High Serum Levels of Toxin A Correlate with Disease Severity in Patients with Clostridioides difficile Infection
Abstract
:1. Introduction
2. Results
2.1. Method Set-Up
2.2. Clinical Features of CDI Patients
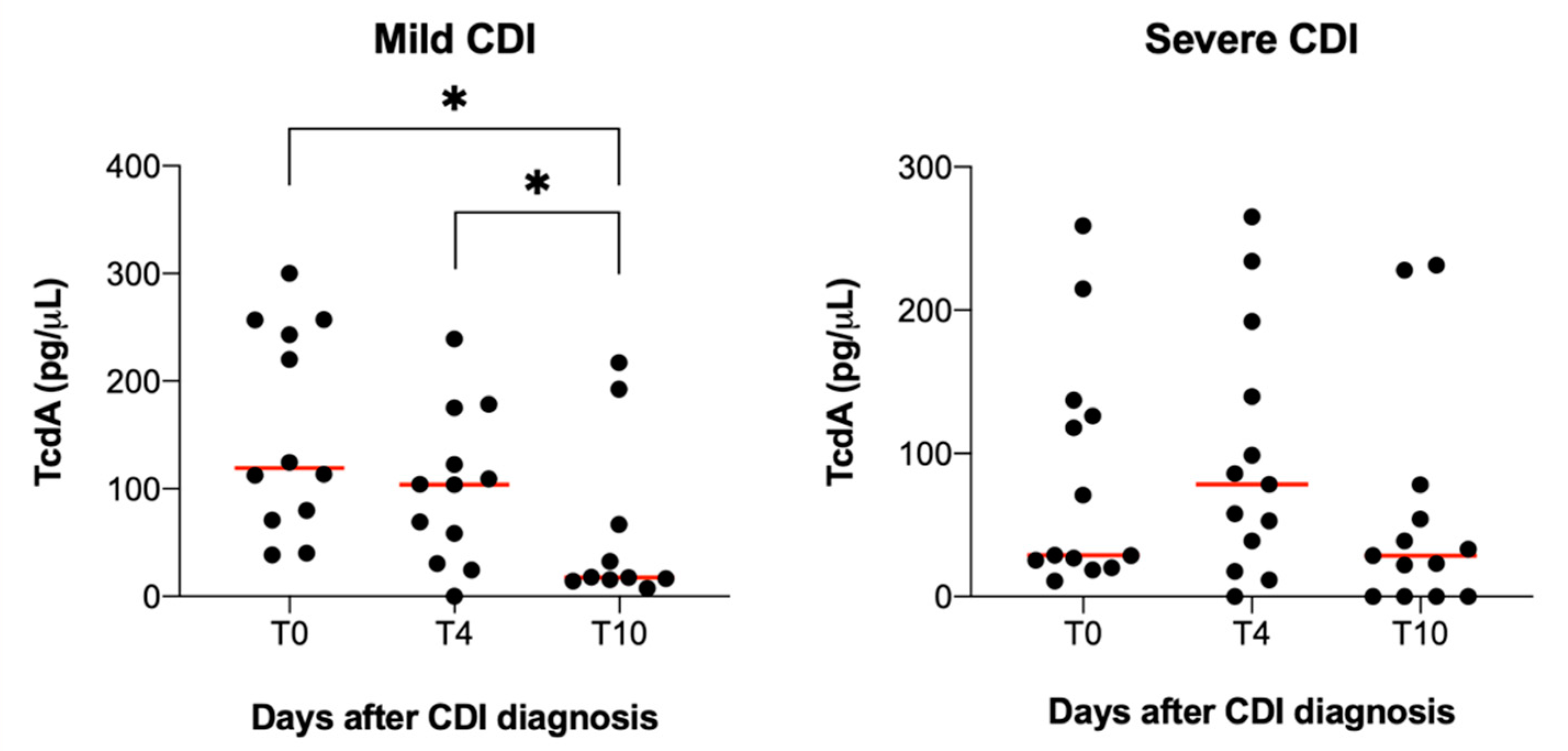
2.3. TcdA and TcdB Serum Levels and CDI Severity
3. Discussion
4. Materials and Methods
4.1. Study Design
4.1.1. Healthy Blood Donors Enrolment
4.1.2. CDI Patient Enrolment
4.2. Experimental Methodology
4.2.1. Preparation of Serum Samples from Healthy Controls and CDI Patients
4.2.2. Reagents and Antibodies
4.2.3. Dot-Blot Analysis
Standard Reference
Determination of Toxins Concentration in Serum Samples Derived from CDI Patients and Healthy Controls
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, C.T.; Safdar, N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin. Inf. Dis. 2015, 60, S66–S71. [Google Scholar] [CrossRef] [Green Version]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 373, 287–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guery, B.; Galperine, T.; Barbut, F. Clostridioides difficile: Diagnosis and treatments. BMJ 2019, 366, l4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shellito, A.D.; Russell, M.M. Diverting loop ileostomy for Clostridium Difficile colitis: A systematic review and meta-analysis. Am. Surg. 2020, 86, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Abou Chakra, C.N.; Pépin, J.; Sirard, S.; Valiquette, L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: A systematic review. PLoS ONE 2014, 9, e98400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheitoyan-Pesant, C.; Abou Chakra, C.N.; Pepin, J.; Marcil-Heguy, A.; Nault, V.; Valiquette, L. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin. Infect. Dis. 2016, 62, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Kuehne, S.A.; Cartman, S.T.; Heap, J.T.; Kelly, M.L.; Cockayne, A.; Minton, N.P. The role of toxin A and toxin B in Clostridium difficile infection. Nature 2010, 467, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef]
- Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; di Masi, A. Clostridium difficile toxins A and B: Insights into pathogenic properties and extraintestinal effects. Toxins 2016, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.P. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin. Microbiol. Infect. 2012, 18, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Steele, J.; Chen, K.; Sun, X.; Zhang, Y.; Wang, H.; Tzipori, S.; Feng, H. Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J. Infect. Dis. 2011, 205, 384–391. [Google Scholar] [CrossRef]
- Qualman, S.J.; Petric, M.; Karmali, M.A.; Smith, C.R.; Hamilton, S.R. Clostridium difficile invasion and toxin circulation in fatal pediatric pseudomembranous colitis. Am. J. Clin. Pathol. 1990, 94, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Chen, K.; Wu, J.; Yang, Z.; Shi, L.; Barlow, L.L.; Aronoff, D.M.; Garey, K.W.; Savidge, T.C.; von Rosenvinge, E.C.; et al. Identification of toxemia in patients with Clostridium difficile infection. PLoS ONE 2015, 10, e0124235. [Google Scholar] [CrossRef] [PubMed]
- Burnham, C.-A.D.; Carroll, K.C. Diagnosis of Clostridium difficile infection: An ongoing conundrum for clinicians and for clinical laboratories. Clin. Microbiol. Rev. 2013, 26, 604–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprague, R.; Warny, K.; Pollock, N.; Daugherty, K.; Lin, Q.; Xu, H.; Cuddemi, C.; Barrett, C.; Chen, X.; Banz, A.; et al. Absence of toxemia in Clostridioides difficile infection: Results from ultrasensitive toxin assay of serum. Dig. Dis. Sci. 2020, 810, 1–4. [Google Scholar] [CrossRef]
- Graff, G.; Anderson, L.A.; Jaques, L.W. Preparation and purification of soybean lipoxygenase-derived unsaturated hydroperoxy and hydroxy fatty acids and determination of molar absorptivities of hydroxy fatty acids. Anal. Biochem. 1990, 188, 38–47. [Google Scholar] [CrossRef]
- Jin, L.; Engelhart, A.E.; Adamala, K.; Szostak, J.W. Preparation, purification, and use of fatty acid-containing liposomes. JoVE 2018, 132, e57324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, K.; Malani, P.N. Diagnosis and treatment of Clostridioides (Clostridium) difficile infection in adults in 2020. JAMA 2020, 323, 1403–1404. [Google Scholar] [CrossRef]
- Lyras, D.; O’Connor, J.R.; Howarth, P.M.; Sambol, S.P.; Carter, G.P.; Phumoonna, T.; Poon, R.; Adams, V.; Vedantam, G.; Johnson, S.; et al. Toxin B is essential for virulence of Clostridium difficile. Nature 2009, 458, 1176–1179. [Google Scholar] [CrossRef]
- Pollock, N.R.; Banz, A.; Chen, X.; Williams, D.; Xu, H.; Cuddemi, C.A.; Cui, A.X.; Perrotta, M.; Alhassan, E.; Riou, B.; et al. Comparison of Clostridioides difficile stool toxin concentrations in adults with symptomatic infection and asymptomatic carriage using an ultrasensitive quantitative immunoassay. Clin. Infect. Dis. 2019, 68, 78–86. [Google Scholar] [CrossRef]
- Di Masi, A.; Leboffe, L.; Polticelli, F.; Tonon, F.; Zennaro, C.; Caterino, M.; Stano, P.; Fischer, S.; Hägele, M.; Müller, M.; et al. Human serum albumin is an essential component of the host defense mechanism against Clostridium difficile intoxication. J. Infect. Dis. 2018, 218, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, S.; di Masi, A.; Turla, S.; Ascenzi, P.; Gouliouris, T.; Petrosillo, N. The protective role of albumin in Clostridium difficile infection: A step toward solving the puzzle. Infect. Control. Hosp. Epidemiol. 2015, 36, 1478–1479. [Google Scholar] [CrossRef] [Green Version]
- Kumarappa, V.S.; Patel, H.; Shah, A.; Baddoura, W.; DeBari, V.A. Temporal changes in serum albumin and total protein in patients with hospital-acquired Clostridium difficile infection. Ann. Clin. Lab. Sci. 2014, 44, 32–37. [Google Scholar]
- Carter, G.P.; Chakravorty, A.; Pham Nguyen, T.A.; Mileto, S.; Schreiber, F.; Li, L.; Howarth, P.; Clare, S.; Cunningham, B.; Sambol, S.P.; et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio 2015, 6, e00551. [Google Scholar] [CrossRef] [Green Version]
- Tonon, F.; Di Bella, S.; Grassi, G.; Luzzati, R.; Ascenzi, P.; di Masi, A.; Zennaro, C. Extra-intestinal Effects of C.difficile toxin A and B: An in vivo study using the Zebrafish embryo model. Cells 2020, 9, 2575. [Google Scholar] [CrossRef]
- Solomon, K.; Martin, A.J.; O’Donoghue, C.; Chen, X.; Fenelon, L.; Fanning, S.; Kelly, C.P.; Kyne, L. Mortality in patients with Clostridium difficile infection correlates with host pro-inflammatory and humoral immune responses. J. Med. Microbiol. 2013, 62, 1453–1460. [Google Scholar] [CrossRef]
- Vita, G.M.; De Simone, G.; Leboffe, L.; Montagnani, F.; Mariotti, D.; Di Bella, S.; Luzzati, R.; Gori, A.; Ascenzi, P.; di Masi, A. Human serum albumin binds Streptolysin O (SLO) toxin produced by Group a Streptococcus and inhibits its cytotoxic and hemolytic effects. Front. Immunol. 2020, 11, 2969. [Google Scholar] [CrossRef]
- Kuehne, S.A.; Collery, M.M.; Kelly, M.L.; Cartman, S.T.; Cockayne, A.; Minton, N.P. Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J. Infect. Dis. 2014, 209, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Savidge, T.C.; Pan, W.H.; Newman, P.; O’brien, M.; Anton, P.M.; Pothoulakis, C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 2003, 125, 413–420. [Google Scholar] [CrossRef]
- Carman, R.J.; Stevens, A.L.; Lyerly, M.W.; Hiltonsmith, M.F.; Stiles, B.G.; Wilkins, T.D. Clostridium difficile binary toxin (CDT) and diarrhea. Anaerobe 2011, 17, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, O.; Benz, R.; Popoff, M.R. Pore-forming activity of clostridial binary toxins. Biochim. Biophys. Acta 2016, 1858, 512–525. [Google Scholar] [CrossRef] [PubMed]
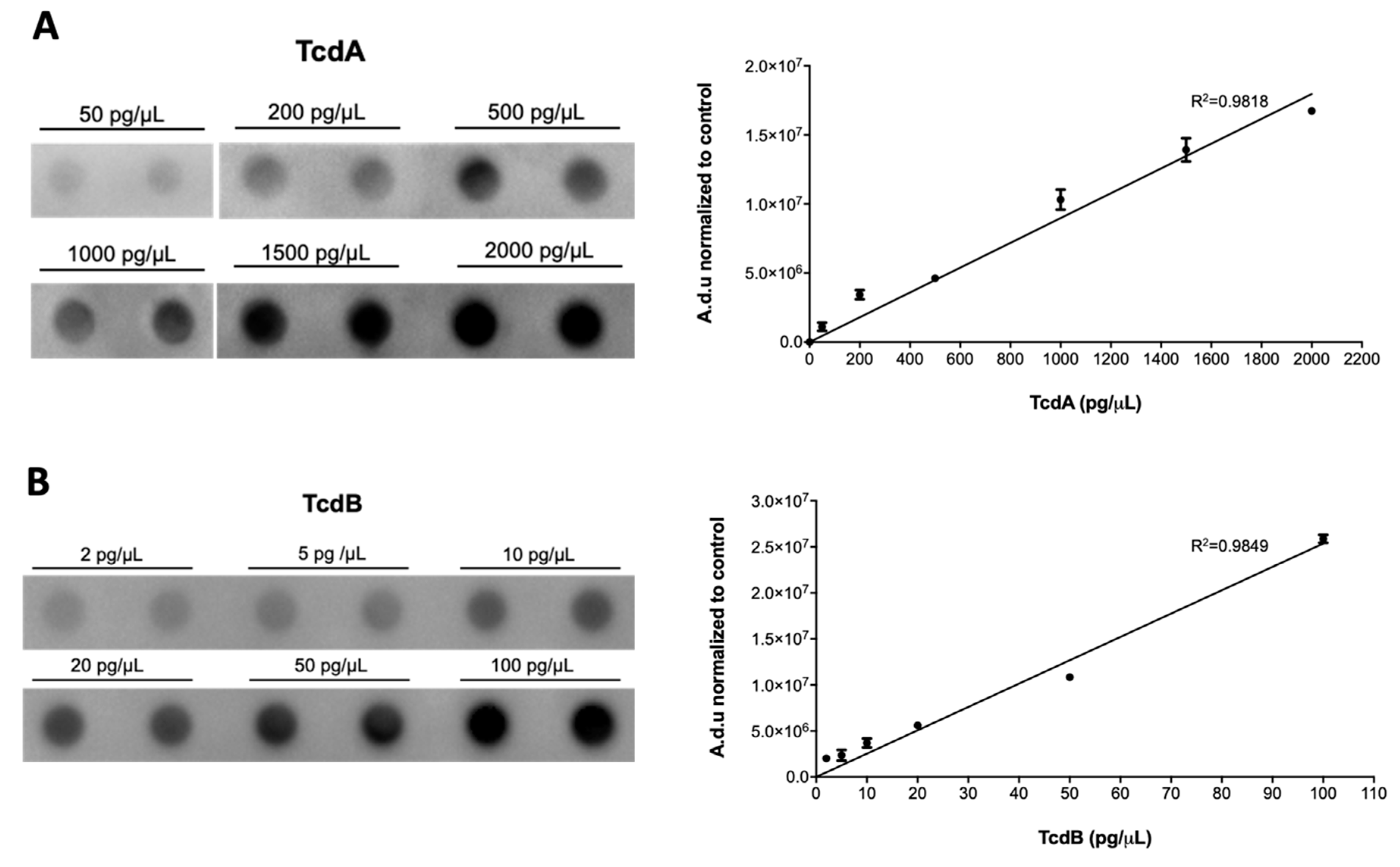
| Laboratory Findings | T0 | T4 | T10 |
|---|---|---|---|
| Total white blood cells peripheral count (103 cells/µL ± SD) | 11.04 ± 5.99 | 7.03 ± 2.59 | 6.33 ± 2.64 |
| Neutrophils peripheral count (103 cells/µL ± SD) | 8.18 ± 5.45 | 4.41 ± 2.25 | 4.11 ± 2.46 |
| Blood creatinine value (mg/dL ± SD) | 1.0 ± 0.6 | 0.8 ± 0.3 | 0.8 ± 0.2 |
| Blood albumin value (g/dL ± SD) | 3.3 ± 0.5 | 3.4 ± 0.6 | 3.6 ± 0.7 |
| Total toxemia (TcdA + TcdB, pg/µL ± SD) | 99.24 ± 103.24 | 89.74 ± 78.18 | 57.70 ± 72.70 |
| TcdA (pg/µL ± SD) | 92.28 ± 96.08 | 83.75 ± 74.81 | 49.96 ± 70.96 |
| TcdB (pg/µL ± SD) | 6.96 ± 25.71 | 5.99 ± 22.2 | 7.74 ± 22.6 |
| Mild CDI (N = 17) | Severe CDI (N = 18) | RR (95% CI) | Fisher’s Test * | |
|---|---|---|---|---|
| Female gender | 11 (64.7%) | 7 (38.8%) | 0.5 (0.2–1.2) | p = 0.1 |
| Mean age (years) | 55.7 | 63.5 | - | p = 0.2 |
| Mean age-adjusted CCI at admission ± SD | 2.4 ± 2.3 | 4.7 ± 2.9 | - | p = 0.01 |
| Comorbidities | ||||
| No comorbidities | 7 (41.1%) | 3 (16.6%) | 1.7 (0.9–3.2) | p = 0.1 |
| Cardiovascular disease | 4 (23.5%) | 9 (50.0%) | 1.9 (0.7–4.6) | p = 0.1 |
| Heart failure | 1 (5.9%) | 3 (16.6%) | 2.0 (0.3–11.6) | p = 0.3 |
| Diabetes | 2 (11.7%) | 5 (27.7%) | 1.8 (0.5–6.3) | p = 0.4 |
| Renal failure | 0 (0%) | 3 (16.6%) | - | p = 0.2 |
| Inflammatory bowel disease | 1 (5.8%) | 1 (5.5%) | 0.9 (0.2–4.0) | p = 1 |
| Chronic liver failure | 1 (5.8%) | 1 (5.5%) | 0.9 (0.2–4.0) | p = 1 |
| Neurological disease | 0 (0%) | 4 (22.2%) | - | p = 0.1 |
| Vasculitis | 0 (0%) | 2 (11.1%) | - | p = 0.4 |
| COPD | 6 (35.3%) | 3 (16.6%) | 0.6 (0.3–1.2) | p = 0.2 |
| Solid cancer | 1 (5.8%) | 2 (11.1%) | 1.5 (0.2–7) | p = 1 |
| Blood cancer | 1 (5.8%) | 2 (11.1%) | 1.5 (0.2–7.7) | p = 1 |
| Transplant, immunodeficiency, immunosuppression | 1 (5.8%) | 6 (33.3%) | 4 (0.6–25.2) | p = 0.08 |
| Other bacteria infections at admission | 6 (35.3%) | 7 (38.8%) | 1.0 (0.5–2.2) | p = 1 |
| Laboratory findings before CDI diagnosis | ||||
| Basal Albumin (g/dL ± SD) | 3.7 ± 0.6 | 3.5 ± 0.6 | - | p = 0.5 |
| Basal Creatinine (mg/dL ± SD) | 0.70 ± 0.1 | 0.8 ± 0.3 | - | p = 0.2 |
| Laboratory findings at CDI diagnosis (T0) | ||||
| White blood cell peripheral count (103 cells/µL ± SD) | 8.81 ± 3.52 | 13.15 ± 7.10 | - | p = 0.02 |
| Neutrophils peripheral count (103 cells/µL ± SD) | 5.70 ± 3.14 | 10.52 ± 6.18 | - | p = 0.007 |
| Creatinine (mg/dL ± SD) | 0.8 ± 0.3 | 1.2 ± 0.7 | - | p = 0.02 |
| Albumin (g/dL ± SD) | 3.5 ± 0.5 | 3.2 ± 0.5 | - | p = 0.2 |
| TcdA (pg/µL ± SD) | 64.68 ± 92.22 | 116.81 ± 95.19 | - | p = 0.1 |
| TcdB (pg/µL ± SD) | 5.95 ± 16.31 | 7.86 ± 32.34 | - | p = 0.8 |
| TcdA + TcdB (pg/µL ± SD) | 70.63 ± 71.65 | 124.67 ± 89.23 | - | p = 0.1 |
| TcdA > 60 pg/µL | 5 (29.4%) | 12 (66.6%) | 2.0 (1.0–4.2) | p = 0.04 |
| Laboratory findings at T4 | ||||
| White blood cell peripheral count (103 cells/µL ± SD) | 6.95 ± 2.38 | 7.09 ± 2.84 | - | p = 0.8 |
| Neutrophils peripheral count (103 cells/µL ± SD) | 4.25 ± 2.18 | 4.56 ± 2.37 | - | p = 0.6 |
| Creatinine (mg/dL ± SD) | 0.7 ± 0.2 | 0.9 ± 0.3 | - | p = 0.1 |
| Albumin (g/dL ± SD) | 3.6 ± 0.5 | 3.2 ± 0.6 | - | p = 0.09 |
| TcdA (pg/µL ± SD) | 51.76 ± 58.48 | 115.74 ± 77.14 | - | p = 0.01 |
| TcdB (pg/µL ± SD) | 4.21 ± 12.85 | 7.76 ± 29.07 | - | p = 0.7 |
| TcdA + TcdB (pg/µL ± SD) | 55.98 ± 48.17 | 123.51 ± 79.36 | - | p = 0.01 |
| TcdA > 60 pg/µL | 5 (29.4%) | 12 (66.6%) | 2.4 (1.08–5.3) | p = 0.03 |
| Laboratory findings at T10 | ||||
| White blood cell peripheral count (103 cells/µL ± SD) | 6.14 ± 1.81 | 6.49 ± 3.36 | - | p = 0.7 |
| Neutrophils peripheral count (103 cells/µL ± SD) | 4.06 ± 1.66 | 4.15 ± 3.05 | - | p = 0.9 |
| Creatinine (mg/dL ± SD) | 0.7 ± 0.2 | 0.8 ± 0.2 | - | p = 0.2 |
| Albumin (g/dL ± SD) | 3.7 ± 0.7 | 3.5 ± 0.7 | - | p = 0.4 |
| TcdA (pg/µL ± SD) | 30.80 ± 52.92 | 66.73 ± 81.65 | - | p = 0.1 |
| TcdB (pg/µL ± SD) | 4.59 ± 12.74 | 10.50 ± 28.79 | - | p = 0.4 |
| TcdA + TcdB (pg/µL ± SD) | 35.39 ± 40.06 | 77.23 ± 66.65 | - | p = 0.1 |
| TcdA > 60 pg/µL | 3 (17.6%) | 4 (22.2%) | 1.1 (0.4–2.9) | p = 1 |
| Patients outcome | ||||
| Deceased | 0 (0%) | 1 (5.5%) | 0.5 (0.3–0.7) | p = 1 |
| rCDI | 3 (17.6%) | 2 (11.1%) | 1.5 (0.2–10.3) | p = 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granata, G.; Mariotti, D.; Ascenzi, P.; Petrosillo, N.; di Masi, A. High Serum Levels of Toxin A Correlate with Disease Severity in Patients with Clostridioides difficile Infection. Antibiotics 2021, 10, 1093. https://doi.org/10.3390/antibiotics10091093
Granata G, Mariotti D, Ascenzi P, Petrosillo N, di Masi A. High Serum Levels of Toxin A Correlate with Disease Severity in Patients with Clostridioides difficile Infection. Antibiotics. 2021; 10(9):1093. https://doi.org/10.3390/antibiotics10091093
Chicago/Turabian StyleGranata, Guido, Davide Mariotti, Paolo Ascenzi, Nicola Petrosillo, and Alessandra di Masi. 2021. "High Serum Levels of Toxin A Correlate with Disease Severity in Patients with Clostridioides difficile Infection" Antibiotics 10, no. 9: 1093. https://doi.org/10.3390/antibiotics10091093
APA StyleGranata, G., Mariotti, D., Ascenzi, P., Petrosillo, N., & di Masi, A. (2021). High Serum Levels of Toxin A Correlate with Disease Severity in Patients with Clostridioides difficile Infection. Antibiotics, 10(9), 1093. https://doi.org/10.3390/antibiotics10091093










