Diagnostic Methods of Clostridioides difficile Infection and Clostridioides difficile Ribotypes in Studied Sample
Abstract
:1. Introduction
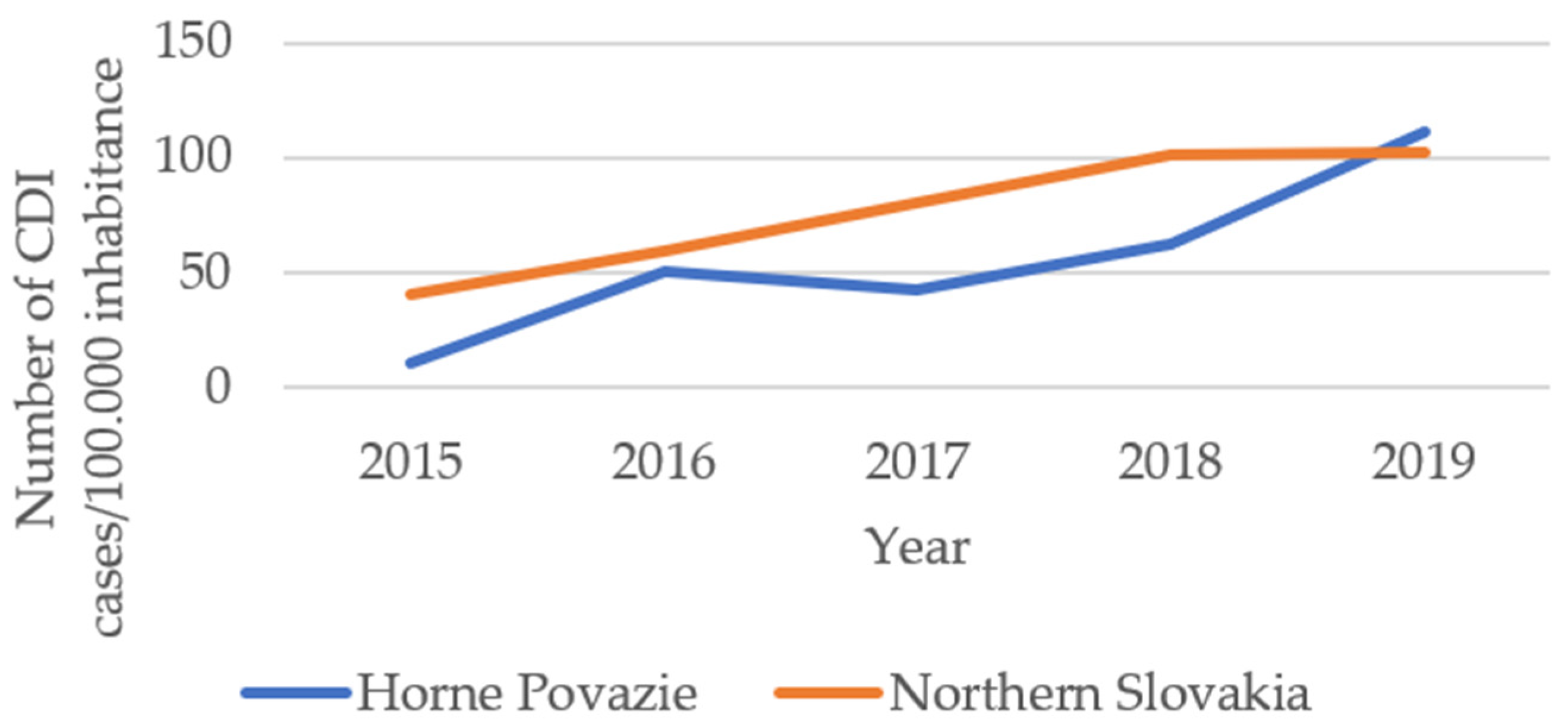
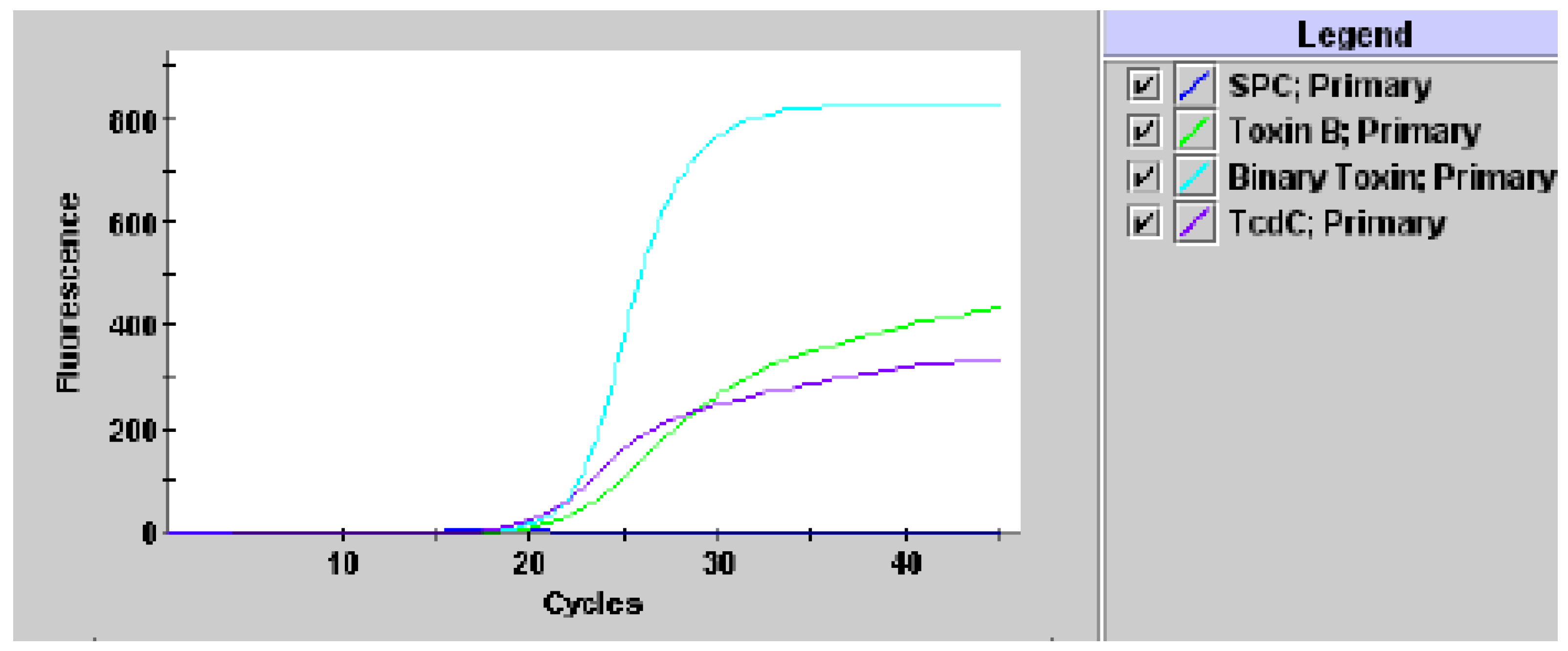
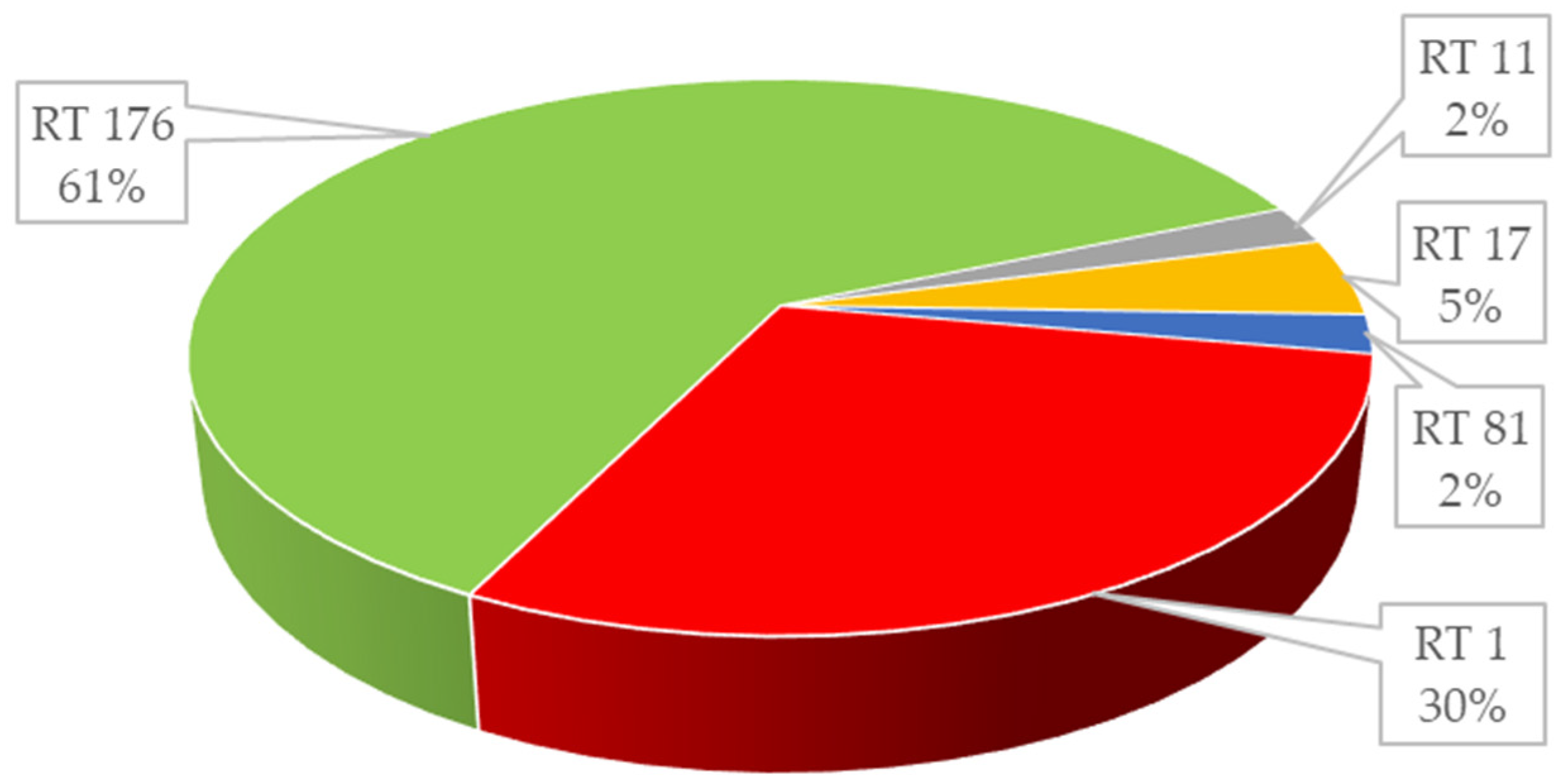
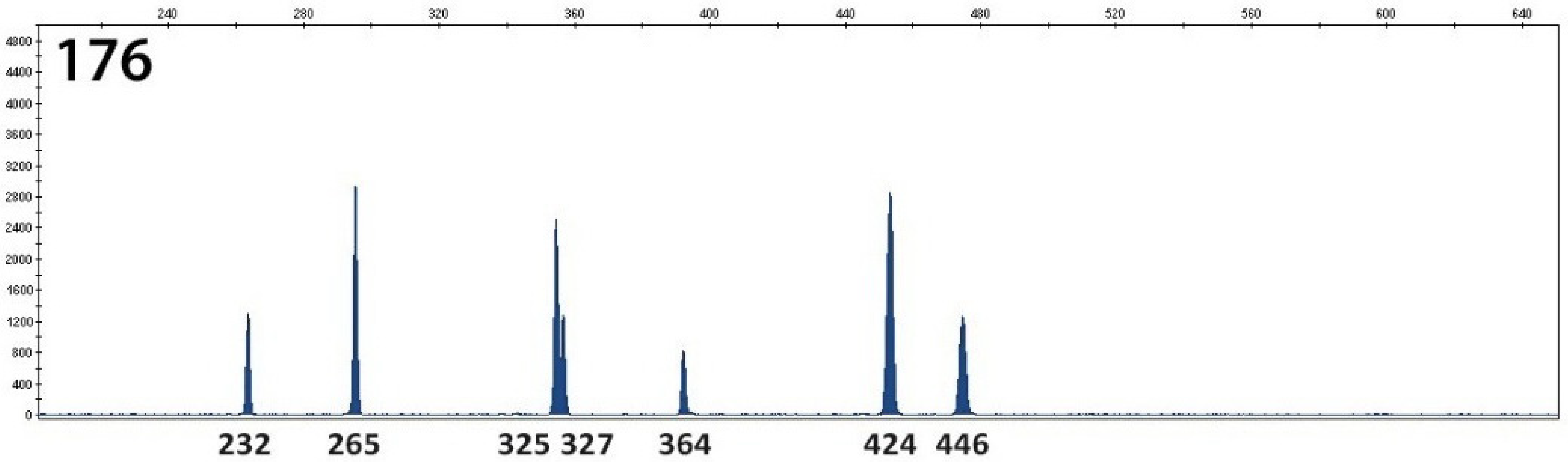
2. Results
3. Discussion
4. Materials and Methods
4.1. Sampling
4.2. Enzymatic and Immunoenzymatic Assays and Cultivation
4.3. MALDI-TOF MS Identification
4.4. Antibiotic Susceptibility Testing
4.5. Polymerase Chain Reaction PCR Ribotyping
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, K.A.; Longshaw, C.; Davis, G.L.; Bouza, E.; Barbut, F.; Barna, Z.; Delmée, M.; Fitzpatrick, F.; Ivanova, K.; Kuijper, E.; et al. Underdiagnosis of Clostridium difficile across Europe: The European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect. Dis. 2014, 14, 1208–1219. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. European Surveillance of Clostridium Difficile Infections; Surveillance Protocol Version 2.3.; ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- Krůtová, M.; Wilcox, M.; Kuijper, E. The pitfalls of laboratory diagnostics of Clostridium difficile infection. Clin. Microbiol. Infect. 2018, 24, 682–683. [Google Scholar] [CrossRef] [Green Version]
- Novakova, E.; Stefkovicova, M.; Kopilec, M.G.; Novak, M.; Kotlebová, N.; Kuijper, E.; Krutova, M.; Garabasova, M.K. The emergence of Clostridium difficile ribotypes 027 and 176 with a predominance of the Clostridium difficile ribotype 001 recognized in Slovakia following the European standardized Clostridium difficile infection surveillance of 2016. Int. J. Infect. Dis. 2020, 90, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Mazakova, I.; Sadlonova, V.; Cervenova, T.; Hudeckova, H. Appearance of Clostridium difficile infections in health care institutions in Slovakia and in the district of martin. Acta Med. Martiniana 2018, 18, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Crobach, M.; Planche, T.; Eckert, C.; Barbut, F.; Terveer, E.; Dekkers, O.; Wilcox, M.; Kuijper, E. European Society of Clinical Microbiology and Infectious Diseases: Update of the diagnostic guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2016, 22, S63–S81. [Google Scholar] [CrossRef] [Green Version]
- Grześkowiak, M.; Pieper, R.; Huynh, H.A.; Cutting, S.M.; Vahjen, W.; Zentek, J. Impact of early-life events on the susceptibility to Clostridium difficile colonisation and infection in the offspring of the pig. Gut Microbes 2018, 10, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Gateau, C.; Couturier, J.; Coia, J.; Barbut, F. How to: Diagnose infection caused by Clostridium difficile. Clin. Microbiol. Infect. 2018, 24, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Carey-Ann, B.D.; Carroll, K.C. Diagnosis of Clostridium difficile Infection: An Ongoing Conundrum for Clinicians and for Clinical Laboratories. Clin. Microbiol. Rev. 2013, 26, 604–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terveer, E.M.; Crobach, M.J.T.; Sanders, I.M.J.G.; Vos, M.C.; Verduin, C.M.; Kuijper, E.J. Detection of Clostridium difficile in Feces of Asymptomatic Patients Admitted to the Hospital. J. Clin. Microbiol. 2017, 55, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debast, S.; Bauer, M.; Kuijper, E. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Origüen, J.; Corbella, L.; Orellana, M.D.L.A.; Fernández-Ruiz, M.; López-Medrano, F.; Juan, R.S.; Lizasoain, M.; Ruiz-Merlo, T.; Morales-Cartagena, A.; Maestro, G.; et al. Comparison of the clinical course of Clostridium difficile infection in glutamate dehydrogenase-positive toxin-negative patients diagnosed by PCR to those with a positive toxin test. Clin. Microbiol. Infect. 2018, 24, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Jarčuška, P.; Bátovský, M.; Drgoňa, L.; Lišková, A.; Holečková, K. Odporúčaný Postup Diagnostiky a Liečby Kolitídy Spôsobenej Clostridium Difficile; Slovenská Zdravotnícka Univerzita v Bratislave: Bratislava, Slovakia, 2015; Volume 12, p. S1. ISSN 1336-4790. [Google Scholar]
- Brazier, J.S.; Borriello, S.P. Microbiology, Epidemiology and Diagnosis of Clostridium difficile Infection. Curr. Top. Microbiol. Immunol. 2000, 250, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Breznicky, J.; Novak, M. The most common etiological agents of prosthetic joint infections in orthopaedics. Med. Glas. 2019, 16, 185–189. [Google Scholar] [CrossRef]
- Walk, S.T.; Micic, D.; Jain, R.; Lo, E.S.; Trivedi, I.; Liu, E.; Almassalha, L.M.; Ewing, S.A.; Ring, C.; Galecki, A.T.; et al. Clostridium difficile Ribotype Does Not Predict Severe Infection. Clin. Infect. Dis. 2012, 55, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Xpert® C.difficile/Epi 1 300-9680, Rev. J April 2020, Cepheid 904 Caribbean Drive Sunnyvale, CA 9408, USA. 2016. Available online: https://www.cepheid.com/Package%20Insert%20Files/300-9680-Xpert-C.%20diff-Epi%20US-IVD%20PI%20Rev%20J.pdf (accessed on 7 August 2021).
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Robotham, J.; Wilcox, M. Updated Guidance on the Diagnosis and Reporting of Clostridium Difficile. NHS, Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection (ARHAI). 2012. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment.data/file/146808/dh_133016.pdf.pdf (accessed on 20 January 2020).
- Zou, J.; Leung, V.; Champagne, S.; Hinch, M.; Wong, A.; Lloyd-Smith, E.; Nguyen, T.T.; Romney, M.G.; Sharma, A.; Payne, M.; et al. Clinical heterogeneity of patients with stool samples testing PCR+/Tox− from a two-step Clostridium difficile diagnostic algorithm. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2355–2359. [Google Scholar] [CrossRef]
- Freeman, J.; Vernon, J.; Pilling, S.; Morris, K.; Nicholson, S.; Shearman, S.; Longshaw, C.; Wilcox, M. The ClosER study: Results from a three-year pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes, 2011–2014. Clin. Microbiol. Infect. 2017, 24, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Krehelova, M.; Nyč, O.; Sinajová, E.; Krutova, M. The predominance and clustering of Clostridioides (Clostridium) difficile PCR ribotype 001 isolates in three hospitals in Eastern Slovakia, 2017. Folia Microbiol. 2018, 64, 49–54. [Google Scholar] [CrossRef]
- Nyc, O.; Krutova, M.; Liskova, A.; Matejkova, J.; Drabek, J.; Kuijper, E. The emergence of Clostridium difficile PCR-ribotype 001 in Slovakia. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1701–1708. [Google Scholar] [CrossRef]
- Kecerova, Z.; Cizek, A.; Nyc, O.; Krutova, M. Clostridium difficile isolates derived from Czech horses are resistant to enrofloxacin; cluster to clades 1 and 5 and ribotype 033 predominates. Anaerobe 2019, 56, 17–21. [Google Scholar] [CrossRef]
- Novakova, E.; Kotlebova, N.; Gryndlerova, A.; Novak, M.; Vladarova, M.; Wilcox, M.; Kuijper, E.; Krutova, M. An Outbreak of Clostridium (Clostridioides) difficile Infections within an Acute and Long-Term Care Wards due to Moxifloxacin-Resistant PCR Ribotype 176 Genotyped as PCR Ribotype 027 by a Commercial Assay. J. Clin. Med. 2020, 9, 3738. [Google Scholar] [CrossRef]
- Krutova, M.; Matejkova, J.; Kuijper, E.J.; Drevinek, P.; Nyc, O.; Czech Clostridium Difficile Study Group. Clostridium difficile PCR ribotypes 001 and 176—The common denominator of C. difficile infection epidemiology in the Czech Republic, 2014. EuroSurveillance 2016, 21, 29. [Google Scholar] [CrossRef]
- Krutova, M.; Matejkova, J.; Tkadlec, J.; Nyc, O. Antibiotic profiling of Clostridium difficile ribotype 176—A multidrug resistant relative to C. difficile ribotype 027. Anaerobe 2015, 36, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Dresler, J.; Krutova, M.; Fucikova, A.; Klimentova, J.; Hruzova, V.; Duracova, M.; Houdkova, K.; Salovska, B.; Matejkova, J.; Hubalek, M.; et al. Analysis of proteomes released from in vitro cultured eight Clostridium difficile PCR ribotypes revealed specific expression in PCR ribotypes 027 and 176 confirming their genetic relatedness and clinical importance at the proteomic level. Gut Pathog. 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Reigadas, E.; Alcalá, L.; Valerio, M.; Marín, M.; Martin, A.; Bouza, E. Toxin B PCR cycle threshold as a predictor of poor outcome ofClostridium difficileinfection: A derivation and validation cohort study. J. Antimicrob. Chemother. 2016, 71, 1380–1385. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.; Micic, D.; Natarajan, M.; Winters, S.; Kiel, M.J.; Walk, S.T.; Santhosh, K.; Mogle, J.A.; Galecki, A.T.; Lebar, W.; et al. Clostridium difficileRibotype 027: Relationship to Age, Detectability of Toxins A or B in Stool with Rapid Testing, Severe Infection, and Mortality. Clin. Infect. Dis. 2015, 61, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Murad, Y.M.; Perez, J.; Ybazeta, G.; Mavin, S.; Lefebvre, S.; Weese, J.S.; Rousseau, J.; Diaz-Mitoma, F.; Nokhbeh, R. False Negative Results in Clostridium difficile Testing. BMC Infect. Dis. 2016, 16, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Fawley, W.N.; Knetsch, W.; MacCannell, D.R.; Harmanus, C.; Du, T.; Mulvey, M.R.; Paulick, A.; Anderson, L.; Kuijper, E.; Wilcox, M.H. Development and Validation of an Internationally-Standardized, High-Resolution Capillary Gel-Based Electrophoresis PCR-Ribotyping Protocol for Clostridium difficile. PLoS ONE 2015, 10, e0118150. [Google Scholar] [CrossRef] [Green Version]
- Janežič, S.; Štrumbelj, I.; Rupnik, M. Use of Modified PCR Ribotyping for Direct Detection of Clostridium difficile Ribotypes in Stool Samples. J. Clin. Microbiol. 2011, 49, 3024–3025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krutova, M.; Wilcox, M.; Kuijper, E. A two-step approach for the investigation of a Clostridium difficile outbreak by molecular methods. Clin. Microbiol. Infect. 2019, 25, 1300–1301. [Google Scholar] [CrossRef] [Green Version]
| Number of C. difficile Isolates | Fenotype Characteristics of C. difficile isolates | Genotype Characteristics of C. difficile isolates | ||||||
|---|---|---|---|---|---|---|---|---|
| GDH/ Culture | Toxins A/B (Rapid Test, ELISA) | MIC90 µg/mL MTZ | MIC90 µg/mL VAN | Gene for B Toxin (tcdB) | Genes cdtA, cdtB for Binary Toxins | Deletion of nt 117 in tcdC Gene (Susp. RT 027) | RT Ribotypes | |
| 13 | positive | positive | 0.047 | 1.5 | positive | Negative | Negative | 001 |
| 27 | positive | positive | 0.5 | 1.0 | positive | Positive | Positive | 176 |
| 1 | positive | positive | 0.125 | 0.5 | positive | Negative | Negative | 011 |
| 2 | positive | positive | 0.125 | 2.0 | positive | Negative | Negative | 017 |
| 1 | positive | positive | 0.125 | 0.5 | positive | Negative | Negative | 081 |
| Laboratory and Other Variables | Ribotypes | Mean (Median) Years | 95% Confidence Interval for Mean | Pearson p < 0.001 |
|---|---|---|---|---|
| Total proteins g/L | 001 | 61.10 (60.0) | (56.1–66.1) | 0.300 |
| 176 | 57.27 (59.0) | (51.2–63.0) | ||
| Albumin g/L | 001 | 29.39 (28.95) | (27.56–31.21) | 0.682 |
| 176 | 28.65 (28.95) | (24.94–32.36) | ||
| CRP mg/L | 001 | 78.07 (82.0) | (54.80–101.32) | 0.295 |
| 176 | 56.75 (26) | (18.36–95.14) | ||
| Glomerular filtration mL/s | 001 | 0.861 (0.73) | (0.687–1.034) | 0.054 |
| 176 | 1.156 (1.32) | (0.875–1.436) | ||
| Length of hospitalization days | 001 | 43.78 (44.0) | (36.04–51.52) | 0.175 |
| 176 | 35.10 (30.5) | (23.72–46.47) | ||
| Creatinine μmol/L | 001 | 122.21 (112.0) | (90.6–153.7) | 0.524 |
| 176 | 106.85 (76.0) | (66.9–146.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novakova, E.; Stofkova, Z.; Sadlonova, V.; Hleba, L. Diagnostic Methods of Clostridioides difficile Infection and Clostridioides difficile Ribotypes in Studied Sample. Antibiotics 2021, 10, 1035. https://doi.org/10.3390/antibiotics10091035
Novakova E, Stofkova Z, Sadlonova V, Hleba L. Diagnostic Methods of Clostridioides difficile Infection and Clostridioides difficile Ribotypes in Studied Sample. Antibiotics. 2021; 10(9):1035. https://doi.org/10.3390/antibiotics10091035
Chicago/Turabian StyleNovakova, Elena, Zuzana Stofkova, Vladimira Sadlonova, and Lukas Hleba. 2021. "Diagnostic Methods of Clostridioides difficile Infection and Clostridioides difficile Ribotypes in Studied Sample" Antibiotics 10, no. 9: 1035. https://doi.org/10.3390/antibiotics10091035
APA StyleNovakova, E., Stofkova, Z., Sadlonova, V., & Hleba, L. (2021). Diagnostic Methods of Clostridioides difficile Infection and Clostridioides difficile Ribotypes in Studied Sample. Antibiotics, 10(9), 1035. https://doi.org/10.3390/antibiotics10091035







