Anti-Inflammatory Effects of RTD-1 in a Murine Model of Chronic Pseudomonas aeruginosa Lung Infection: Inhibition of NF-κB, Inflammasome Gene Expression, and Pro-IL-1β Biosynthesis
Abstract
:1. Introduction
2. Methods
2.1. In Vivo Studies
2.1.1. Chronic Murine Infection Model
2.1.2. RNA Microarray Analysis
2.2. In Vitro Studies
2.2.1. Cell Culture
2.2.2. NF-κB Reporter Assay
2.2.3. Gene Expression Analysis
2.2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.2.5. Immunoblot Analyses
2.2.6. Caspase-1 Activity
2.2.7. Data and Statistical Analysis
3. Results
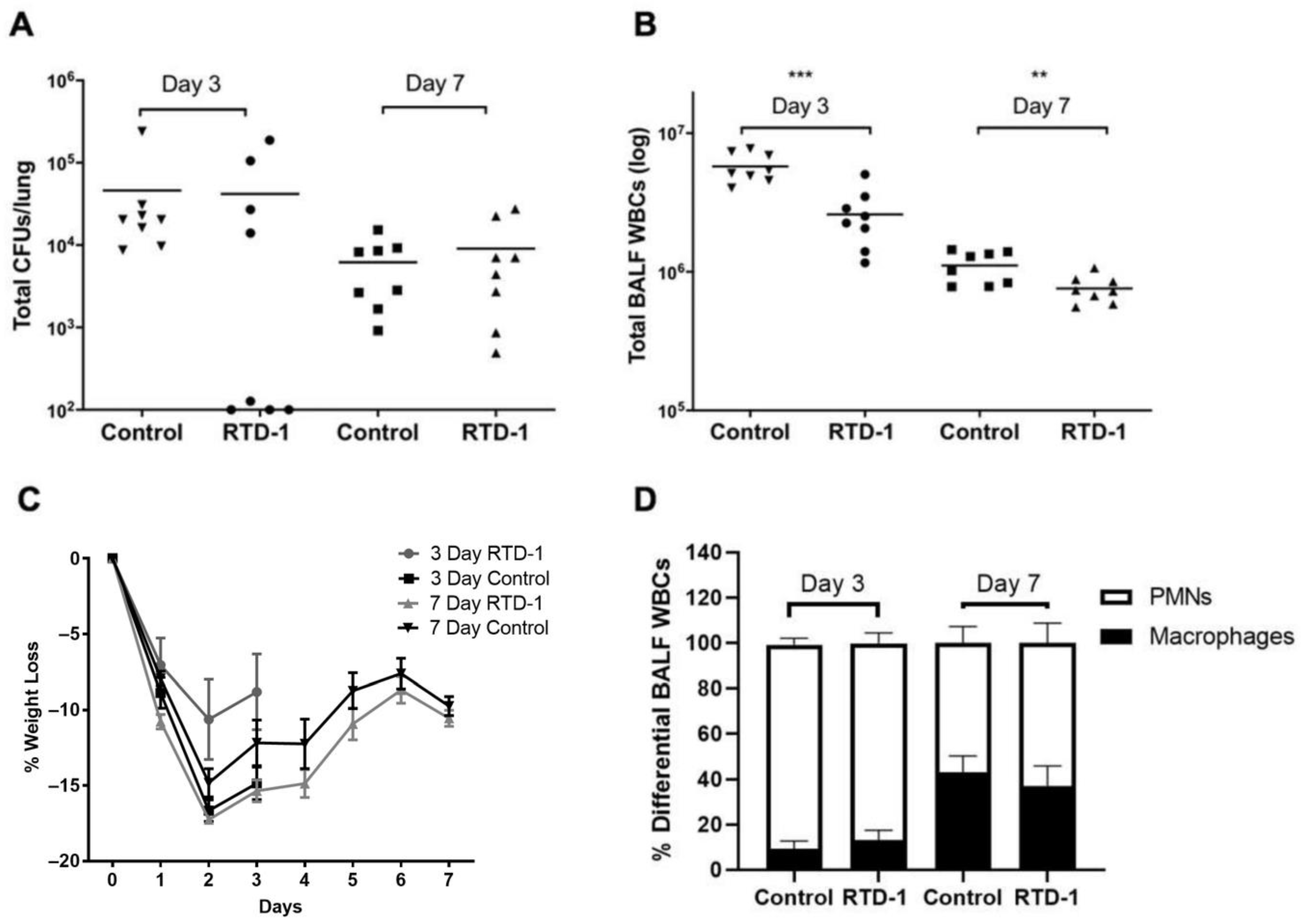
3.1. In Vivo Efficacy of Aerosolized RTD-1 in Mice with Chronic Pseudomonas aeruginosa Lung Infection
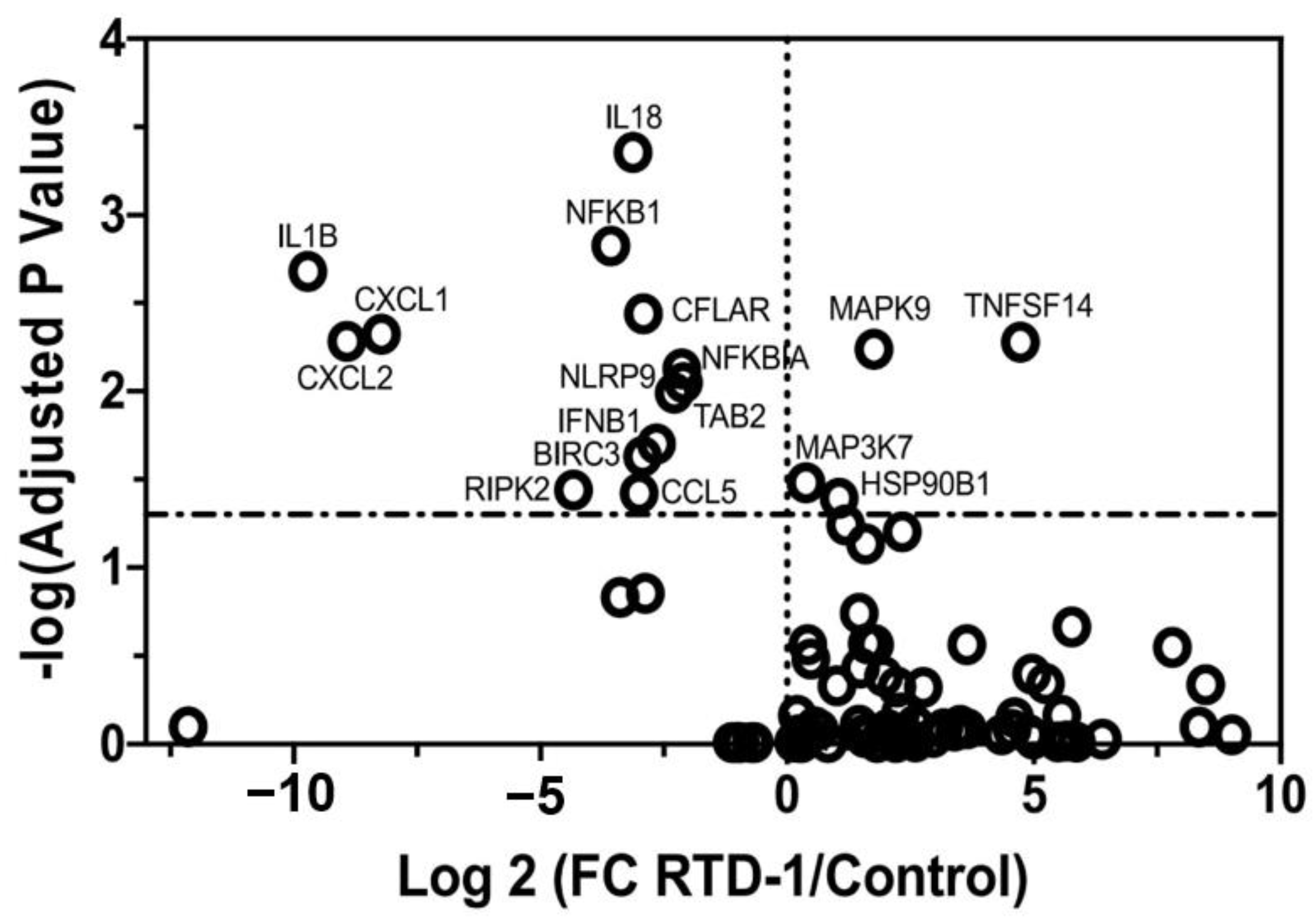
3.2. Biological Targets of Anti-Inflammatory Activity of RTD-1 during Chronic Pseudomonas aeruginosa Lung Infection
3.3. RTD-1 Inhibits NF-κB Activity
3.4. RTD-1 Inhibits Inflammatory Gene Expression
3.5. RTD-1 Treatment Reduces Cytokine Production In Vitro
3.6. RTD-1 Downregulates Inflammasome-Associated Gene Expression
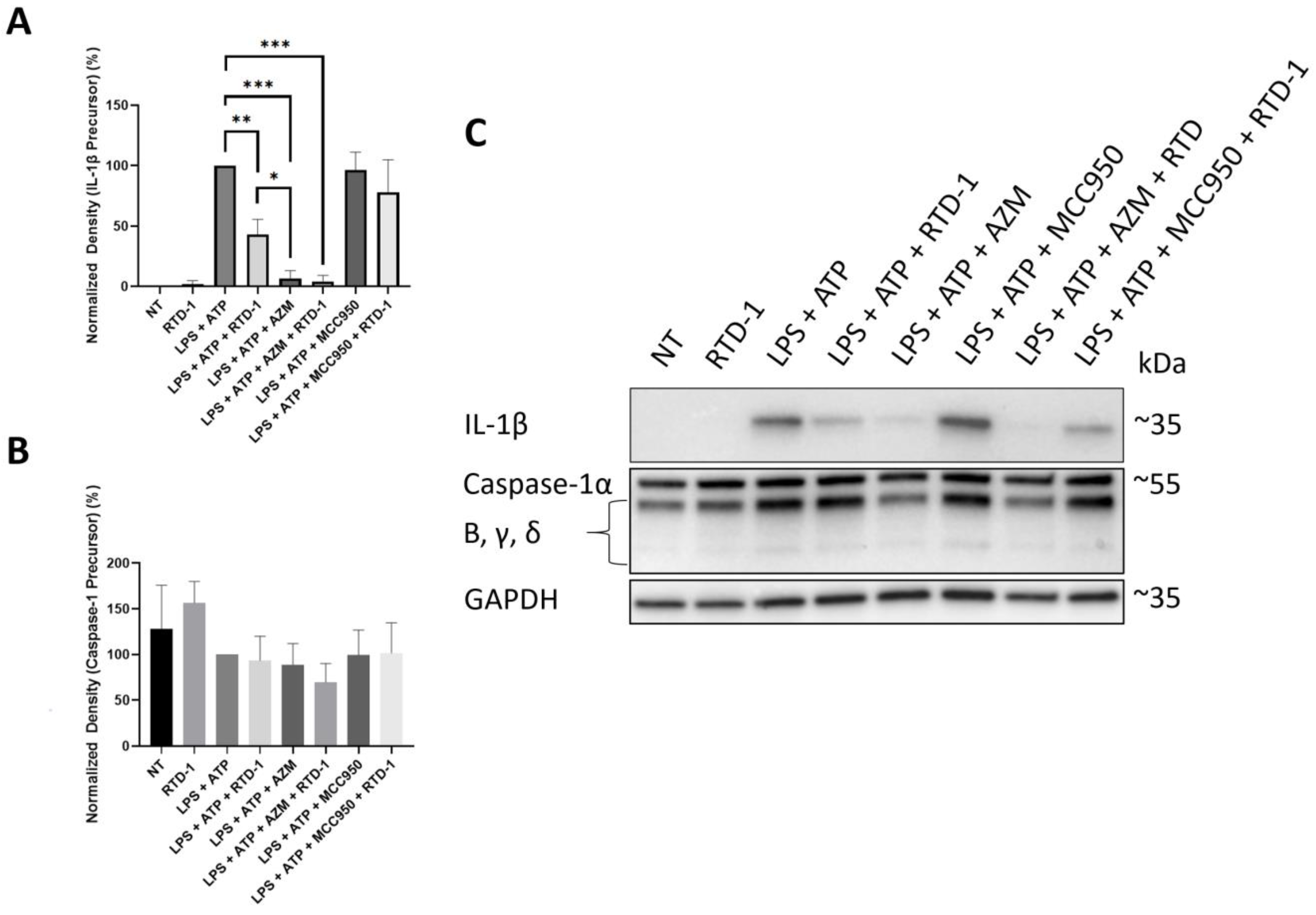
3.7. RTD-1 Decreases IL-1β Precursor Formation
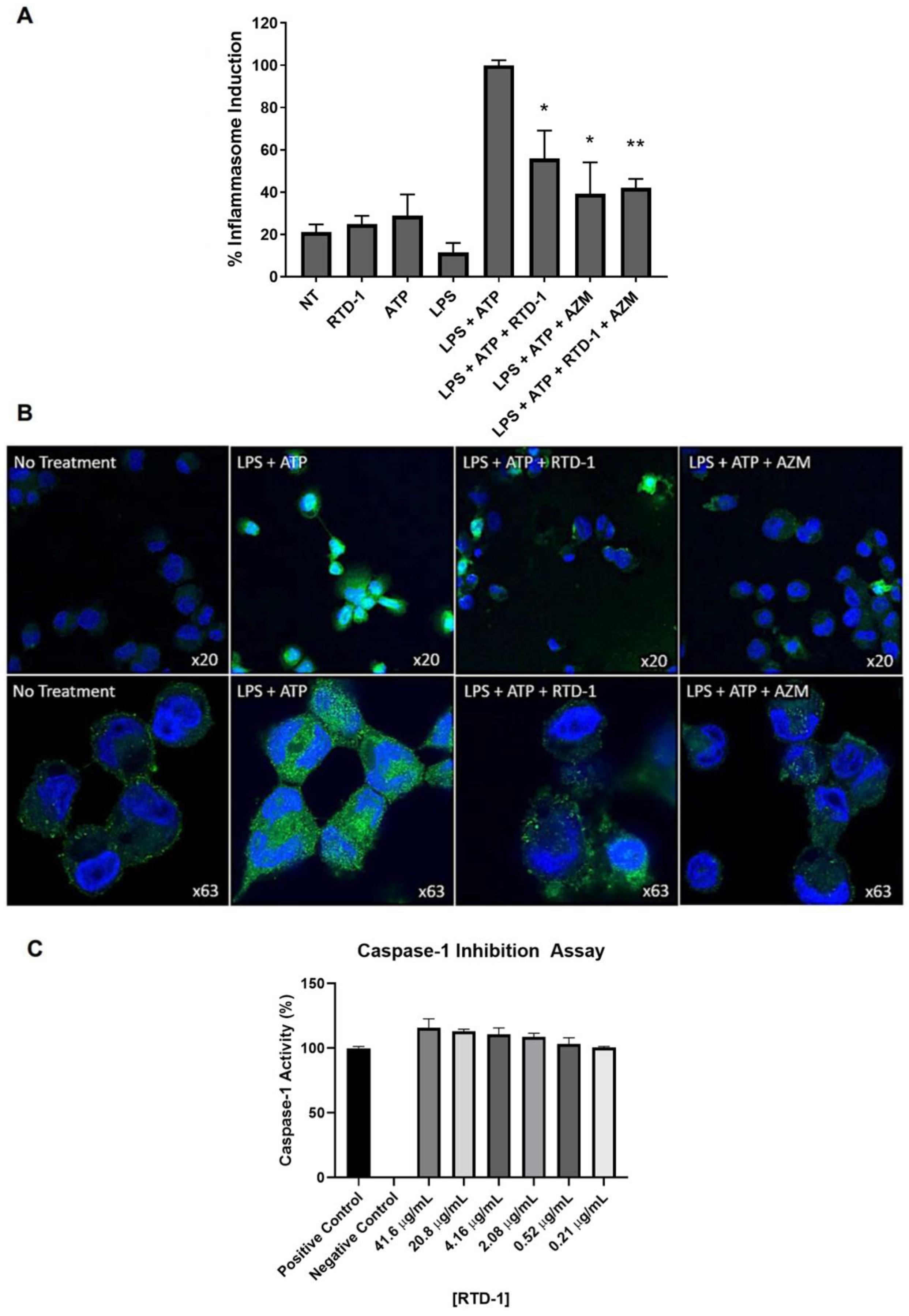
3.8. RTD-1 Inhibits Inflammasome Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
Abbreviations
References
- De Boeck, K.; Zolin, A.; Cuppens, H.; Olesen, H.V.; Viviani, L. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Courtney, J.M.; Ennis, M.; Elborn, J.S. Cytokines and inflammatory mediators in cystic fibrosis. J. Cyst. Fibros. 2004, 3, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, T.A.; Brennan, S.; Gard, S.; Berry, L.; Gangell, C.; Stick, S.M.; Clements, B.S.; Sly, P.D. Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. Eur. Respir. J. 2009, 33, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfield, T.L.; Panuska, J.R.; Konstan, M.W.; Hilliard, K.A.; Hilliard, J.B.; Ghnaim, H.; Berger, M. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995, 152, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Osika, E.; Cavaillon, J.M.; Chadelat, K.; Boule, M.; Fitting, C.; Tournier, G.; Clement, A. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur. Respir. J. 1999, 14, 339–346. [Google Scholar] [CrossRef]
- Eickmeier, O.; Huebner, M.; Herrmann, E.; Zissler, U.; Rosewich, M.; Baer, P.C.; Buhl, R.; Schmitt-Grohe, S.; Zielen, S.; Schubert, R. Sputum biomarker profiles in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) and association between pulmonary function. Cytokine 2010, 50, 152–157. [Google Scholar] [CrossRef]
- Tang, A.; Sharma, A.; Jen, R.; Hirschfeld, A.F.; Chilvers, M.A.; Lavoie, P.M.; Turvey, S.E. Inflammasome-mediated IL-1beta production in humans with cystic fibrosis. PLoS ONE 2012, 7, e37689. [Google Scholar] [CrossRef] [Green Version]
- Verhoef, P.A.; Kertesy, S.B.; Lundberg, K.; Kahlenberg, J.M.; Dubyak, G.R. Inhibitory effects of chloride on the activation of caspase-1, IL-1beta secretion, and cytolysis by the P2X7 receptor. J. Immunol. 2005, 175, 7623–7634. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.C.; FitzSimmons, S.C.; Allen, D.B.; Kosorok, M.R.; Rosenstein, B.J.; Campbell, P.W.; Farrell, P.M. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N. Engl. J. Med. 2000, 342, 851–859. [Google Scholar] [CrossRef]
- Konstan, M.W.; Schluchter, M.D.; Xue, W.; Davis, P.B. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Lands, L.C.; Dauletbaev, N. High-Dose Ibuprofen in Cystic Fibrosis. Pharmaceuticals 2010, 3, 2213–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, C.; Tamalet, A.; Thien, H.V.; Taytard, J.; Perisson, C.; Nathan, N.; Clement, A.; Boelle, P.Y.; Corvol, H. Long-term effects of azithromycin in patients with cystic fibrosis. Respir. Med. 2016, 117, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, G.Y.; He, Q.; Xiang-Lian, L.; Ya-Nan, Y.; Si-Te, F. Prolonged treatment with macrolides in adult patients with non-cystic fibrosis bronchiectasis: Meta-analysis of randomized controlled trials. Pulm. Pharmacol. Ther. 2014, 29, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.E.; Guha, K.; Piper, S.; Banya, W.; Bilton, D.; Hodson, M.E. A retrospective analysis of the impact of azithromycin maintenance therapy on adults attending a UK cystic fibrosis clinic. J. Cyst. Fibros. 2013, 12, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, L.; Wang, H.; Tai, S.; Liu, H.; Zhang, D. AWRK6, A Synthetic Cationic Peptide Derived from Antimicrobial Peptide Dybowskin-2CDYa, Inhibits Lipopolysaccharide-Induced Inflammatory Response. Int. J. Mol. Sci. 2018, 19, 600. [Google Scholar] [CrossRef] [Green Version]
- Jayne, J.G.; Bensman, T.J.; Schaal, J.B.; Park, A.Y.J.; Kimura, E.; Tran, D.; Selsted, M.E.; Beringer, P.M. Rhesus theta-Defensin-1 Attenuates Endotoxin-induced Acute Lung Injury by Inhibiting Proinflammatory Cytokines and Neutrophil Recruitment. Am. J. Respir. Cell Mol. Biol. 2018, 58, 310–319. [Google Scholar] [CrossRef]
- Wuerth, K.C.; Falsafi, R.; Hancock, R.E.W. Synthetic host defense peptide IDR-1002 reduces inflammation in Pseudomonas aeruginosa lung infection. PLoS ONE 2017, 12, e0187565. [Google Scholar] [CrossRef] [Green Version]
- Bensman, T.J.; Jayne, J.G.; Sun, M.; Kimura, E.; Meinert, J.; Wang, J.C.; Schaal, J.B.; Tran, D.; Rao, A.P.; Akbari, O.; et al. Efficacy of Rhesus Theta-Defensin-1 in Experimental Models of Pseudomonas aeruginosa Lung Infection and Inflammation. Antimicrob. Agents Chemother. 2017, 61, e00154-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beringer, P.M.; Bensman, T.J.; Ho, H.; Agnello, M.; Denovel, N.; Nguyen, A.; Wong-Beringer, A.; She, R.; Tran, D.Q.; Moskowitz, S.M.; et al. Rhesus theta-defensin-1 (RTD-1) exhibits in vitro and in vivo activity against cystic fibrosis strains of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2016, 71, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, K.P.; Kamdar, K.; Yamaki, J.; Le, V.V.; Tran, D.; Tran, P.; Selsted, M.E.; Ouellette, A.J.; Wong-Beringer, A. Microbicidal effects of alpha- and theta-defensins against antibiotic-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Innate Immun. 2015, 21, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tongaonkar, P.; Trinh, K.K.; Schaal, J.B.; Tran, D.; Gulko, P.S.; Ouellette, A.J.; Selsted, M.E. Rhesus macaque theta-defensin RTD-1 inhibits proinflammatory cytokine secretion and gene expression by inhibiting the activation of NF-kappaB and MAPK pathways. J. Leukoc. Biol. 2015, 98, 1061–1070. [Google Scholar] [CrossRef]
- Schaal, J.B.; Tran, D.; Tran, P.; Osapay, G.; Trinh, K.; Roberts, K.D.; Brasky, K.M.; Tongaonkar, P.; Ouellette, A.J.; Selsted, M.E. Rhesus macaque theta defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis. PLoS ONE 2012, 7, e51337. [Google Scholar] [CrossRef] [PubMed]
- Schaal, J.B.; Maretzky, T.; Tran, D.Q.; Tran, P.A.; Tongaonkar, P.; Blobel, C.P.; Ouellette, A.J.; Selsted, M.E. Macrocyclic theta-defensins suppress tumor necrosis factor-alpha (TNF-alpha) shedding by inhibition of TNF-alpha-converting enzyme. J. Biol. Chem. 2018, 293, 2725–2734. [Google Scholar] [CrossRef] [Green Version]
- Basso, V.; Garcia, A.; Tran, D.Q.; Schaal, J.B.; Tran, P.; Ngole, D.; Aqeel, Y.; Tongaonkar, P.; Ouellette, A.J.; Selsted, M.E. Fungicidal Potency and Mechanisms of theta-Defensins against Multidrug-Resistant Candida Species. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Schaal, J.B.; Tran, D.Q.; Subramanian, A.; Patel, R.; Laragione, T.; Roberts, K.D.; Trinh, K.; Tongaonkar, P.; Tran, P.A.; Minond, D.; et al. Suppression and resolution of autoimmune arthritis by rhesus theta-defensin-1, an immunomodulatory macrocyclic peptide. PLoS ONE 2017, 12, e0187868. [Google Scholar] [CrossRef] [Green Version]
- Bragonzi, A.; Wiehlmann, L.; Klockgether, J.; Cramer, N.; Worlitzsch, D.; Doring, G.; Tummler, B. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 2006, 152, 3261–3269. [Google Scholar] [CrossRef] [Green Version]
- Facchini, M.; De Fino, I.; Riva, C.; Bragonzi, A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Danziger, L.H.; Gotfried, M.H. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 2003, 47, 2450–2457. [Google Scholar] [CrossRef] [Green Version]
- Kretzschmar, A.; Schulke, J.P.; Masana, M.; Durre, K.; Muller, M.B.; Bausch, A.R.; Rein, T. The Stress-Inducible Protein DRR1 Exerts Distinct Effects on Actin Dynamics. Int. J. Mol. Sci. 2018, 19, 3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, N.L.; Lin, D.; Pelleymounter, L.; Moon, I.; Stilling, G.; Eckloff, B.W.; Wieben, E.D.; Redfield, M.M.; Burnett, J.C., Jr.; Yee, V.C.; et al. Natriuretic peptide receptor-3 gene (NPR3): Nonsynonymous polymorphism results in significant reduction in protein expression because of accelerated degradation. Circ. Cardiovasc. Genet. 2013, 6, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S186–S195. [Google Scholar] [CrossRef]
- Bruscia, E.M.; Bonfield, T.L. Cystic Fibrosis Lung Immunity: The Role of the Macrophage. J. Innate Immun. 2016, 8, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Lendermon, E.A.; Coon, T.A.; Bednash, J.S.; Weathington, N.M.; McDyer, J.F.; Mallampalli, R.K. Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir. Res. 2017, 18, 131. [Google Scholar] [CrossRef] [Green Version]
- Tukaj, S.; Wegrzyn, G. Anti-Hsp90 therapy in autoimmune and inflammatory diseases: A review of preclinical studies. Cell Stress Chaperones. 2016, 21, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- McElvaney, O.J.; Palsson-McDermott, Z.Z.; Gunaratnam, E.; O’Neill, L.A.J.; Reeves, O.; McElvaney, N.G. Metabolic Reprogramming of the Cystic Fibrosis Neutrophil Drives Interleukin-1β via the NLRP3 Inflammasome. Am. J. Respir. Crit. Care Med. 2019, 199, A6188. [Google Scholar]
- Rimessi, A.; Bezzerri, V.; Patergnani, S.; Marchi, S.; Cabrini, G.; Pinton, P. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 2015, 6, 6201. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, Y.; Zhang, Y.; Li, M.; Li, D.; Huang, X.; Wu, Y.; Pu, J.; Wu, M. Pseudomonas aeruginosa Triggers Macrophage Autophagy To Escape Intracellular Killing by Activation of the NLRP3 Inflammasome. Infect. Immun. 2016, 84, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomo, J.; Marchiol, T.; Piotet, J.; Fauconnier, L.; Robinet, M.; Reverchon, F.; Le Bert, M.; Togbe, D.; Buijs-Offerman, R.; Stolarczyk, M.; et al. Role of IL-1beta in experimental cystic fibrosis upon P. aeruginosa infection. PLoS ONE 2014, 9, e114884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, S.T.; Dittrich, A.S.; Garratt, L.W.; Turkovic, L.; Frey, D.L.; Stick, S.M.; Mall, M.A.; Kicic, A. Interleukin-1 is associated with inflammation and structural lung disease in young children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.; Murphy, A.; Zou, F.; Gerard, C.; Klanderman, B.; Schuemann, B.; Lazarus, R.; Garcia, K.C.; Celedon, J.C.; Drumm, M.; et al. IL1B polymorphisms modulate cystic fibrosis lung disease. Pediatr. Pulmonol. 2009, 44, 580–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef]
- Veliz Rodriguez, T.; Moalli, F.; Polentarutti, N.; Paroni, M.; Bonavita, E.; Anselmo, A.; Nebuloni, M.; Mantero, S.; Jaillon, S.; Bragonzi, A.; et al. Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection. Infect. Immun. 2012, 80, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Balazs, A.; Mall, M.A. Mucus obstruction and inflammation in early cystic fibrosis lung disease: Emerging role of the IL-1 signaling pathway. Pediatr. Pulmonol. 2019, 54 (Suppl. 3), S5–S12. [Google Scholar] [CrossRef] [Green Version]
- Scambler, T.; Jarosz-Griffiths, H.H.; Lara-Reyna, S.; Pathak, S.; Wong, C.; Holbrook, J.; Martinon, F.; Savic, S.; Peckham, D.; McDermott, M.F. Excessive ENaC-mediated sodium influx drives NLRP3 inflammasome-dependent autoinflammation in cystic fibrosis. bioRxiv 2018. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dughbaj, M.A.; Jayne, J.G.; Park, A.Y.J.; Bensman, T.J.; Algorri, M.; Ouellette, A.J.; Selsted, M.E.; Beringer, P.M. Anti-Inflammatory Effects of RTD-1 in a Murine Model of Chronic Pseudomonas aeruginosa Lung Infection: Inhibition of NF-κB, Inflammasome Gene Expression, and Pro-IL-1β Biosynthesis. Antibiotics 2021, 10, 1043. https://doi.org/10.3390/antibiotics10091043
Dughbaj MA, Jayne JG, Park AYJ, Bensman TJ, Algorri M, Ouellette AJ, Selsted ME, Beringer PM. Anti-Inflammatory Effects of RTD-1 in a Murine Model of Chronic Pseudomonas aeruginosa Lung Infection: Inhibition of NF-κB, Inflammasome Gene Expression, and Pro-IL-1β Biosynthesis. Antibiotics. 2021; 10(9):1043. https://doi.org/10.3390/antibiotics10091043
Chicago/Turabian StyleDughbaj, Mansour A., Jordanna G. Jayne, A Young J. Park, Timothy J. Bensman, Marquerita Algorri, Andre J. Ouellette, Michael E. Selsted, and Paul M. Beringer. 2021. "Anti-Inflammatory Effects of RTD-1 in a Murine Model of Chronic Pseudomonas aeruginosa Lung Infection: Inhibition of NF-κB, Inflammasome Gene Expression, and Pro-IL-1β Biosynthesis" Antibiotics 10, no. 9: 1043. https://doi.org/10.3390/antibiotics10091043
APA StyleDughbaj, M. A., Jayne, J. G., Park, A. Y. J., Bensman, T. J., Algorri, M., Ouellette, A. J., Selsted, M. E., & Beringer, P. M. (2021). Anti-Inflammatory Effects of RTD-1 in a Murine Model of Chronic Pseudomonas aeruginosa Lung Infection: Inhibition of NF-κB, Inflammasome Gene Expression, and Pro-IL-1β Biosynthesis. Antibiotics, 10(9), 1043. https://doi.org/10.3390/antibiotics10091043





