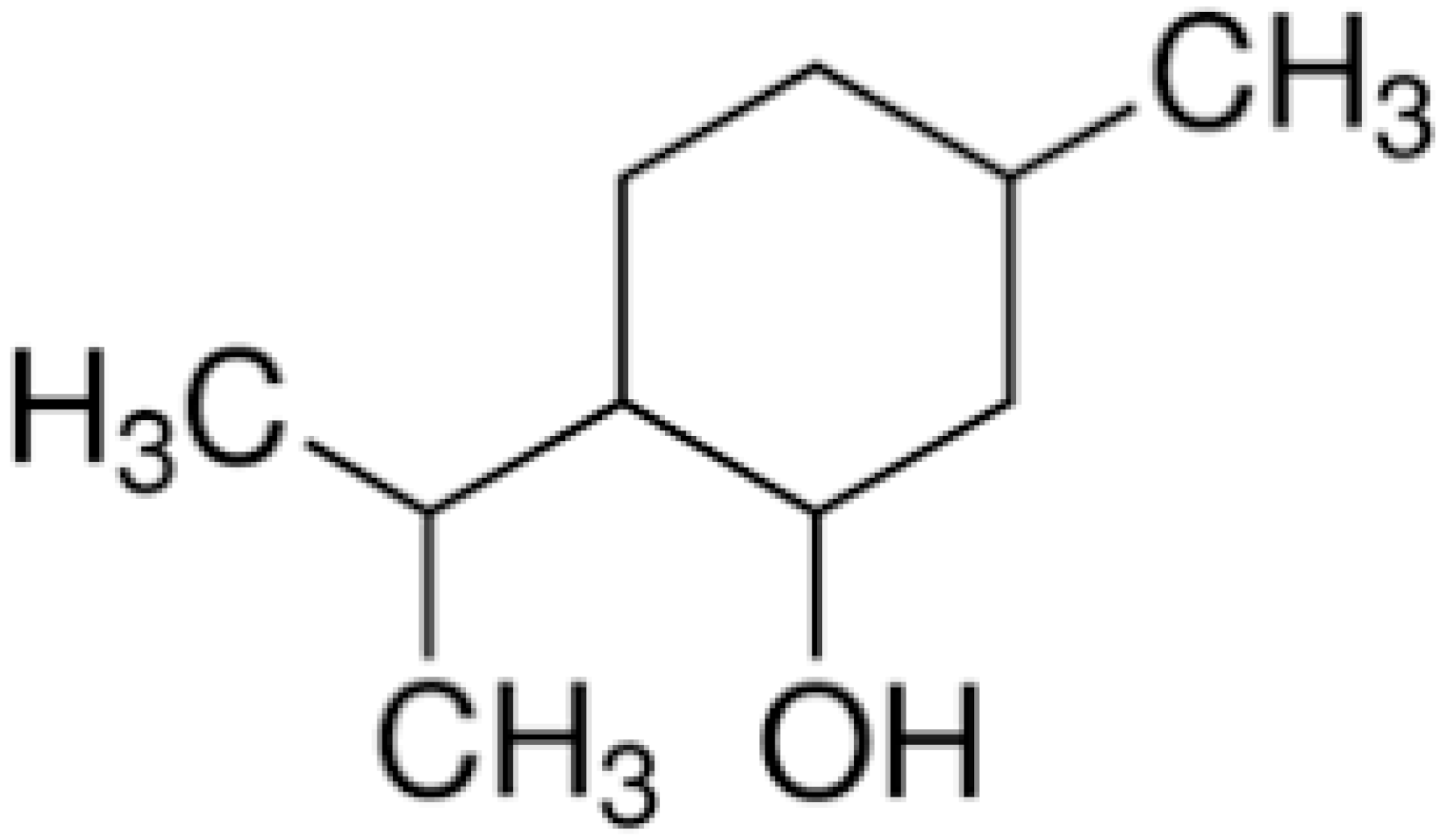 | C. albicans (MIC 90 were 1.6 to 25 μg/mL) | |||
| Farnesol | 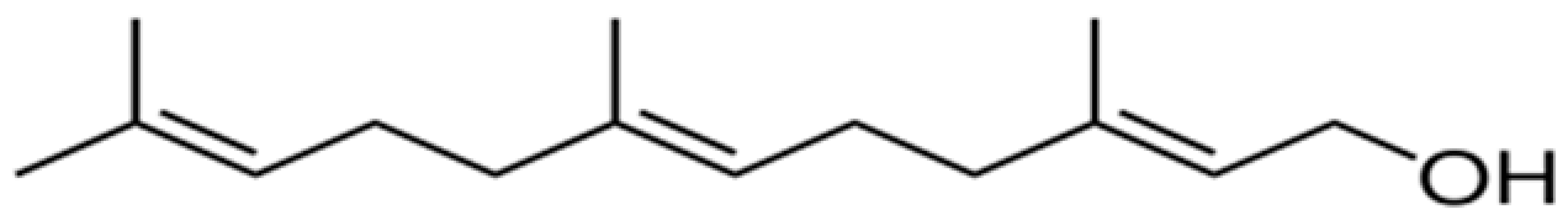 | Lactobacillus spp. (MIC = 1500 µM) | Disrupting cell membrane integrity | [148,149,150,151,152,153] | |
| Nerolidol | 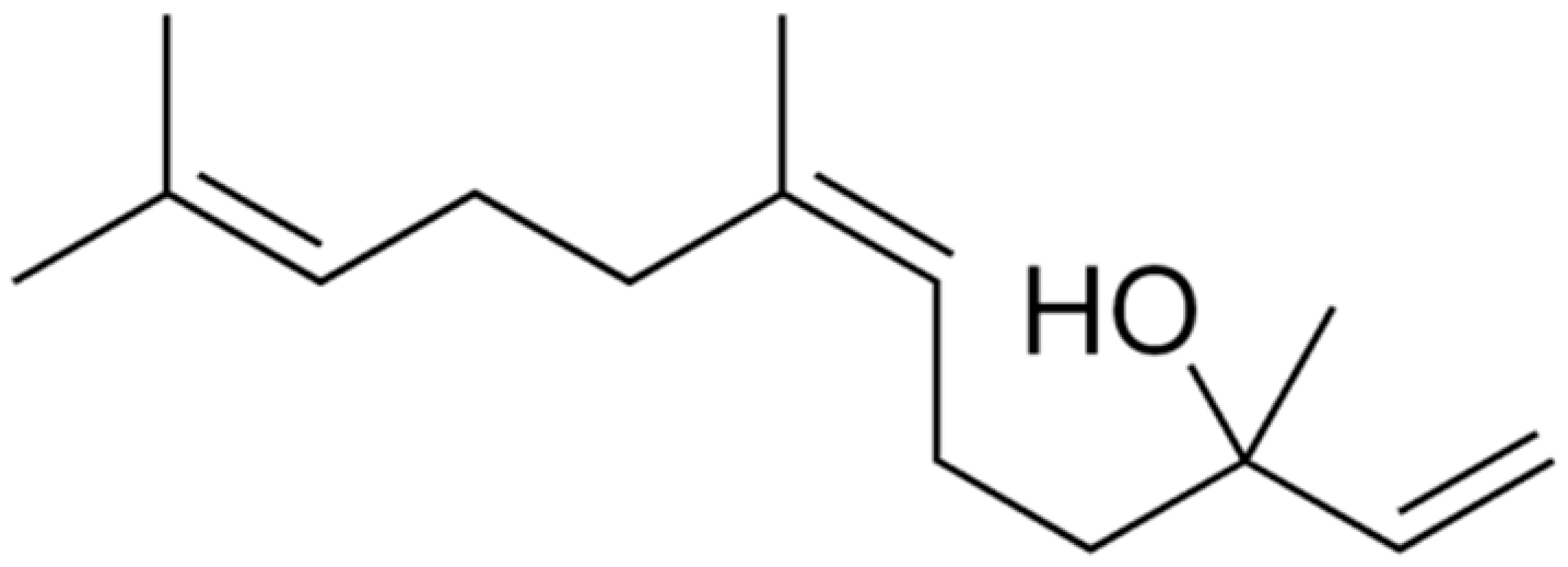 | S. aureus (MIC = 1 mg/mL), S. mutans (MIC = 4 mg/mL), P. aeruginosa (MIC = 0.5 mg/mL), and K. pneumoniae (MIC = 0.5 mg/mL). | |||
| Carvone |  | Inhibiting the transformation of cellular yeast to the filamentous | [154] | ||
| Ursolic acid |  | Carbapenem-resistant E. cloacae (MIC = 0.1 mg/mL) | Disrupting cell membrane integrity and inhibition of β-lactamase | [155,156] | |
| α-Amyrin | 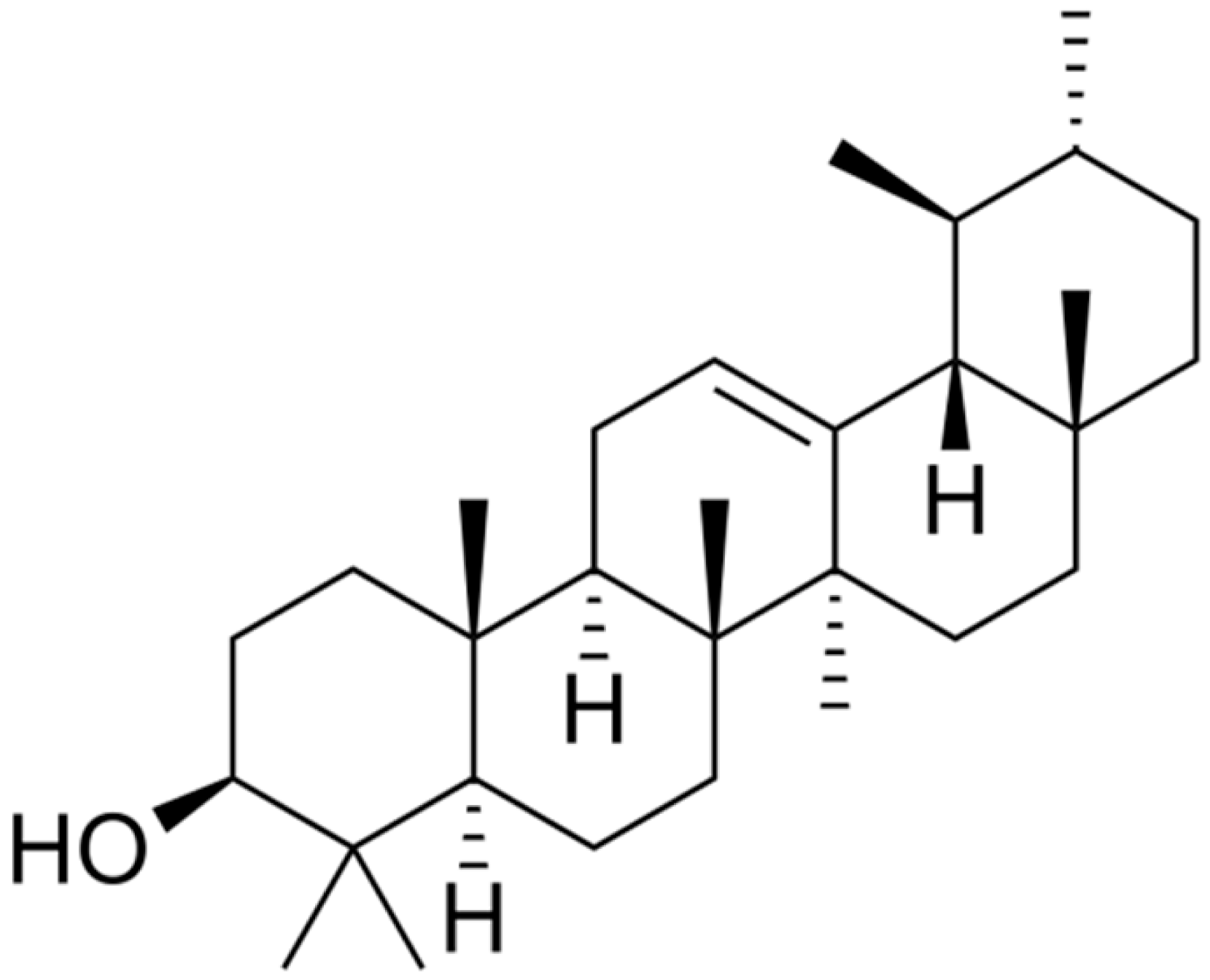 | [157] | |||
| Cinnamaldehyde | 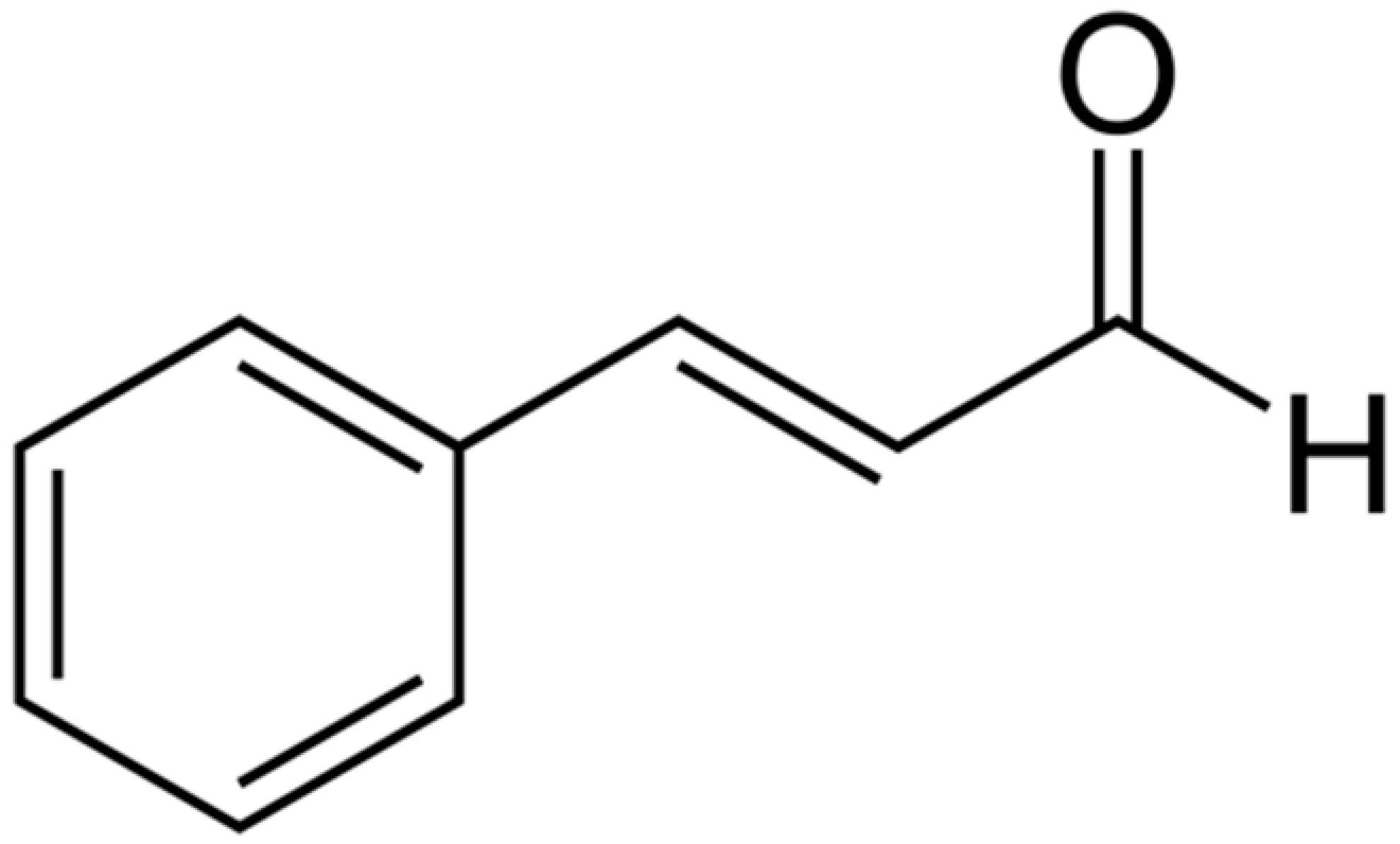 | E. coli (MIC = 780 µg/mL) | Disrupting cell membrane integrity, Decreasing membrane potential, and metabolic activity | [158,159] | |
| Artemisinin | 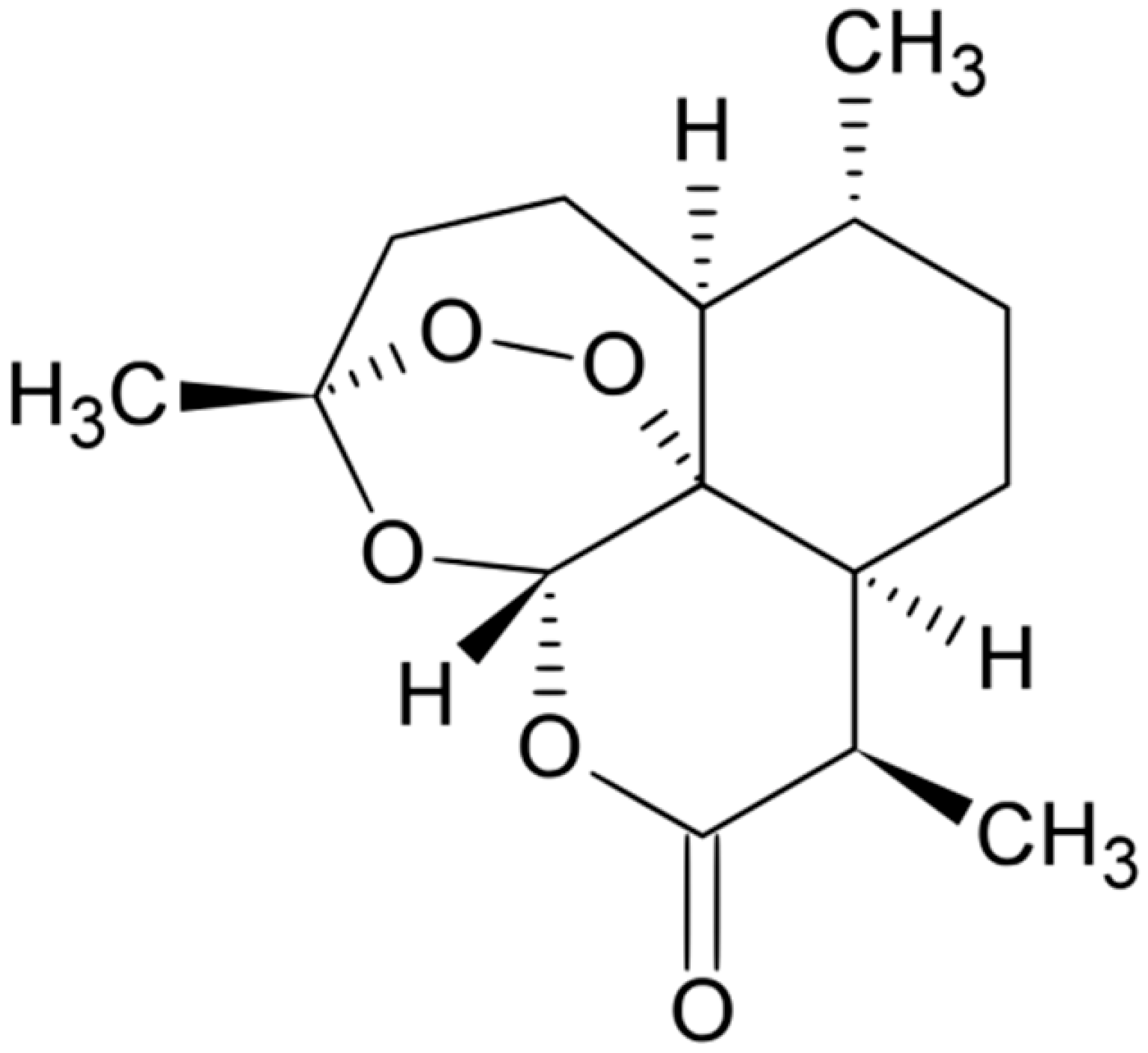 | Free radicals formation | [160] | ||
| Linalool |  | P. aeruginosa (MIC = 431 μg/mL) | Disrupting cell membrane integrity, changing in the nucleoid morphology, and interfering with cellular respiration | [161,162,163] | |
| Sabinene |  | Multi drug-resistant strains (MIC ≥ 1024 μg/mL) | Disrupting cell membrane integrity and inhibiting DNA synthesis | [164,165] | |
| α-Terpineol | 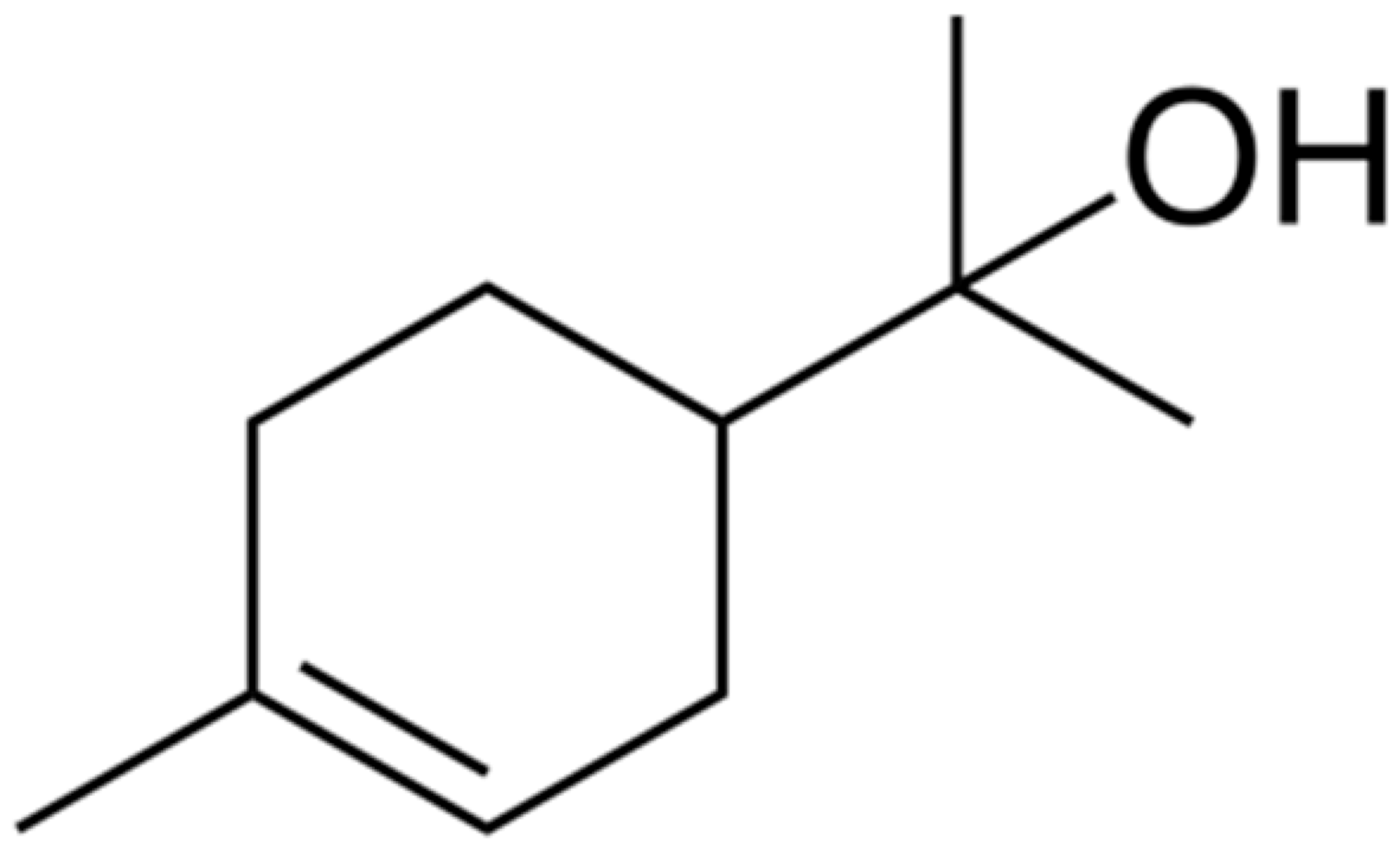 | E. coli (MIC ≥ 0.78 μg/mL) | Lossing membrane-bound autolytic enzymes, the cytoplasm leakage and inability to osmoregulate | [166,167] | |
| Citronellol |  | Trichophyton rubrum (MIC values from 8 to 1024μg/mL) | Deteriorating membrane integrity | [168,169] | |
| α-Bisabolol | 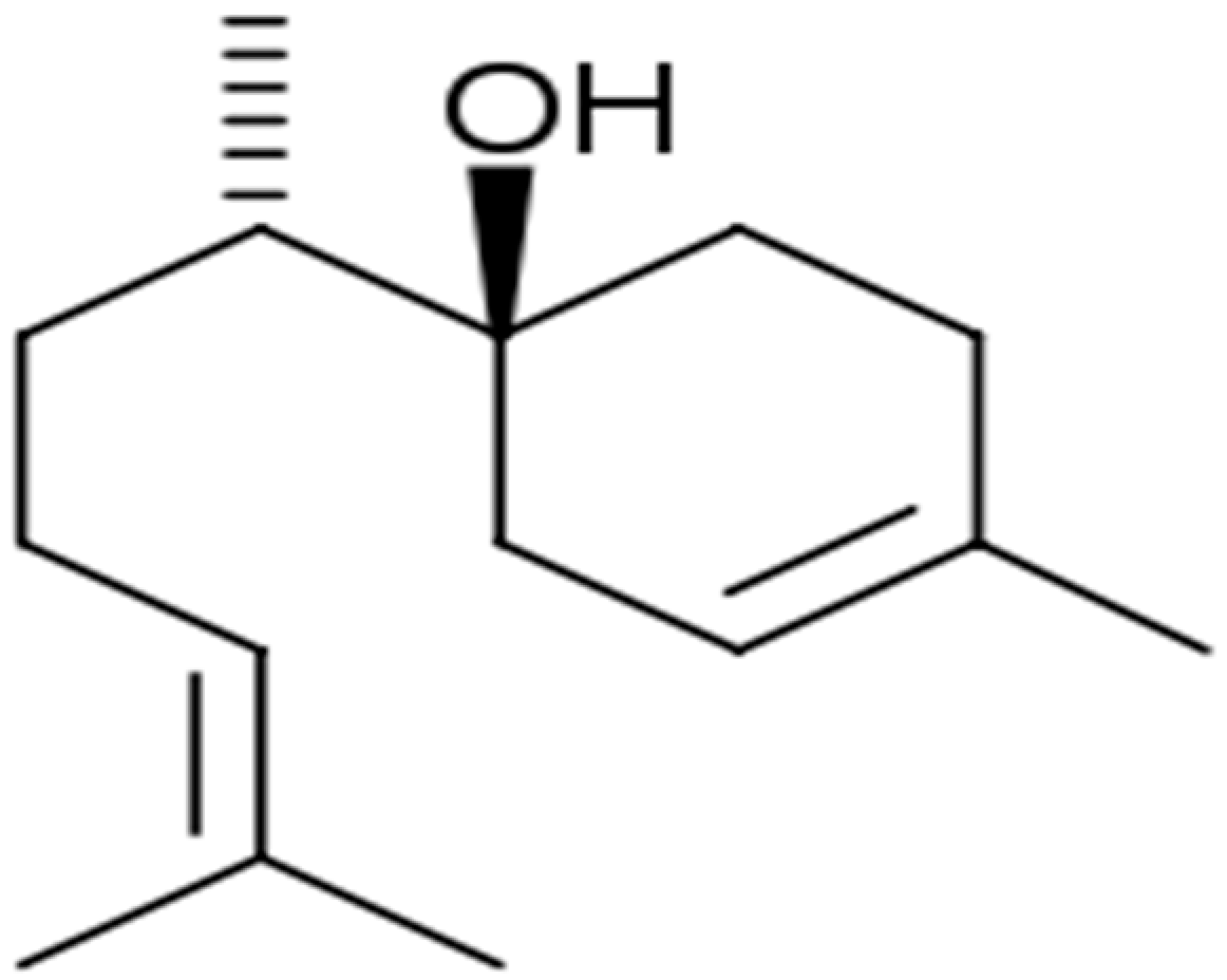 | Propionibacterium acnes and S. epidermidis (MIC = 75 and 37.5 μg/mL) | Disrupting cell membrane integrity | [170,171] |
4. Preclinical and Clinical Studies on Antibacterial Effects of Phytochemicals
4.1. Concentrated Herbal Extract Granules TRA
4.2. Uva Ursi Extract
4.3. Vaccinium spp.
4.4. Sanguiritrin
4.5. Eucalimin
4.6. Scutellaria baicalensis Georgi
4.7. Houttuynia Cordata Thunb.
4.8. Berberine
4.9. Mastic
4.10. GutGard
4.11. Listerine
4.12. Parodontax
4.13. Myrtol
4.14. Tea Tree Oil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Date Availability Statement
Conflicts of Interest
References
- Sarwar, A.; Butt, M.A.; Hafeez, S.; Danish, M.Z. Rapid emergence of antibacterial resistance by bacterial isolates from patients of gynecological infections in Punjab, Pakistan. J. Infect. Public Health 2020, 13, 1972–1980. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Han, X.; Wang, N.; Cai, Y.; Wang, H.; Yu, J.; Zhang, X.; Zhang, K. Impact of antibiotic prescription on the resistance of Klebsiella pneumoniae at a tertiary hospital in China, 2012–2019. Am. J. Infect. Control 2020, 49, 65–69. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 1–28. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Bazzaz, B.S.F. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Control CFD and Prevention. Antibiotic Resistance Threats in the United States; US Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Fleming, A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An Enzyme from Bacteria able to Destroy Penicillin. Nature 1940, 146, 837. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Fatemi, N.; Sharifmoghadam, M.R.; Bahreini, M.; Khameneh, B.; Shadifar, H. Antibacterial and Synergistic Effects of Herbal Extracts in Combination with Amikacin and Imipenem Against Multidrug-Resistant Isolates of Acinetobacter. Curr. Microbiol. 2020, 77, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Shadifar, H.; Bahreini, M.; Khameneh, B.; Emami, S.A.; Fatemi, N.; Sharifmoghadam, M.R. Antibacterial and synergistic effects of aqueous and methanol extracts of artemisia annua against multidrug-resistant isolates of acinetobacter. Anti-Infect. Agents 2021, 19, 28–35. [Google Scholar] [CrossRef]
- Jaktaji, R.P.; Ghalamfarsa, F. Antibacterial activity of honeys and potential synergism of honeys with antibiotics and alkaloid extract of Sophora alopecuroides plant against antibiotic-resistant Escherichia coli mutant. Iran. J. Basic Med. Sci. 2021, 24, 623–628. [Google Scholar] [CrossRef]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Abbas Bukhari, S.N. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran. J. Basic Med. Sci. 2020, 23, 1501–1526. [Google Scholar] [CrossRef] [PubMed]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, B.S.F.; Haririzadeh, G.; Imami, S.A.; Rashed, M.H. Survey of Iranian Plants for Alkaloids, Flavonoids, Saponins, and Tannins [Khorasan Province]. Int. J. Pharmacogn. 1997, 35, 17–30. [Google Scholar] [CrossRef]
- El Kolli, M.; Laouer, H.; El Kolli, H.; Akkal, S.; Sahli, F. Chemical analysis, antimicrobial and anti-oxidative properties of Daucus gracilis essential oil and its mechanism of action. Asian Pac. J. Trop. Biomed. 2016, 6, 8–15. [Google Scholar] [CrossRef]
- Salarbashi, D.; Fazly Bazzaz, B.S.; Karimkhani, M.M.; Sabeti Noghabi, Z.; Khanzadeh, F.; Sahebkar, A. Oil stability index and biological activities of Achillea biebersteinii and Achillea wilhelmsii extracts as influenced by various ultrasound intensities. Ind. Crops. Prod. 2014, 55, 163–172. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Seyedi, S.; Goki, N.H.; Khameneh, B. Human Antimicrobial Peptides: Spectrum, Mode of Action and Resistance Mechanisms. Int. J. Pept. Res. Ther. 2021, 27, 801–816. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Khameneh, B.; Namazi, N.; Iranshahi, M.; Davoodi, D.; Golmohammadzadeh, S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: The novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett. Appl. Microbiol. 2018, 66, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Gemeda, N.; Tadele, A.; Lemma, H.; Girma, B.; Addis, G.; Tesfaye, B.; Abebe, A.; Gemechu, W.; Yirsaw, K.; Teka, F.; et al. Development, Characterization, and Evaluation of Novel Broad-Spectrum Antimicrobial Topical Formulations from Cymbopogon martini (Roxb.) W. Watson Essential Oil. Evid-Based Complement. Altern. Med. 2018, 2018, 9812093. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharm. Rev. 2017, 11, 57–72. [Google Scholar]
- Chitsazian-Yazdi, M.; Agnolet, S.; Lorenz, S.; Schneider, B.; Es’haghi, Z.; Kasaian, J.; Khameneh, B.; Iranshahi, M. Foetithiophenes C-F, thiophene derivatives from the roots of Ferula foetida. Pharm. Biol. 2015, 53, 710–714. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Dhayakaran, R.; Neethirajan, S.; Weng, X. Investigation of the antimicrobial activity of soy peptides by developing a high throughput drug screening assay. Biochem. Biophys. Rep. 2016, 6, 149–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eduati, F.; Utharala, R.; Madhavan, D.; Neumann, U.P.; Longerich, T.; Cramer, T.; Saez-Rodriguez, J.; Merten, C.A. A microfluidics platform for combinatorial drug screening on cancer biopsies. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Kleymann, G.; Werling, H.O. A generally applicable, high-throughput screening-compatible assay to identify, evaluate, and optimize antimicrobial agents for drug therapy. J. Biomol. Screen. 2004, 9, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and Characterisation of Antimicrobial Properties of Semisynthetic Betulin Derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef] [PubMed]
- Ymele-Leki, P.; Cao, S.; Sharp, J.; Lambert, K.G.; McAdam, A.J.; Husson, R.N.; Tamayo, G.; Clardy, J.; Watnick, P.I. A high-throughput screen identifies a new natural product with broad-spectrum antibacterial activity. PLoS ONE 2012, 7, e31307. [Google Scholar] [CrossRef]
- Sorokina, M.; Steinbeck, C. Review on natural products databases: Where to find data in 2020. J. Chemin. 2020, 12, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Phatak, S.S.; Stephan, C.C.; Cavasotto, C.N. High-throughput and in silico screenings in drug discovery. Expert Opin. Drug Discov. 2009, 4, 947–959. [Google Scholar] [CrossRef]
- Skariyachan, S.; Muddebihalkar, A.G.; Badrinath, V.; Umashankar, B.; Eram, D.; Uttarkar, A.; Niranjan, V. Natural epiestriol-16 act as potential lead molecule against prospective molecular targets of multidrug resistant Acinetobacter baumannii-Insight from in silico modelling and in vitro investigations. Infect. Genet. Evol. 2020, 82, 104314. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2020, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Khameneh, B.; Ostad, M.R.Z.; Hosseinzadeh, H. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J. Phytomedicine 2018, 8, 246–253. [Google Scholar]
- Ohene-Agyei, T.; Mowla, R.; Rahman, T.; Venter, H. Phytochemicals increase the antibacterial activity of antibiotics by acting on a drug efflux pump. Microbiologyopen 2014, 3, 885–896. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Teng, L.; Fateeva, T.V.; Brown, J.C.; Job, K.M.; Bortnikova, V.V.; Krepkova, L.; Gubarev, M.I.; Sherwin, C. Phytotherapy as an alternative to conventional antimicrobials: Combating microbial resistance. Expert Rev. Clin. Pharmacol. 2017, 10, 1203–1214. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Hu, Z.-Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef]
- Zhao, W.H.; Asano, N.; Hu, Z.Q.; Shimamura, T. Restoration of antibacterial activity of beta-lactams by epigallocatechin gallate against beta-lactamase-producing species depending on location of beta-lactamase. J. Pharm. Pharmacol. 2003, 55, 735–740. [Google Scholar] [CrossRef]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef]
- Eumkeb, G.; Sakdarat, S.; Siriwong, S. Reversing β-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum Hance and synergism with ceftazidime. Phytomedicine 2010, 18, 40–45. [Google Scholar] [CrossRef]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De Vliegher, S.; Fox, L.; Piepers, S.; McDougall, S.; Barkema, H. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Maia, N.L.; De Barros, M.; De Oliveira, L.L.; Cardoso, S.; Dos Santos, M.; Pieri, F.; Ramalho, T.C.; Da Cunha, E.F.F.; Moreira, M.A.S. Synergism of Plant Compound With Traditional Antimicrobials Against Streptococcus spp. Isolated From Bovine Mastitis. Front. Microbiol. 2018, 9, 1203. [Google Scholar] [CrossRef]
- Dias, K.S.; Januário, J.P.; Dego, J.L.D.; Dias, A.L.T.; dos Santos, M.H.; Camps, I.; Coelho, L.F.; Viegas, C., Jr. Semisynthesis and antimicrobial activity of novel guttiferone-A derivatives. Bioorganic Med. Chem. 2012, 20, 2713–2720. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, S.R.S.; Sivakumar, N.; Vijayaraghavan, P. Enzymatic Inhibition of Phytochemical from Garcinia imberti on Homology Modelled Beta-lactamase Protein in Staphylococcus sciuri. J. Young Pharm. 2020, 12, 37–41. [Google Scholar] [CrossRef]
- Klancnik, A.; Sikic Pogacar, M.; Trost, K.; Tusek Znidaric, M.; Mozetic Vodopivec, B.; Smole Mozina, S. Anti-Campylobacter activity of resveratrol and an extract from waste Pinot noir grape skins and seeds, and resistance of Camp. jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J. Appl. Microbiol. 2017, 122, 65–77. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol enhances the antimicrobial effect of polymyxin B on Klebsiella pneumoniae and Escherichia coli isolates with polymyxin B resistance. BMC Microbiol. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, S.-B.; Park, S.-J.; Seo, Y.-S.; Gong, R.; Choi, J.-G.; Shin, N.-W.; Rho, J.-R.; Kang, O.-H.; et al. The Mechanism of Antimicrobial Activity of Sophoraflavanone B Against Methicillin-ResistantStaphylococcus aureus. Foodborne Pathog. Dis. 2014, 11, 234–239. [Google Scholar] [CrossRef]
- Cha, J.D.; Moon, S.-E.; Kim, J.-Y.; Jung, E.-K.; Lee, Y.-S. Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2009, 23, 1326–1331. [Google Scholar] [CrossRef]
- Yun, B.-Y.; Zhou, L.; Xie, K.-P.; Wang, Y.-J.; Xie, M.-J. Antibacterial activity and mechanism of baicalein. Yao Xue Xue Bao Acta Pharm. Sin. 2012, 47, 1587–1592. [Google Scholar]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and Mode of Action of Ceftazidime plus Quercetin or Luteolin onStreptococcus pyogenes. Evid.-Based Complement. Altern. Med. 2015, 2015, 759459. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant Defense Against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, J.-S.; Fu, Q.; Zhang, H.-T. Antibacterial Activity and Mechanism of Berberine from the Fruit of Berberis poiretii. Shipin Kexue/Food Sci. 2020, 41, 29–34. [Google Scholar]
- Kristiansen, M.M.; Leandro, C.; Ordway, D.; Martins, M.; Viveiros, M.; Pacheco, T.; Molnar, J.E.; Kristiansen, J.; Amaral, L. Thioridazine reduces resistance of methicillin-resistant staphylococcus aureus by inhibiting a reserpine-sensitive efflux pump. In Vivo 2006, 20, 361–366. [Google Scholar] [PubMed]
- Beuria, T.K.; Santra, A.M.K.; Panda, D. Sanguinarine Blocks Cytokinesis in Bacteria by Inhibiting FtsZ Assembly and Bundling. Biochemistry 2005, 44, 16584–16593. [Google Scholar] [CrossRef] [PubMed]
- Evstigneev, M.; Rybakova, K.; Davies, D. Complexation of norfloxacin with DNA in the presence of caffeine. Biophys. Chem. 2006, 121, 84–95. [Google Scholar] [CrossRef][Green Version]
- Iranshahi, M.; Hassanzadeh, K.M.; Bazzaz, S.F.B.; Sabeti, Z. High Content of Polysulphides in the Volatile Oil of Ferula latisecta Rech. F. et Aell. Fruits and Antimicrobial Activity of the Oil. J. Essent. Oil Res. 2008, 20, 183–185. [Google Scholar] [CrossRef]
- Sarfraz, M.; Nasim, M.J.; Jacob, C.; Gruhlke, M.C.H. Efficacy of Allicin against Plant Pathogenic Fungi and Unveiling the Underlying Mode of Action Employing Yeast Based Chemogenetic Profiling Approach. Appl. Sci. 2020, 10, 2563. [Google Scholar] [CrossRef]
- Lin, C.-M.; Preston, J.F.; Wei, C.-I. Antibacterial Mechanism of Allyl Isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant Activities of Coumarins from the Roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Patents Anti-Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Contreras, M.D.M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Ghandadi, M.; Atashbeyk, D.G.; Bazzaz, B.S.F.; Iranshahi, M. Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistantStaphylococcus aureus. Drug Dev. Ind. Pharm. 2014, 41, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.N.H.W.; Hashim, N.A.; Fabarani, N.P.; Ahmad, F. Antibacterial activity of constituents from piper retrofractum vahl. and piper arborescens roxb. Agric. Conspec. Sci. 2020, 85, 269–280. [Google Scholar]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine Targets Assembly of Escherichia coli Cell Division Protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.-B.; Yu, Y.; Liang, Y.-Z.; Zeng, B. Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. J. Pharm. Biomed. Anal. 2007, 44, 301–304. [Google Scholar] [CrossRef]
- Poopedi, E.; Marimani, M.; AlOmar, S.Y.; Aldahmash, B.; Ahmad, A. Modulation of antioxidant defence system in response to berberine in Candida albicans. Yeast 2020, 38, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef]
- Guo, N.; Yu, L.; Meng, R.; Fan, J.-W.; Wang, D.-C.; Sun, G.; Deng, X.-M. Global gene expression profile of Saccharomyces cerevisiae induced by dictamnine. Yeast 2008, 25, 631–641. [Google Scholar] [CrossRef]
- Sridevi, D.; Shankar, C.; Prakash, P.; Park, J.H.; Thamaraiselvi, K. Inhibitory effects of reserpine against efflux pump activity of antibiotic resistance bacteria. Chem. Biol. Lett. 2017, 4, 69–72. [Google Scholar]
- Awasthi, D.; Kumar, K.; Ojima, I. Therapeutic potential of FtsZ inhibition: A patent perspective. Expert Opin. Ther. Pat. 2011, 21, 657–679. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.; Liu, M.; Zhang, J.; Yang, M.; Wang, T.; Qian, W. In vitro anti-biofilm efficacy of sanguinarine against carbapenem-resistant Serratia marcescens. Biofouling 2021, 37, 341–351. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Maurya, A.; Yadav, D.K.; Singh, V.; Khan, F.; Gupta, M.K.; Singh, M.; Darokar, M.P.; Srivastava, S.K. Synergy of clavine alkaloid ‘chanoclavine’ with tetracycline against multi-drug-resistant E. coli. J. Biomol. Struct. Dyn. 2019, 37, 1307–1325. [Google Scholar] [CrossRef]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.-E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.S.; Ali, S.T.; Rizwani, G.H.; Begum, S.; Tauseef, S.; Ahmad, A. Antimicrobial activity of the methanolic bark extract of Holarrhena pubescens (Buch. Ham), its fractions and the pure compound conessine. Nat. Prod. Res. 2012, 26, 987–992. [Google Scholar] [CrossRef]
- He, N.; Wang, P.; Wang, P.; Ma, C.; Kang, W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Ma, B.; Ni, Y.; Xue, X.; Li, M.; Meng, J.; Luo, X.; Fang, C.; Hou, Z. Restoration of the Antibiotic Susceptibility of Methicillin-Resistant Staphylococcus aureus and Extended-Spectrum β-Lactamases Escherichia coli Through Combination with Chelerythrine. Microb. Drug Resist. 2021, 27, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H. In vitro antibacterial activity of alkaloids from Sophora flavescens. Chin. Anim. Health 2010, 12, 28–30. [Google Scholar]
- Liu, J.; Ding, Z.; Yang, Y.; Wang, F.; Deng, L. Antimicrobial activity of Sophora alopecuroides alkaloids. J. Beijing Univ. Chem. Technol. 2011, 38, 84–88. [Google Scholar]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dastidar, D.G.; Paul, P.; Dutta, S.; Basu, D.; Sharma, S.R.; Basu, S.; Sarker, R.K.; Sen, A.; Sarkar, A.; et al. Inhibition of biofilm formation of Pseudomonas aeruginosa by caffeine: A potential approach for sustainable management of biofilm. Arch. Microbiol. 2019, 202, 623–635. [Google Scholar] [CrossRef]
- Anjani, G.; Widyastuti, N.; Masruroh, Z.; Ayu Dwi Yuliana, R.; Gustin Almira, V.; Arif Tsani, A.F.; Nissa, C.; Prawira-Atmaja, M. Bioactive components and antibacterial activity in robusta coffee leaves (Coffea canephora). Int. J. Pharm. Res. 2020, 12, 1374–1382. [Google Scholar]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C.; Thompson, N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Zainal, M.; Zain, N.; Amin, I.M.; Ahmad, V.N. The antimicrobial and antibiofilm properties of allicin against Candida albicans and Staphylococcus aureus—A therapeutic potential for denture stomatitis. Saudi Dent. J. 2020, 33, 105–111. [Google Scholar] [CrossRef]
- Torres, J.; Romero, H. In vitro antifungal activity of ajoene on five clinical isolates of Histoplasma capsulatum var. capsulatum. Rev. Iberoam. Micol. 2012, 29, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Velliyagounder, K.; Ganeshnarayan, K.; Velusamy, S.K.; Fine, D.H. In Vitro Efficacy of Diallyl Sulfides against the Periodontopathogen Aggregatibacter actinomycetemcomitans. Antimicrob. Agents Chemother. 2012, 56, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Ma, Y.-K.; Xie, X.-B.; Shi, Q.-S.; Wen, X.; Sun, T.-L.; Peng, H. Diallyl Disulfide From Garlic Oil Inhibits Pseudomonas aeruginosa Quorum Sensing Systems and Corresponding Virulence Factors. Front. Microbiol. 2019, 9, 3222. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, F.; Gu, D.; Wang, W.; Huang, J.; Jiao, X. Antimicrobial Effect and the Mechanism of Diallyl Trisulfide against Campylobacter jejuni. Antibiotics 2021, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Chu, X.-L.; Su, J.-Q.; Cui, Z.-Q.; Zhang, L.-Y.; Yu, Z.-J.; Wu, Z.-M.; Cai, M.-L.; Li, H.-X.; Zhang, Z.-J. Baicalin protects mice against Salmonella typhimurium infection via the modulation of both bacterial virulence and host response. Phytomedicine 2018, 48, 21–31. [Google Scholar] [CrossRef]
- Cannalire, R.; Machado, D.; Felicetti, T.; Costa, S.S.; Massari, S.; Manfroni, G.; Barreca, M.L.; Tabarrini, O.; Couto, I.; Viveiros, M.; et al. Natural isoflavone biochanin A as a template for the design of new and potent 3-phenylquinolone efflux inhibitors against Mycobacterium avium. Eur. J. Med. Chem. 2017, 140, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Cashman, K.K.; Halligan, K.M.; Morel, C.; Tegos, G.P.; Lewis, K. Polyacylated neohesperidosides From Geranium caespitosum: Bacterial multidrug resistance pump inhibitors. Bioorganic Med. Chem. Lett. 2003, 13, 1915–1918. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Beeson, T.D.; Mueller, P.J.; Hsiang, J.-F.; Lewis, K. Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensu strictu) species. Biochem. Syst. Ecol. 2001, 29, 793–798. [Google Scholar] [CrossRef]
- Holler, J.G.; Christensen, S.B.; Slotved, H.-C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.E.; Petersen, B.; Mølgaard, P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Peng, X.; Lin, J.; Zhang, Y.; Zhang, J.; Gao, H.; Tian, X.; Zhang, R.; Zhao, G. Quercetin ameliorates Aspergillus fumigatus keratitis by inhibiting fungal growth, toll-like receptors and inflammatory cytokines. Int. Immunopharmacol. 2021, 93, 107435. [Google Scholar] [CrossRef]
- Li, K.; Guan, G.; Zhu, J.; Wu, H.; Sun, Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan–gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control 2018, 96, 234–243. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Sarabandi, S.; Khameneh, B.; Hosseinzadeh, H. Effect of catechins, green tea extract and methylxanthines in combination with gentamicin agair staphylococcus aureus and pseudomonas aeruginosa-Combination therapy against resistant bacteria. J. Pharmacopunct. 2016, 19, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Knidel, C.; Pereira, M.F.; Barcelos, D.H.F.; Gomes, D.C.D.O.; Guimarães, M.C.C.; Schuenck, R.P. Epigallocatechin gallate has antibacterial and antibiofilm activity in methicillin resistant and susceptible Staphylococcus aureus of different lineages in non-cytotoxic concentrations. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.; Neergheen, V.; Luximon-Ramma, A.; Aruoma, O.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Li, B.H.; Zhang, R.; Du, Y.T.; Sun, Y.H.; Tian, W.X. Inactivation mechanism of the beta-ketoacyl-[acyl carrier protein] reductase of bacterial type-II fatty acid synthase by epigallocatechin gallate. Biochem. Cell Biol. 2006, 84, 755–762. [Google Scholar] [CrossRef]
- Yun, Y.F.; Aisyah, L.S.; Purbaya, S.; Agustini, D.M.; Wardhani, I.P.; Alawiyah, N.; Supratman, U.; Shiono, Y. Identification of flavonoid compounds from ethyl acetate extract of Kalanchoe millotii (Crassulaceae) and endodontics antibacterial activity. Res. J. Chem. Environ. 2020, 24, 53–55. [Google Scholar]
- Moura, F.C.S.; Cechinel-Filho, V.; Greco, F.A.; Venzon, L.; Meurer, M.C.; França, T.C.d.S.; Longo, B.; Somensi, L.B.; Mariano, L.N.B.; Cruz, A.B.; et al. Taxifolin and gastro-adhesive microparticles containing taxifolin promotes gastric healing in vivo, inhibits Helicobacter pylori in vitro and proton pump reversibly in silico. Chem. Interact. 2021, 339, 109445. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.; Mahmood, S.S.; Hindi, N. Effects of Curcumin and Silymarin on the Shigella dysenteriae and Campylobacter jejuni In vitro. J. Gastrointest. Cancer 2019, 51, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. d-Alanine:d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Szatmári, Á.; Móricz, Á.M.; Schwarczinger, I.; Nagy, J.K.; Alberti, Á.; Pogány, M.; Bozsó, Z. A pattern-triggered immunity-related phenolic, acetosyringone, boosts rapid inhibition of a diverse set of plant pathogenic bacteria. BMC Plant Biol. 2021, 21, 1–20. [Google Scholar] [CrossRef]
- Saravanakumar, T.; Park, H.-S.; Mo, A.-Y.; Choi, M.-S.; Kim, D.-H.; Park, S.-M. Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J. Mol. Catal. B Enzym. 2016, 134, 196–205. [Google Scholar] [CrossRef]
- Neetu, N.; Katiki, M.; Dev, A.; Gaur, S.; Tomar, S.; Kumar, P. Structural and Biochemical Analyses Reveal that Chlorogenic Acid Inhibits the Shikimate Pathway. J. Bacteriol. 2020, 202, e00248-20. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Xie, Y.; Ren, L.; Chen, Y. Antimicrobial Activity of Galangin and Its Effects on Murein Hydrolases of Vancomycin-Intermediate Staphylococcus aureus (VISA) Strain Mu50. Chemotherapy 2017, 63, 20–28. [Google Scholar] [CrossRef]
- Morel, C.; Stermitz, F.R.; Tegos, A.G.; Lewis, K. Isoflavones As Potentiators of Antibacterial Activity. J. Agric. Food Chem. 2003, 51, 5677–5679. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta 2013, 1828, 2751–2756. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–Activity Relationship of Flavonoids on Their Anti-Escherichia coli Activity and Inhibition of DNA Gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Park, I.-H.; Yim, D.; Choi, S.S. Comprehensive Evaluation of the Anti- Helicobacter pylori Activity of Scutellariae Radix. Nat. Prod. Sci. 2017, 23, 46. [Google Scholar] [CrossRef][Green Version]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Silva, H.T.; Magnani, M.; de Siqueira, S.; de Souza, E.L.; de Siqueira-Júnior, J.P. Fruit flavonoids as modulators of norfloxacin resistance in Staphylococcus aureus that overexpresses norA. LWT 2017, 85, 324–326. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M. Antibacterial Activity of Totarol and Its Potentiation. J. Nat. Prod. 1992, 55, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Jr, S.E.S.; Lin, W.F.; Wei, C.I. Growth inhibition of selected food-borne bacteria by tannic acid, propyl gallate and related compounds. Lett. Appl. Microbiol. 1993, 17, 29–32. [Google Scholar] [CrossRef]
- Diniz-Silva, H.T.; Cirino, I.C.D.S.; Falcão-Silva, V.D.S.; Magnani, M.; De Souza, E.L.; Siqueira-Júnior, J.P. Tannic Acid as a Potential Modulator of Norfloxacin Resistance in Staphylococcus Aureus Overexpressing norA. Chemotherapy 2016, 61, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Hisano, M.; Yamaguchi, K.; Inoue, Y.; Ikeda, Y.; Iijima, M.; Adachi, M.; Shimamura, T. Inhibitory effect of catechin against the superantigen staphylococcal enterotoxin B (SEB). Arch. Dermatol. Res. 2003, 295, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sinsinwar, S.; Vadivel, V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Imanzadeh, G.; Jahed, F.S.; Zarrini, G. Pyranocoumarins from Zosima absinthifolia (Vent) link roots. Russ. J. Bioorganic Chem. 2013, 39, 215–217. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Tsai, F.T.; Singh, O.M.; Skarzynski, T.; Wonacott, A.J.; Weston, S.; Tucker, A.A.; Pauptit, R.; Breeze, A.L.; Poyser, J.P.; O’Brien, R.; et al. The high-resolution crystal structure of a 24-kDa gyrase B fragment from E. coli complexed with one of the most potent coumarin inhibitors, clorobiocin. Proteins Struct. Funct. Bioinform. 1997, 28, 41–52. [Google Scholar] [CrossRef]
- Yadav, N.; Agarwal, D.; Kumar, S.; Dixit, A.; Gupta, R.D.; Awasthi, S.K. In vitro antiplasmodial efficacy of synthetic coumarin-triazole analogs. Eur. J. Med. Chem. 2018, 145, 735–745. [Google Scholar] [CrossRef]
- Lv, X.-H.; Liu, H.; Ren, Z.-L.; Wang, W.; Tang, F.; Cao, H.-Q. Design, synthesis and biological evaluation of novel flavone Mannich base derivatives as potential antibacterial agents. Mol. Divers. 2018, 23, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, B.S.F.; Memariani, Z.; Khashiarmanesh, Z.; Iranshahi, M.; Naderinasab, M. Effect Of Galbanic Acid, A Sesquiterpene Coumarin From Ferula Szowitsiana, As An Inhibitor Of Efflux Mechanism In Resistant Clinical Isolates of Staphylococcus Aureus. Braz. J. Microbiol. 2010, 41, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, B.S.F.; Iranshahi, M.; Naderinasab, M.; Hajian, S.; Sabeti, Z.; Masumi, E. Evaluation of the effects of galbanic acid from Ferula szowitsiana and conferol from F. badrakema, as modulators of multi-drug resistance in clinical isolates of Escherichia coli and Staphylococcus aureus. Res. Pharm. Sci. 2010, 5, 21–28. [Google Scholar]
- Yang, L.; Ding, W.; Xu, Y.; Wu, D.; Liang, Y.; Chen, J.; Guo, B. New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules 2016, 21, 468. [Google Scholar] [CrossRef]
- Liu, W.; Mei, J.; Xie, J. Elucidating Antibacterial Activity and Mechanism of Daphnetin against Pseudomonas fluorescens and Shewanella putrefaciens. J. Food Qual. 2020, 2020, 6622355. [Google Scholar] [CrossRef]
- Yang, L.; Wu, L.; Yao, X.; Zhao, S.; Wang, J.; Li, S.; Ding, W. Hydroxycoumarins: New, effective plant-derived compounds reduce Ralstonia pseudosolanacearum populations and control tobacco bacterial wilt. Microbiol. Res. 2018, 215, 15–21. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Horie, H.; Chiba, A.; Wada, S. Inhibitory effect of soy saponins on the activity of β-lactamases, including New Delhi metallo-β-lactamase 1. J. Food Sci. Technol. 2018, 55, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, L.; Fu, J.; Liao, S.; Yang, S.; Jia, X.; Gong, G. Evaluation of the membrane damage mechanism of thymol against Bacillus cereus and its application in the preservation of skim milk. Food Control 2021, 131, 108435. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of Eugenol against Escherichia coli. J. Herb. Med. 2020, 26, 100406. [Google Scholar] [CrossRef]
- Togashi, N.; Inoue, Y.; Hamashima, H.; Takano, A. Effects of Two Terpene Alcohols on the Antibacterial Activity and the Mode of Action of Farnesol against Staphylococcus aureus. Molecules 2008, 13, 3069–3076. [Google Scholar] [CrossRef]
- Memariani, Z.; Sharifzadeh, M.; Bozorgi, M.; Hajimahmoodi, M.; Farzaei, M.H.; Gholami, M.; Siavoshi, F.; Saniee, P. Protective effect of essential oil of Pistacia atlantica Desf. on peptic ulcer: Role of α-pinene. J. Tradit. Chin. Med. 2017, 37, 57–63. [Google Scholar] [CrossRef]
- Costa, M.D.S.; Rocha, J.E.; Campina, F.F.; Silva, A.R.; Da Cruz, R.P.; Pereira, R.L.; Quintans-Júnior, L.J.; De Menezes, I.R.; Araújo, A.A.D.S.; De Freitas, T.S.; et al. Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. Eur. J. Pharm. Sci. 2018, 128, 158–161. [Google Scholar] [CrossRef]
- Shayegan, S.; Khodavandi, A. Inhibitory effect of menthol on expression of aspartyl proteinase 1 in fluconazole-resistant Candida albicans. J. Herb. Med. Pharmacol. 2019, 8, 35–40. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Zhang, D.; Niu, X. In vitro activity of farnesol against vaginal Lactobacillus spp. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 25–29. [Google Scholar] [CrossRef]
- de Moura, D.F.; Rocha, T.A.; Barros, D.d.M.; Silva, M.M.d.; Santana, M.D.S.; Neta, B.M.; Cavalcanti, I.M.F.; Martins, R.D.; Silva, M.V. Evaluation of the antioxidant, antibacterial, and antibiofilm activity of the sesquiterpene nerolidol. Arch. Microbiol. 2021, 203, 4303–4311. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.; Da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Li, X.; Shen, L.; Wang, T.; Liu, M.; Zhang, J.; Yang, M.; Li, X.; Cai, C. RETRACTED: Antibacterial and antibiofilm activity of ursolic acid against carbapenem-resistant Enterobacter cloacae. J. Biosci. Bioeng. 2019, 129, 528–534. [Google Scholar] [CrossRef]
- Catteau, L.; Reichmann, N.T.; Olson, J.; Pinho, M.G.; Nizet, V.; Van Bambeke, F.; Quetin-Leclercq, J. Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and β-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms. Molecules 2017, 22, 2245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Liu, X.Y.; Jiang, P.P.; Li, W.D.; Wang, Y.F. Mechanism and antibacterial activity of cinnamaldehyde against Escherichia coli and Staphylococcus aureus. Mod. Food Sci. Technol. 2015, 31, 31–35. [Google Scholar] [CrossRef]
- Pereira, W.; Pereira, C.; Assunção, R.; da Silva, I.; Rego, F.; Alves, L.; Santos, J.; Nogueira, F.; Zagmignan, A.; Thomsen, T.; et al. New Insights into the Antimicrobial Action of Cinnamaldehyde towards Escherichia coli and Its Effects on Intestinal Colonization of Mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.-J.; Ni Chia, W.; Loh, C.C.Y.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.-X.; Lim, T.K.; Liu, M.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.; Meile, J.C.; Lebrun, M.; Caruso, D.; Chu-Ky, S.; Sarter, S. Litsea cubeba leaf essential oil from Vietnam: Chemical diversity and its impacts on antibacterial activity. Lett. Appl. Microbiol. 2018, 66, 207–214. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Silva, V.A.; Sousa, J.P.; Guerra, F.Q.S.; Pessôa, H.L.F.; Freitas, A.F.R.; Coutinho, H.D.M.; Alves, L.B.N.; Lima, E.O. Antibacterial activity of the monoterpene linalool: Alone and in association with antibiotics against bacteria of clinical importance. Int. J. Pharm. Phytochem. Res. 2015, 7, 1022–1026. [Google Scholar]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Xiao, Z.; Lin, L.; Li, C. Antibacterial Properties of Nutmeg Oil in Pork and Its Possible Mechanism. J. Food Saf. 2015, 35, 370–377. [Google Scholar] [CrossRef]
- Matias, E.F.; Alves, E.F.; Silva, M.K.; Carvalho, V.R.; Figueredo, F.G.; Ferreira, J.V.; Coutinho, H.D.; Silva, J.M.; Ribeiro-Filho, J.; Costa, J.G. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crop. Prod. 2016, 87, 45–53. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2014, 45, 1409–1413. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef]
- Pereira, F.d.O.; Mendes, J.M.; Lima, I.O.; de Lira Mota, K.S.; de Oliveira, W.A.; de Oliveira Lima, E. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef]
- Sieniawska, E.; Swatko-Ossor, M.; Sawicki, R.; Ginalska, G. Morphological Changes in the Overall Mycobacterium tuberculosis H37Ra Cell Shape and Cytoplasm Homogeneity due to Mutellina purpurea L. Essential Oil and Its Main Constituents. Med. Princ. Pract. 2015, 24, 527–532. [Google Scholar] [CrossRef]
- Firat, Z.; Demirci, F.; Demirci, B.; Baser, K.H.C. Microbial transformation of α-Bisabolol towards bioactive metabolites. J. Biotechnol. 2017, 256, S52–S53. [Google Scholar] [CrossRef]
- e Nogueira, J.O.; Campolina, G.A.; Batista, L.R.; Alves, E.; Caetano, A.R.S.; Brandão, R.M.; Nelson, D.L.; Cardoso, M.D.G. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021, 368, fnab052. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Fork, S.D.; Ahmadi, R.; Khameneh, B. Deep insights into urinary tract infections and effective natural remedies. Afr. J. Urol. 2021, 27, 1–13. [Google Scholar] [CrossRef]
- Afshar, K.; Fleischmann, N.; Schmiemann, G.; Bleidorn, J.; Hummers-Pradier, E.; Friede, T.; Wegscheider, K.; Moore, M.; Gágyor, I. Reducing antibiotic use for uncomplicated urinary tract infection in general practice by treatment with uva-ursi (REGATTA)—A double-blind, randomized, controlled comparative effectiveness trial. BMC Complement. Altern. Med. 2018, 18, 203. [Google Scholar] [CrossRef]
- Larsson, B.; Jonasson, A.; Fianu, S. Prophylactic effect of UVA-E in women with recurrent cystitis: A preliminary report. Curr. Ther. Res. 1993, 53, 441–443. [Google Scholar] [CrossRef]
- Howell, A.B.; Foxman, B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA 2002, 287, 3082–3083. [Google Scholar] [CrossRef] [PubMed]
- Burger, O.; Ofek, I.; Tabak, M.; Weiss, E.I.; Sharon, N.; Neeman, I. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol. Med. Microbiol. 2000, 29, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, P.; Agniel, R.; David, K.; Templer, C.; Gaillard, J.L.; Denys, P.; Botto, H. Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: A double-blind randomized placebo-controlled cross-over trial. World J. Urol. 2006, 24, 21–27. [Google Scholar] [CrossRef]
- Tao, Y.; Pinzón-Arango, P.A.; Howell, A.B.; Camesano, T.A. Oral Consumption of Cranberry Juice Cocktail Inhibits Molecular-Scale Adhesion of Clinical UropathogenicEscherichia coli. J. Med. Food 2011, 14, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dason, S.; Dason, J.T.; Kapoor, A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can. Urol. Assoc. J. 2013, 5, 316–322. [Google Scholar] [CrossRef]
- Davidson, E.; Zimmermann, B.F.; Jungfer, E.; Chrubasik-Hausmann, S. Prevention of Urinary Tract Infections withVacciniumProducts. Phytother. Res. 2013, 28, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Bhattacharyya, S.; Chattopadhyay, R. Medicinal plants and urinary tract infections: An update. Pharmacogn. Rev. 2008, 2, 277. [Google Scholar]
- Head, K.A. Natural approaches to prevention and treatment of infections of the lower urinary tract. Altern. Med. Rev. 2008, 13, 227–244. [Google Scholar]
- Vichkanova, S.A.; Tolkachev, O.N.; Martynova, R.G.; Arzamastsev, E.V. Sanguiritrin, a new antimicrobial drug. Pharm. Chem. J. 1982, 16, 925–929. [Google Scholar] [CrossRef]
- Dvorak, Z.; Simanek, V. Metabolism of Sanguinarine: The Facts and The Myths. Curr. Drug Metab. 2007, 8, 173–176. [Google Scholar] [CrossRef]
- Spiridonov, N.A.; Foigel, A.G.; Fomkina, M.G.; Arkhipov, V.V.; Shipulina, L.D. Mechanism of action of some antimicrobial preparations of plant origin. Pharm. Chem. J. 1996, 30, 400–403. [Google Scholar] [CrossRef]
- Semkina, O.A.; Sokol’Skaya, T.A.; Krasnyuk, I.I.; Okhotnikova, V.F.; Krutikova, N.M.; Vichkanova, S.A. Eucalimin: Antimicrobial and antiinflammatory medicinal plant preparation. Pharm. Chem. J. 2006, 40, 459–462. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Morita, H.; Takeya, A.K.; Itokawa, H. Macrocarpals H, I, and J from the Leaves of Eucalyptus globulus. J. Nat. Prod. 1996, 59, 823–827. [Google Scholar] [CrossRef]
- Zhukovich, E.N.; Bobrenko, L.M.; Semenova, M.Y.; Bokareva, S.Y. Chemical Studies of Eucalyptus Tincture. Pharm. Chem. J. 2014, 48, 323–327. [Google Scholar] [CrossRef]
- Xing, S.; Wang, M.; Peng, Y.; Li, X. Effects of intestinal microecology on metabolism and pharmacokinetics of oral wogonoside and Baicalin. Nat. Prod. Comm. 2017, 12, 509–514. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Sakamuru, S.; Huang, R.; Teneva, N.; Simmons, S.; Xia, M.; Tice, R.R.; Austin, C.P.; Myung, K. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc. Natl. Acad. Sci. USA 2012, 109, 5423–5428. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.-J.; Cha, S.-M.; Choi, S.-M.; Cha, J.-D. Combination effects of baicalein with antibiotics against oral pathogens. Arch. Oral Biol. 2014, 59, 1233–1241. [Google Scholar] [CrossRef]
- Cai, W.; Fu, Y.; Zhang, W.; Chen, X.; Zhao, J.; Song, W.; Li, Y.; Huang, Y.; Wu, Z.; Sun, R.; et al. Synergistic effects of baicalein with cefotaxime against Klebsiella pneumoniae through inhibiting CTX-M-1 gene expression. BMC Microbiol. 2016, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, S.; Pimchan, T.; Naknarong, W.; Eumkeb, G. Mode of Action and Synergy of Ceftazidime and Baicalein against Streptococcus pyogenes. Trop. J. Pharm. Res. 2015, 14, 641. [Google Scholar] [CrossRef][Green Version]
- Chan, B.C.; Ip, M.; Lau, C.; Lui, S.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.B.; Pergola, G.; Kuenz, J.; Hellwig, E.; Sculean, A.; Auschill, T.M. Clinical and antibacterial effect of an anti-inflammatory toothpaste formulation with Scutellaria baicalensis extract on experimental gingivitis. Clin. Oral Investig. 2010, 15, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Sarveswari, H.; Vasudevan, S.; Shanmugam, K.; Solomon, A.; Neelakantan, P. Baicalein Inhibits Streptococcus mutans Biofilms and Dental Caries-Related Virulence Phenotypes. Antibiotics 2021, 10, 215. [Google Scholar] [CrossRef]
- Liu, T.; Luo, J.; Bi, G.; Du, Z.; Kong, J.; Chen, Y. Antibacterial synergy between linezolid and baicalein against methicillin-resistant Staphylococcus aureus biofilm in vivo. Microb. Pathog. 2020, 147, 104411. [Google Scholar] [CrossRef]
- Hemalatha, S.; Kumar, M.; Prasad, S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharmacogn. Rev. 2014, 8, 22–35. [Google Scholar] [CrossRef]
- Hou, B.-Y.; Zhang, L.; Du, G.H. Houttuynin. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018; pp. 415–420. [Google Scholar]
- Lu, X.; Yang, X.; Li, X.; Lu, Y.; Ren, Z.; Zhao, L.; Hu, X.; Jiang, J.; You, X. In Vitro Activity of Sodium New Houttuyfonate Alone and in Combination with Oxacillin or Netilmicin against Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2013, 8, e68053. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhong, L.; Xie, J.; Sui, Y.; Li, G.; Ma, Z.; Yang, L. Sodium houttuyfonate: A review of its antimicrobial, anti-inflammatory and cardiovascular protective effects. Eur. J. Pharmacol. 2021, 902, 174110. [Google Scholar] [CrossRef]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Mizuguchi, H.; Amoh, T.; Ogino, S.; Matsuo, T.; Miyake, Y.; Fukui, H.; Kashiwada, Y. Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci. Biotechnol. Biochem. 2016, 80, 1205–1213. [Google Scholar] [CrossRef]
- Kim, G.S.; Kim, D.H.; Lim, J.J.; Lee, J.J.; Han, D.Y.; Lee, W.M.; Jung, W.C.; Min, W.G.; Gil Won, C.; Rhee, M.H.; et al. Biological and Antibacterial Activities of the Natural Herb Houttuynia cordata Water Extract against the Intracellular Bacterial Pathogen Salmonella within the RAW 264.7 Macrophage. Biol. Pharm. Bull. 2008, 31, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, X.; Liang, Y.; Zhang, J. Variation in Chemical Composition and Antibacterial Activities of Essential Oils from Two Species of Houttuynia THUNB. Chem. Pharm. Bull. 2006, 54, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Deng, S.; Li, J. Preparation of Flavonoids of Houttuynia cordata Thunb. and the Antibacterial Mechanism on Bacillus subtilis. J. Chin. Inst. Food Sci. Technol. 2017, 17, 82–89. [Google Scholar] [CrossRef]
- Cui, X.H.; Wang, L.; Li, Y.P.; Deng, S.L.; Li, T.Q.; Shang, H.C. Efficacy of Houttuynia cordata Injection for respiratory system diseases: A meta-analysis. Chin. J. Evid. Based Med. 2011, 11, 786–798. [Google Scholar]
- Petronio Petronio, G.; Cutuli, M.A.; Magnifico, I.; Venditti, N.; Pietrangelo, L.; Vergalito, F.; Pane, A.; Scapagnini, G.; Di Marco, R. In Vitro and In Vivo Biological Activity of Berberine Chloride against Uropathogenic E. coli Strains Using Galleria mellonella as a Host Model. Molecules 2020, 25, 5010. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, F.; Huang, F.; Yang, M.; He, D.; Deng, L. Targeting effect of berberine on type I fimbriae of Salmonella Typhimurium and its effective inhibition of biofilm. Appl. Microbiol. Biotechnol. 2021, 105, 1563–1573. [Google Scholar] [CrossRef]
- Shi, C.; Li, M.; Muhammad, I.; Ma, X.; Chang, Y.; Li, R.; Li, C.; He, J.; Liu, F. Combination of berberine and ciprofloxacin reduces multi-resistant Salmonella strain biofilm formation by depressing mRNA expressions of luxS, rpoE, and ompR. J. Veter. Sci. 2018, 19, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Si, S.Y.; Jiang, J.D. Antibacterial activity of berberine. Yaoxue Xuebao 2018, 53, 163–168. [Google Scholar] [CrossRef]
- Salari, R.; Rajabi, O.; Khashyarmanesh, Z.; Najafi, M.F.; Bazzaz, B.S.F. Characterization of Encapsulated Berberine in Yeast Cells of Saccharomyces cerevisiae. Iran. J. Pharm. Res. IJPR 2015, 14, 1247–1256. [Google Scholar]
- Salari, R.; Bazzaz, B.S.F.; Rajabi, O.; Khashyarmanesh, Z. New aspects of Saccharomyces cerevisiae as a novel carrier for berberine. DARU J. Pharm. Sci. 2013, 21, 73. [Google Scholar] [CrossRef]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-Derived Products as Antibacterial and Antifungal Agents in Human Health Care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Chauhan, R.K.S.; Jain, A.M.; Bhandari, B. Berberine in the treatment of childhood diarrhoea. Indian J. Pediatr. 1970, 37, 577–579. [Google Scholar] [CrossRef]
- Chauhan, R.K.S.; Jain, A.M.; Dube, M.K.; Bhandari, B. A combination of sulfadimidine, neomycin and berberine in the treatment of infectious diarrhoea. Indian J. Pediatr. 1969, 36, 242–244. [Google Scholar] [CrossRef]
- Sharma, R.; Joshi, C.K.; Goyal, R.K. Berberine tannate in acute diarrhoea. Indian Pediatr. 1970, 7, 496–501. [Google Scholar]
- Lahiri, S.C.; Dutta, N.K. Berberine and chloramphenicol in the treatment of cholera and severe diarrhoea. J. Indian Med. Assoc. 1967, 48, 1–11. [Google Scholar]
- Khin Maung, U.; Myo, K.; Nyunt Nyunt, W.; Aye, K.; Tin, U. Clinical trial of berberine in acute watery diarrhoea. Br. Med. J. 1985, 291, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical Composition and Antibacterial Activity of the Essential Oil and the Gum ofPistacia lentiscusVar. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Okimoto, T.; Kuwano, M. Chemical Composition of the Essential Oil of Mastic Gum and their Antibacterial Activity Against Drug-Resistant Helicobacter pylori. Nat. Prod. Bioprospecting 2014, 4, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Kottakis, F.; Lamari, F.; Matragkou, C.; Zachariadis, G.; Karamanos, N.; Choli-Papadopoulou, T. Arabino-Galactan Proteins from Pistacia lentiscus var. chia: Isolation, characterization and biological function. Amino Acids 2007, 34, 413–420. [Google Scholar] [CrossRef]
- Al-Habbal, M.J.; Al-Habbal, Z.; Huwez, F.U. A double-blind controlled clinical trial of mastic and placebo in the treatment of duodenal ulcer. Clin. Exp. Pharmacol. Physiol. 1984, 11, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Dabos, K.; Sfika, E.; Vlatta, L.; Giannikopoulos, G. The effect of mastic gum on Helicobacter pylori: A randomized pilot study. Phytomedicine 2010, 17, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Kottakis, F.; Kouzi-Koliakou, K.; Pendas, S.; Kountouras, J.; Choli-Papadopoulou, T. Effects of mastic gum Pistacia lentiscus var. Chia on innate cellular immune effectors. Eur. J. Gastroenterol. Hepatol. 2009, 21, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002, 71, 1449–1463. [Google Scholar] [CrossRef]
- Asha, M.K.; Debraj, D.; Prashanth, D.; Edwin, J.R.; Srikanth, H.; Muruganantham, N.; Dethe, S.; Anirban, B.; Jaya, B.; Deepak, M.; et al. In vitro anti-Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J. Ethnopharmacol. 2013, 145, 581–586. [Google Scholar] [CrossRef]
- Wittschier, N.; Faller, G.; Hensel, A. Aqueous extracts and polysaccharides from Liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Ethnopharmacol. 2009, 125, 218–223. [Google Scholar] [CrossRef]
- Puram, S.; Suh, H.C.; Kim, S.U.; Bethapudi, B.; Joseph, J.A.; Agarwal, A.; Kudiganti, V. Effect of GutGard in the Management of Helicobacter pylori: A Randomized Double Blind Placebo Controlled Study. Evid.-Based Complement. Altern. Med. 2013, 2013, 263805. [Google Scholar] [CrossRef][Green Version]
- Bardhan, K.D.; Cumberland, D.C.; Dixon, R.A.; Holdsworth, C.D. Clinical trial of deglycyrrhizinised liquorice in gastric ulcer. Gut 1978, 19, 779–782. [Google Scholar] [CrossRef]
- Hollanders, D.; Green, G.; Woolf, I.L.; Boyes, B.E.; Wilson, R.Y.; Cowley, D.J.; Dymock, I.W. Prophylaxis with deglycyrrhizinised liquorice in patients with healed gastric ulcer. BMJ 1978, 1, 148. [Google Scholar] [CrossRef]
- Kato, T.; Iijima, H.; Ishihara, K.; Kaneko, T.; Hirai, K.; Naito, Y.; Okuda, K. Antibacterial effects of Listerine on oral bacteria. Bull. Tokyo Dent. Coll. 1990, 31, 301–307. [Google Scholar]
- Sharma, N.C.; Charles, C.H.; Qaqish, J.G.; Galustians, H.J.; Zhao, Q.; Kumar, L.D. Comparative effectiveness of an essential oil mouthrinse and dental floss in controlling interproximal gingivitis and plaque. Am. J. Dent. 2002, 15, 351–355. [Google Scholar]
- Charles, C.H.; Mostler, K.M.; Bartels, L.L.; Mankodi, S.M. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J. Clin. Periodontol. 2004, 31, 878–884. [Google Scholar] [CrossRef]
- Sharma, N.C.; Araujo, M.W.B.; Wu, M.M.; Qaqish, J.; Charles, C.H. Superiority of an essential oil mouthrinse when compared with a 0.05% cetylpyridinium chloride containing mouthrinse: A six-month study. Int. Dent. J. 2010, 60, 175–180. [Google Scholar]
- Singh, A.; Daing, A.; Dixit, J. The effect of herbal, essential oil and chlorhexidine mouthrinse on de novo plaque formation. Int. J. Dent. Hyg. 2012, 11, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Stoeken, J.E.; Paraskevas, S.; Van Der Weijden, G.A. The Long-Term Effect of a Mouthrinse Containing Essential Oils on Dental Plaque and Gingivitis: A Systematic Review. J. Periodontol. 2007, 78, 1218–1228. [Google Scholar] [CrossRef]
- Kraivaphan, P.; Amornchat, C.; Maneepitsa, Y. Bactericidal Effects of Three Mint Essential Oils on Porphyromonas gingivalis in Planktonic and Biofilm Cells. Res. J. Med. Plant 2013, 7, 100–106. [Google Scholar] [CrossRef][Green Version]
- Renggli, H.H. The effect of Parodontax mouthwash and its constituents on the microorganisms of subgingival plaque. J. Clin. Dent. 1988, 1, A30–A33. [Google Scholar] [PubMed]
- Arweiler, N.B.; Auschill, T.M.; Reich, E.; Netuschil, L. Substantivity of toothpaste slurries and their effect on reestablishment of the dental biofilm. J. Clin. Periodontol. 2002, 29, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, F.; Pannuti, C.M.; Imbronito, A.V.; Pessotti, W.; Saraiva, L.; De Freitas, N.M.; Ferrari, G.; Cabral, V.N. Efficacy of a herbal toothpaste on patients with established gingivitis: A randomized controlled trial. Braz. Oral Res. 2006, 20, 172–177. [Google Scholar] [CrossRef]
- Verkaik, M.J.; Busscher, H.J.; Jager, D.; Slomp, A.M.; Abbas, F.; van der Mei, H.C. Efficacy of natural antimicrobials in toothpaste formulations against oral biofilms in vitro. J. Dent. 2010, 39, 218–224. [Google Scholar] [CrossRef]
- Paparoupa, M.; Gillissen, A. Is Myrtol® standardized a new alternative toward antibiotics? Pharm. Rev. 2016, 10, 143–146. [Google Scholar] [CrossRef]
- Sengespeik, H.C.; Zimmermann, T.; Peiske, C.; De Mey, C. Myrtol standardized in the treatment of acute and chronic respiratory infections in children/A multicenter post-marketing surveillance study. Arzneim.-Forsch. Drug Res. 1998, 48, 990–994. [Google Scholar]
- Meister, R.; Wittig, T.; Beuscher, N.; De Mey, C. Efficacy and tolerability of myrtol standardized in long-term treatment of chronic bronchitis: A double-blind, placebo-controlled study. Arzneim. Drug Res. 1999, 49, 351–358. [Google Scholar] [CrossRef]
- Matthys, H.; De Mey, C.; Carls, C.; Rys, A.; Geib, A.; Wittig, T. Efficacy and tolerability of myrtol standardized in acute bronchitis: A multi-centre, randomised, double-blind, placebo-controlled parallel group clinical trial vs. cefuroxime and ambroxol. Arzneim. Drug Res. 2000, 50, 700–711. [Google Scholar] [CrossRef]
- Bassett, I.B.; Barnetson, R.S.C.; Pannowitz, D.L. A comparative study of tea-tree oil versus benzoyl peroxide in the treatment of acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [CrossRef]
- Jooya, A.; Siadat, A.H.; Iraji, F.; Enshaieh, S. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomized, double-blind placebo-controlled study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25. [Google Scholar] [CrossRef]
- Dryden, M.; Dailly, S.; Crouch, M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonization. J. Hosp. Infect. 2004, 56, 283–286. [Google Scholar] [CrossRef]
- Blackwood, B.; Thompson, G.; McMullan, R.; Stevenson, M.; Riley, T.V.; Alderdice, F.A.; Trinder, T.J.; Lavery, G.G.; McAiley, D.F. Tea tree oil (5%) body wash versus standard care (johnson’s baby softwash) to prevent colonization with methicillin-resistant staphylococcus aureus in critically ill adults: A randomized controlled trial. J. Antimicrob. Chemother. 2013, 68, 1193–1199. [Google Scholar] [CrossRef]
- Tong, M.M.; Altman, P.M.; Barnetson, R.S. Tea tree oil in the treatment of tinea pedis. Australas. J. Dermatol. 1992, 33, 145–149. [Google Scholar] [CrossRef]
| Compound or Product | Sources or Ingredient | Indications |
|---|---|---|
| Concentrated herbal extract granules TRA | Traditional Chinese Medicine | Urinary tract infections |
| Uva ursi extract | Uva ursi | Urinary tract infections |
| Monoselect Macrocarpon | Vaccinium spp. | Urinary tract infections |
| Anthocran | Vaccinium spp. | Urinary tract infections |
| Cysticlean | Vaccinium spp. | Urinary tract infections |
| UVA-E | Arctostaphylos uva-ursi, Taraxacum officinale | Urinary tract infections |
| Pylorin | polyherbal formulation | Helicobacter pylori Infection |
| Sanguiritrin | Macleaya cordata and Macleaya microcarpa | Acute intestinal infections and wound infections |
| Eucalimin | Consisted of triterpene phenol aldehyde and triterpenoid that isolated from foliage and shoots of Eucalyptus Viminalis Labill | Pharyngitis, laryngitis, and sinusitis |
| Scutellaria baicalensis Georgi | Scutellaria baicalensis Georgi | Pathopyretic sores, ulcers or pustules |
| Houttuynia cordata Thunb | Pseudorabies herpesvirus | |
| Berberine | Berberis vulgaris | Gastrointestinal infections |
| Mastic | Pistacia lentiscus resin | H. pylori Infection |
| GutGard | Glycyrrhiza glabra extract | H. pylori Infection |
| Listerine | eucalyptol, menthol, methyl salicylate, and thymol | Oral infections |
| Parodontax | Commiphora myrrha, Echinacea purpurea, Krameria triandra, and Matricaria recutita extracts; Mentha arvensis, M. x Piperita and Salvia officinalis essential oils | Oral infections |
| Myrtol | Citrus limon, Citrus sinensis, Eucalyptus globulus, and Myrtus communis essential oils | Chronic and acute bronchitis |
| Tea tree oil | TTO, Melaleuca alternifolia essential oil | Mild to moderate acne |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. https://doi.org/10.3390/antibiotics10091044
Khameneh B, Eskin NAM, Iranshahy M, Fazly Bazzaz BS. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics. 2021; 10(9):1044. https://doi.org/10.3390/antibiotics10091044
Chicago/Turabian StyleKhameneh, Bahman, N. A. Michael Eskin, Milad Iranshahy, and Bibi Sedigheh Fazly Bazzaz. 2021. "Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria" Antibiotics 10, no. 9: 1044. https://doi.org/10.3390/antibiotics10091044
APA StyleKhameneh, B., Eskin, N. A. M., Iranshahy, M., & Fazly Bazzaz, B. S. (2021). Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics, 10(9), 1044. https://doi.org/10.3390/antibiotics10091044







