Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use
Abstract
:1. Introduction
2. A Global Overview of Genes Encoding β-Lactamases
3. Old and New Beta-Lactamase Inhibitors
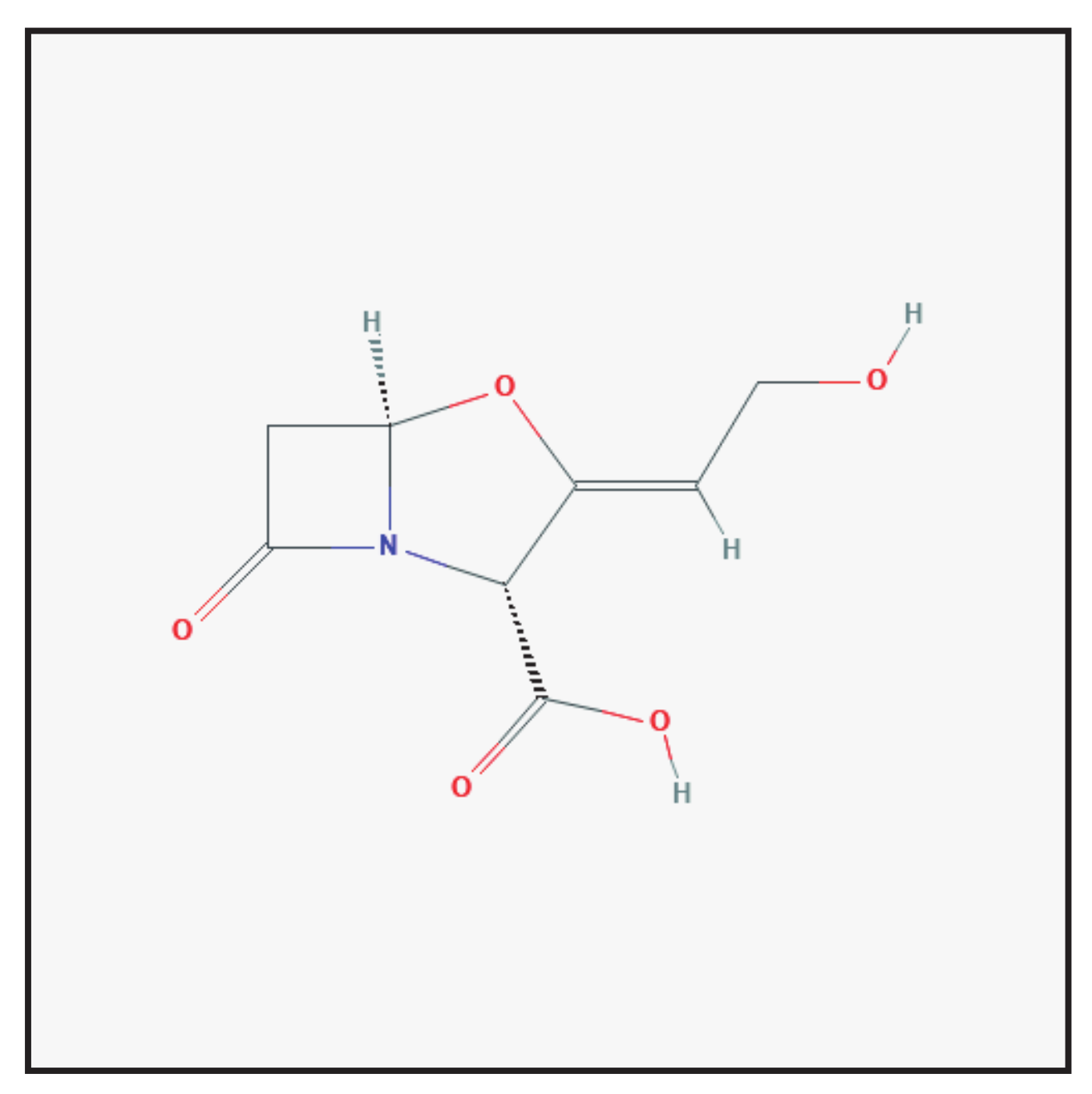
3.1. Clavulanic Acid
3.1.1. Chemical Structure and Mechanism of Action
3.1.2. Clinical Use
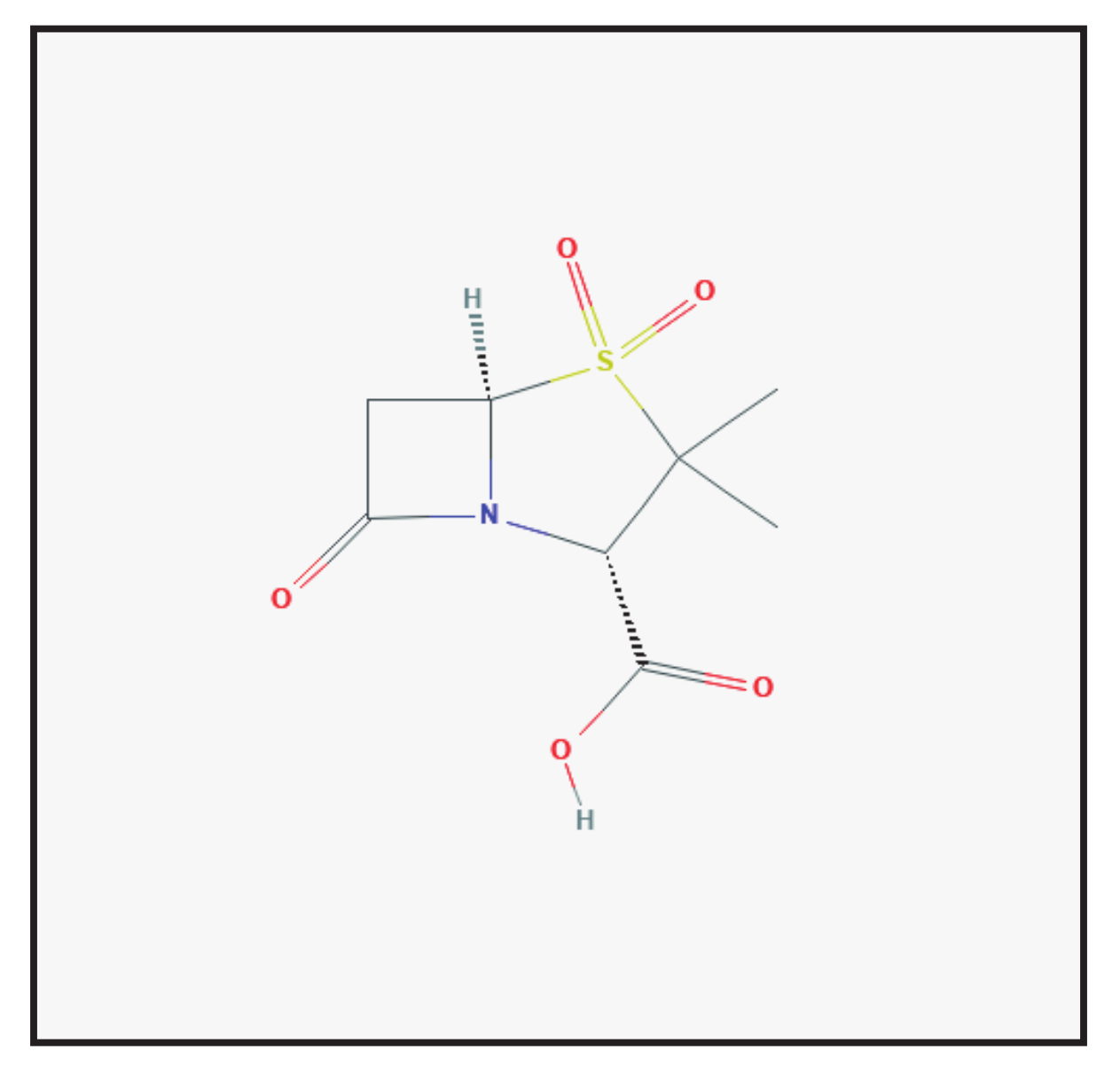
3.2. Sulbactam
3.2.1. Chemical Structure and Mechanism of Action
3.2.2. Clinical Use
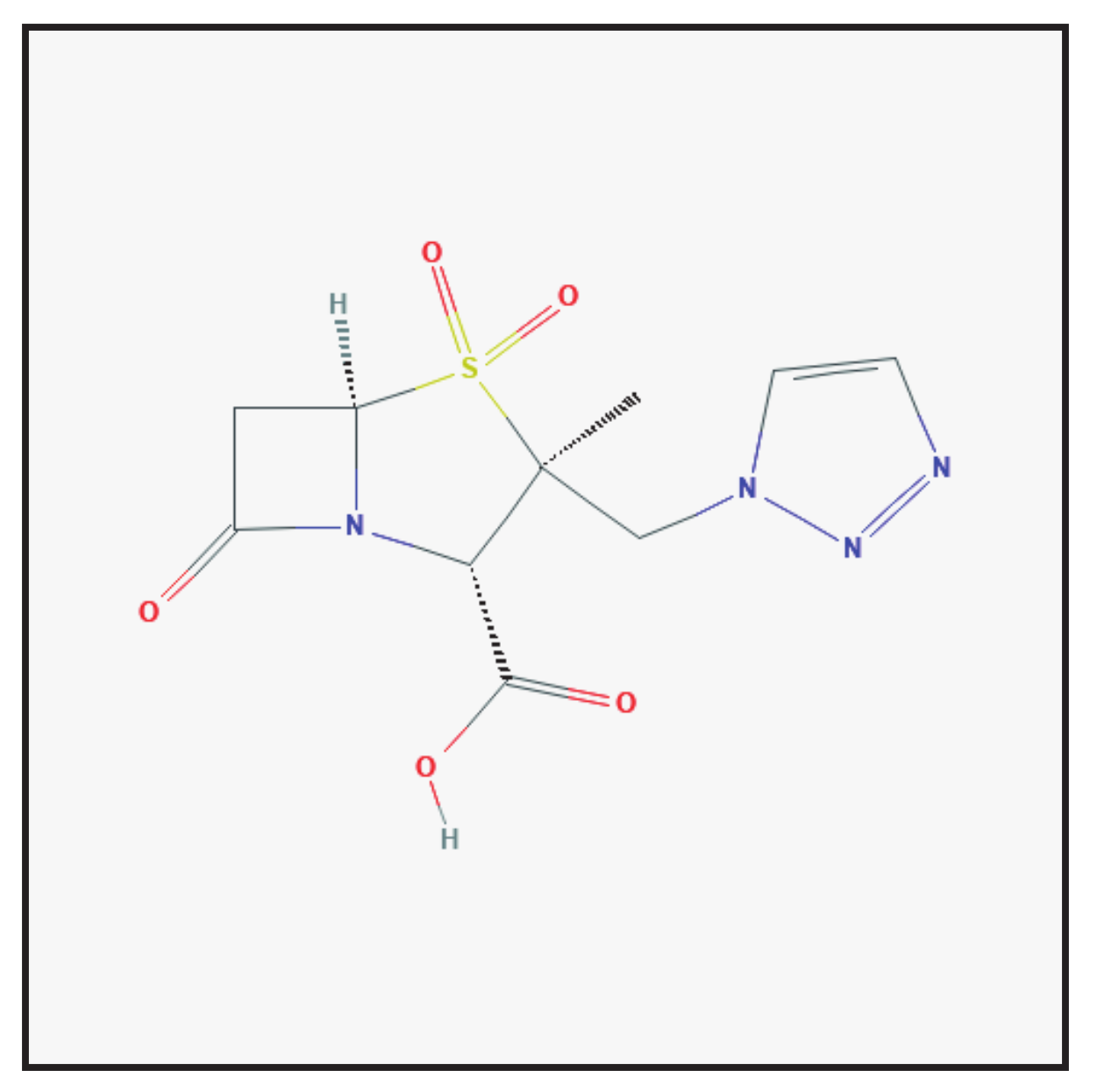
3.3. Tazobactam
3.3.1. Molecular Structure and Mechanism of Action
3.3.2. Clinical Use
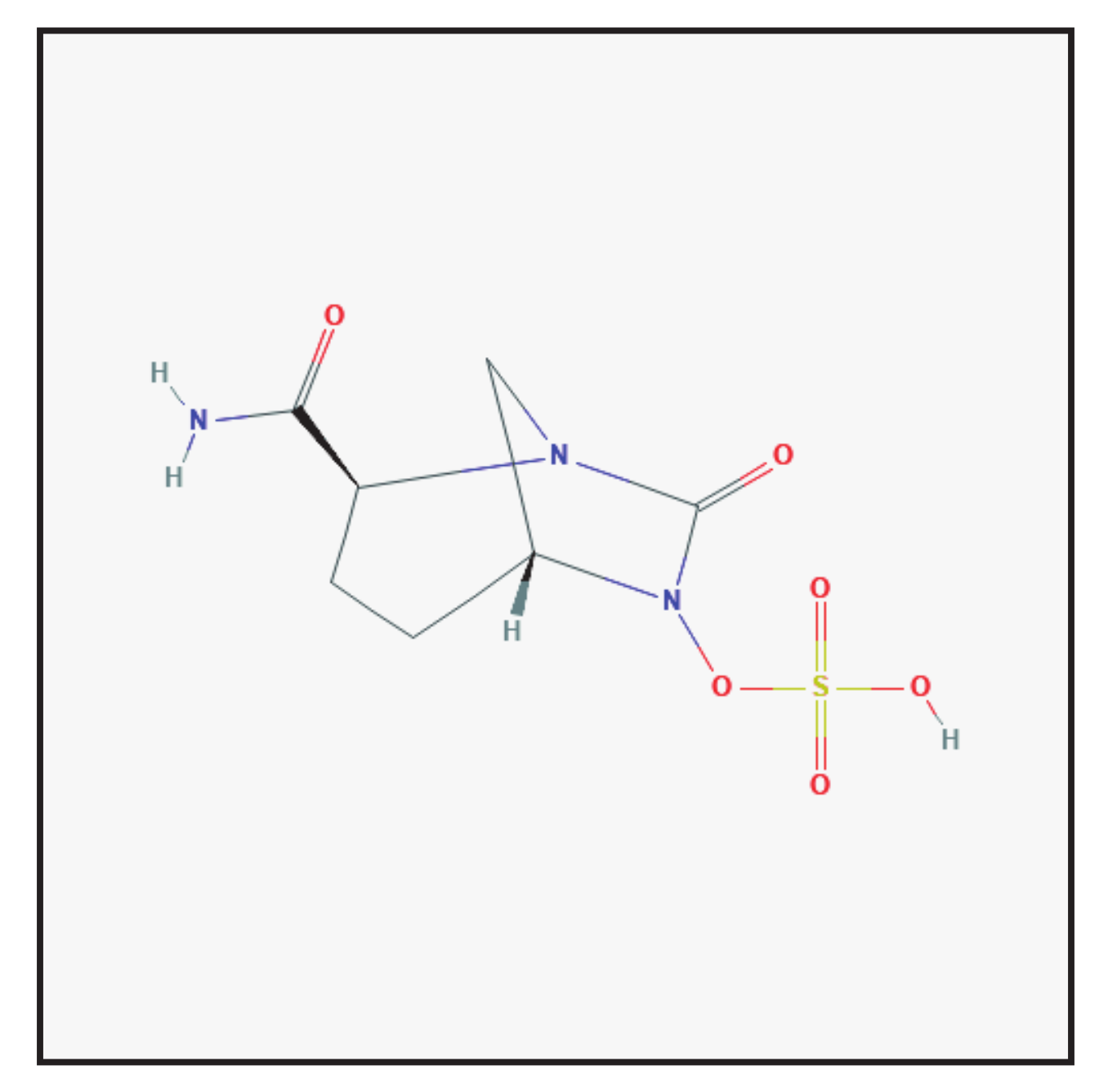
3.4. Avibactam
3.4.1. Chemical Structure and Mechanism of Action
3.4.2. Clinical Use
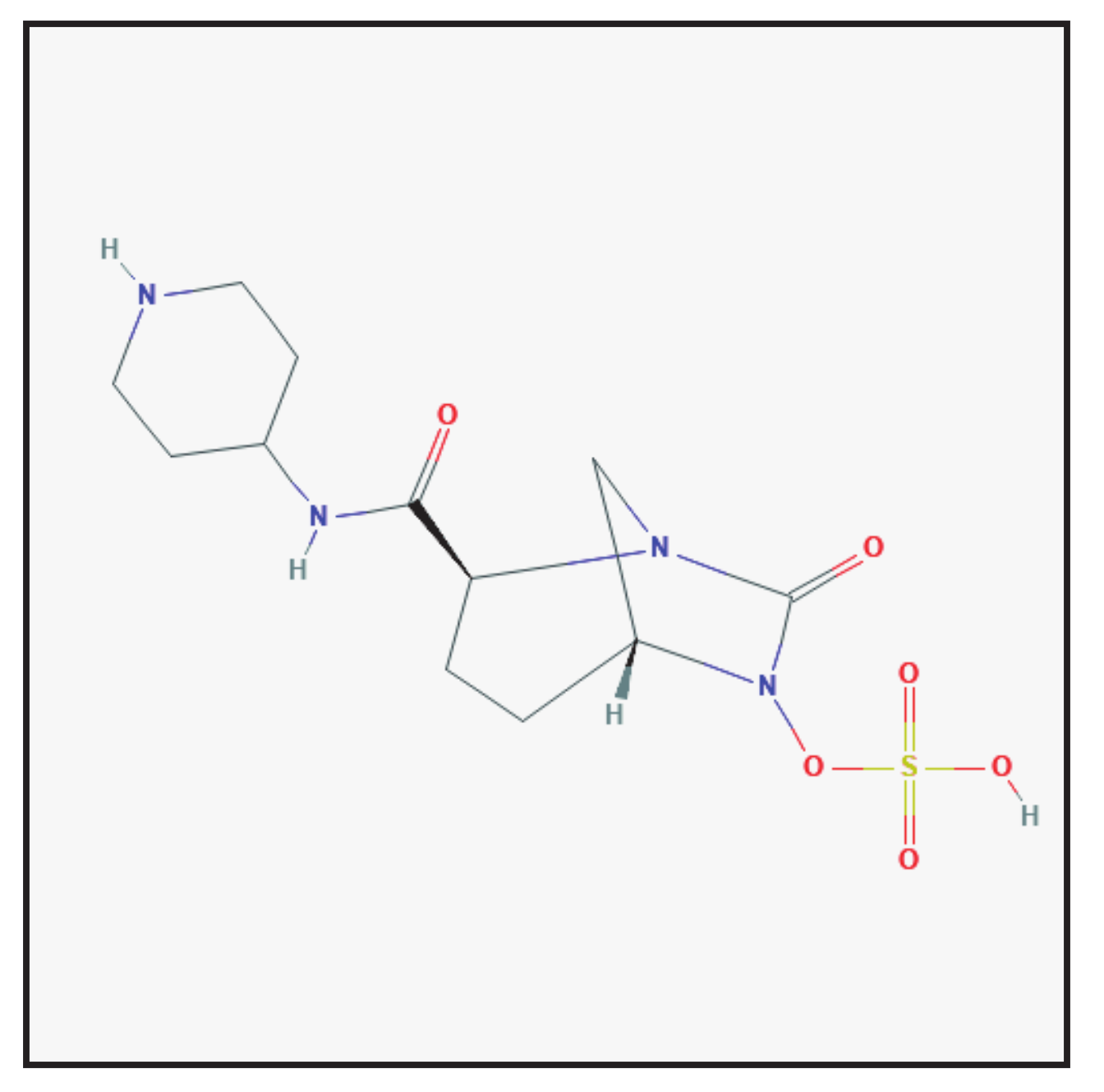
3.5. Relebactam
3.5.1. Chemical Structure and Mechanism of Action
3.5.2. Clinical Use
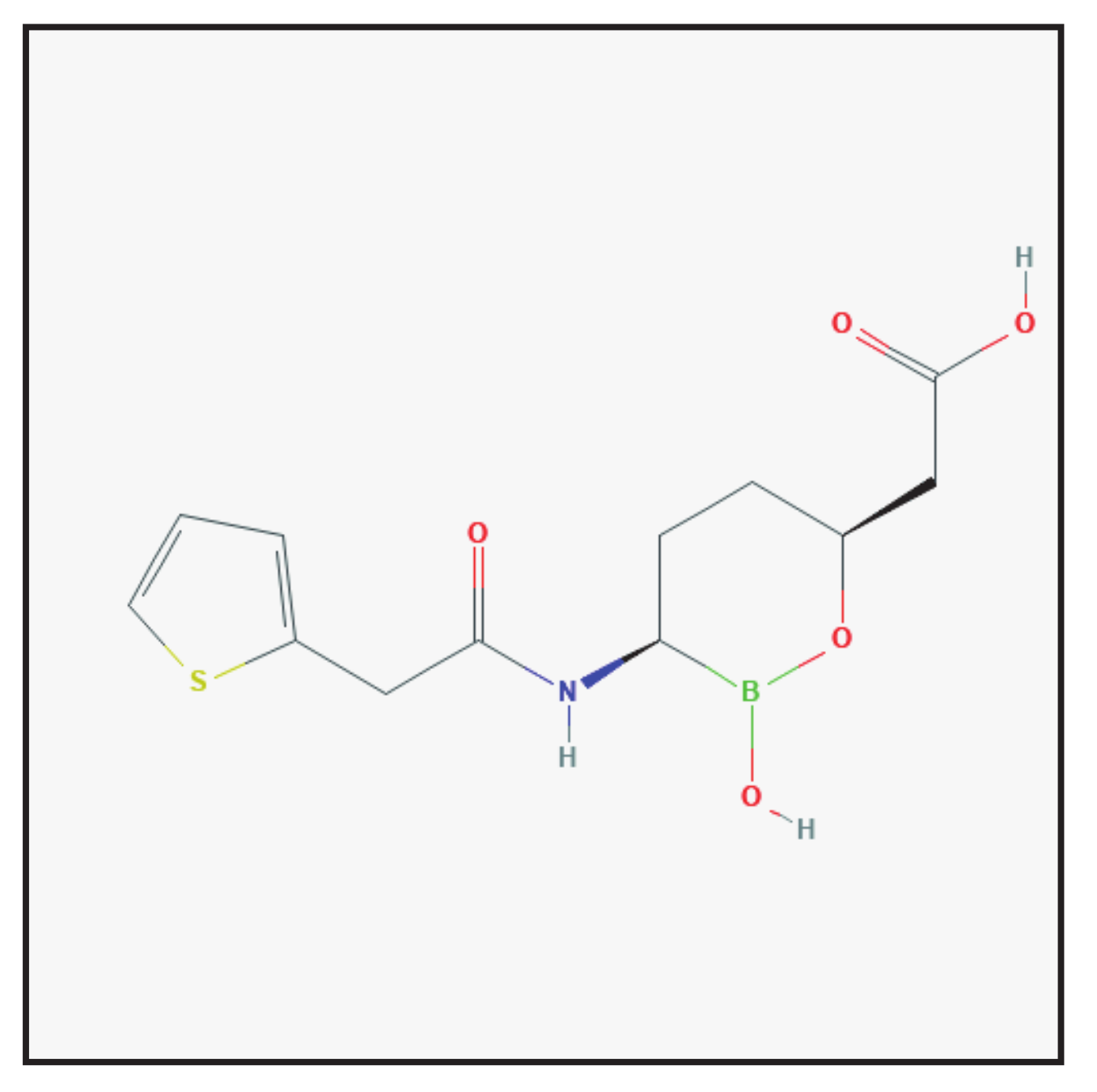
3.6. Vaborbactam
3.6.1. Chemical Structure and Mechanism of Action
3.6.2. Clinical Use
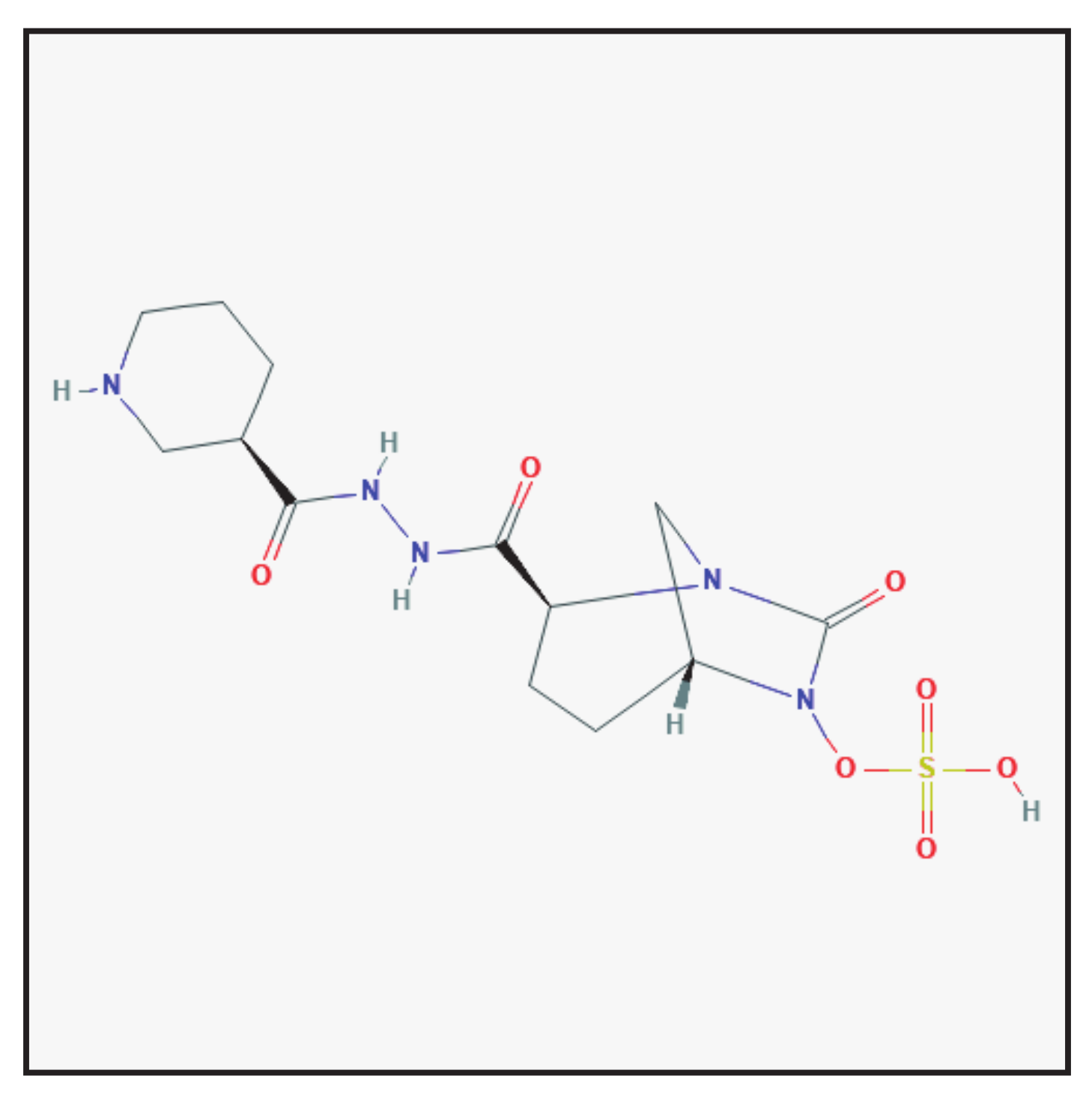
3.7. Zidebactam
3.7.1. Chemical Structure and Mechanism of Action
3.7.2. Clinical Use
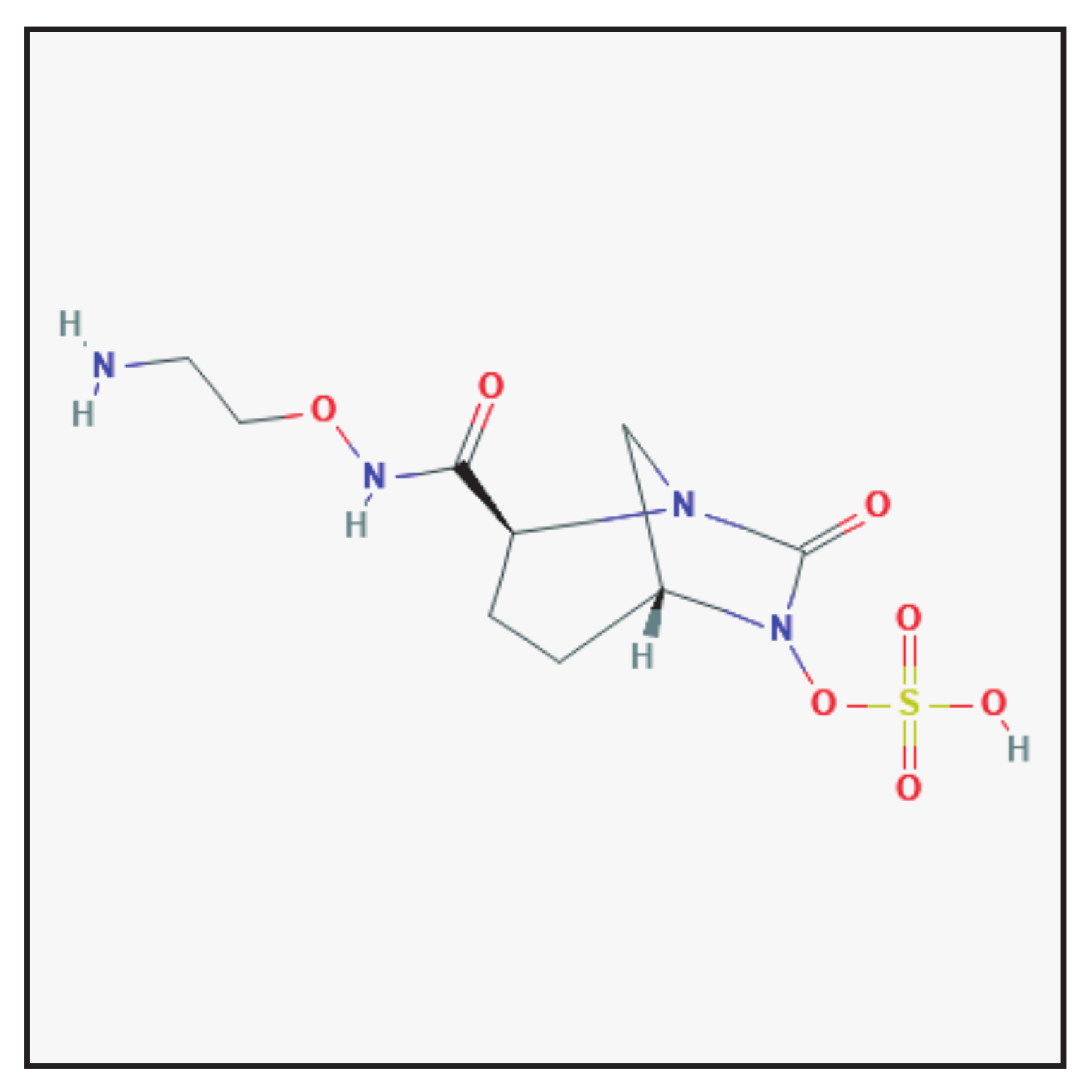
3.8. Nacubactam
3.8.1. Chemical Structure and Mechanism of Action
3.8.2. Clinical Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hvievonen, V.H.A.; Takebayeshi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Tehrani, K.H.M.E.; Martin, N.I. β-lactam/β-lactamase inhibitor combinations: An update. MedChemComm 2018, 9, 1439–1456. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, I.; Papoutsaki, V.; Galani, L.; Giamarellou, H. Carbapenemase producing Klebsiella pneumoniae: Implication on future therapeutic strategies. Expert Rev. Anti. Infect. Ther. 2021, 3. [Google Scholar] [CrossRef]
- Khanna, N.R.; Gerriets, V. Beta Lactamase Inhibitors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Papp-Wallace, K.M.; Bonomo, R.A. New β-Lactamase Inhibitors in the Clinic. Infect. Dis. Clin. N. Am. 2016, 30, 441–464. [Google Scholar] [CrossRef] [Green Version]
- Sonda, T.; Kumburu, H.; van Zwetselaar, M.; Alifrangis, M.; Lund, O.; Kibiki, G.; Aarestrup, F.M. Meta-analysis of proportion estimates of Extended-Spectrum-Beta-Lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob. Resist. Infect. Control. 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onduru, O.G.; Mkakosya, R.S.; Aboud, S.; Rumisha, S.F. Genetic Determinants of Resistance among ESBL-Producing Enterobacteriaceae in Community and Hospital Settings in East, Central, and Southern Africa: A Systematic Review and Meta-Analysis of Prevalence. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 5153237. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.; Hernandez, A.; Parvathi, A.; Kumar, S. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.Y.C.; Barrigas, Z.P.T.; Astutillo, S.G.O.; Jaramillo, A.P.A.; Ausili, A. Detection and molecular characterization of β-lactamase genes in clinical isolates of Gram-negative bacteria in Southern Ecuador. Braz. J. Infect. Dis. 2016, 20, 627–630. [Google Scholar] [CrossRef] [Green Version]
- Coppi, M.; Di Pilato, V.; Monaco, F.; Giani, T.; Conldi, P.G.; Rossolini, G.M. Ceftazidime-Avibactam Resistance Associated with Increased blaKPC-3 Gene Copy Number Mediated by pKpQIL Plasmid Derivatives in Sequence Type 258 Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2020, 64, e01816-19. [Google Scholar] [CrossRef]
- Uto, L.R.; Gerriets, V. Clavulanic Acid. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.-P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Rafailidis, P.I.; Ioannidou, E.N.; Falagas, M.E. Ampicillin/sulbactam: Current status in severe bacterial infections. Drugs 2007, 67, 1829–1849. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Douzinas, E.E. Ampicillin-sulbactam: An update on the use of parenteral and oral forms in bacterial infections. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1099–1112. [Google Scholar] [CrossRef]
- Peechakara, B.V.; Gupta, M. Ampicillin/Sulbactam. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ku, Y.H.; Yu, W.L. Cefoperazone/sulbactam: New composites against multiresistant gram negative bacteria? Infect. Genet. Evol. 2021, 88, 104707. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, Y.; Lan, P.; Xu, Q.; Jiang, Y.; Chen, Y.; Ruan, Z.; Ji, S.; Hua, X.; Yu, Y. Molecular Epidemiology and Mechanism of Sulbactam Resistance in Acinetobacter baumannii Isolates with Diverse Genetic Backgrounds in China. Antimicrob. Agents Chemother. 2018, 62, e01947-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, C.M.; Markham, A. Piperacillin/tazobactam: An updated review of its use in the treatment of bacterial infections. Drugs 1999, 57, 805–843. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Bassetti, M.; De Rosa, F.G.; Del Bono, V.; Grossi, P.; Menichetti, F.; Pea, F.; Rossolini, G.M.; Tumbarello, M.; Viale, P.; et al. Ceftolozane/tazobactam: Place in therapy. Expert Rev. Anti. Infect. Ther. 2018, 16, 307–320. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Mack, A.R.; Taracila, M.A.; Bonomo, R.A. Resistance to Novel β-Lactam-β-Lactamase Inhibitor Combinations: The “Price of Progress”. Infect. Dis. Clin. N. Am. 2020, 34, 773–819. [Google Scholar] [CrossRef]
- Giri, P.; Patel, H.; Srinivas, N.R. Review of Clinical Pharmacokinetics of Avibactam, A Newly Approved non-β lactam β-lactamase Inhibitor Drug, in Combination Use with Ceftazidime. Drug Res. 2019, 69, 245–255. [Google Scholar] [CrossRef]
- Crass, R.L.; Pai, M.P. Pharmacokinetics and Pharmacodynamics of β-Lactamase Inhibitors. Pharmacotherapy 2019, 39, 182–195. [Google Scholar] [CrossRef]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam-β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Campanella, T.A.; Gallagher, J.C. A Clinical Review and Critical Evaluation of Imipenem—Relebactam: Evidence to Date. Infect. Drug Resist. 2020, 13, 4297–4308. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W. Clinical Pharmacokinetics and Pharmacodynamics of Imipenem-Cilastatin/Relebactam Combination Therapy. Clin. Pharmacokinet. 2020, 59, 567–573. [Google Scholar] [CrossRef]
- Petty, L.A.; Henig, O.; Patel, T.S.; Pogue, J.; Kaye, K.S. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect. Drug Resist. 2018, 11, 1461–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, B. A Brief Review of a New Antibiotic: Meropenem-vaborbactam. Sr. Care Pharm. 2019, 34, 187–191. [Google Scholar]
- Novelli, A.; Del Giacomo, P.; Rossolini, G.M.; Tumbarello, M. Meropenem/vaborbactam: A next generation β-lactam β-lactamase inhibitor combination. Expert Rev. Anti. Infect. Ther. 2020, 18, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, E.; Scoble, P.J. An Appraisal of the Pharmacokinetic and Pharmacodynamic Properties of Meropenem-Vaborbactam. Infect. Dis. Ther. 2020, 9, 769–784. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Vickers, A.; Woodford, N. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J. Antimicrob. Chemother. 2017, 72, 1373–1385. [Google Scholar] [CrossRef]
- Jean, S.S.; Gould, I.M.; Lee, W.S.; Hsueh, P.R. International Society of Antimicrobial Chemotherapy (ISAC). New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship. Drugs 2019, 79, 705–714. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Hackel, M.A.; Bouchillon, S.K.; Sahm, D.F. In Vitro Activity of WCK 5222 (Cefepime-Zidebactam) against Worldwide Collected Gram-Negative Bacilli Not Susceptible to Carbapenems. Antimicrob. Agents Chemother. 2020, 64, e01432-20. [Google Scholar] [CrossRef]
- Mallalieu, N.L.; Winter, E.; Fettner, S.; Patel, K.; Zwanziger, E.; Attley, G.; Rodriguez, I.; Kano, A.; Salama, S.M.; Bentley, D.; et al. Safety and Pharmacokinetic Characterization of Nacubactam, a Novel β-Lactamase Inhibitor, Alone and in Combination with Meropenem, in Healthy Volunteers. Antimicrob. Agents Chemother. 2020, 64, e02229-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagihara, M.; Kato, H.; Sugano, T.; Okade, H.; Sato, N.; Shibata, Y.; Sakanashi, D.; Asai, N.; Koizumi, Y.; Suematsu, H.; et al. Pharmacodynamic evaluation of meropenem, cefepime, or aztreonam combined with a novel β-lactamase inhibitor, nacubactam, against carbapenem-resistant and/or carbapenemase-producing Klebsiella pneumoniae and Escherichia coli using a murine thigh-infection model. Int. J. Antimicrob. Agents 2021, 57, 106330. [Google Scholar] [PubMed]
- Barnes, M.D.; Taracila, M.A.; Good, C.E.; Bajaksouzian, S.; Rojas, L.J.; van Duin, D.; Kreiswirth, B.N.; Jacobs, M.R.; Haldimann, A.; Papp-Wallace, K.M.; et al. Nacubactam Enhances Meropenem Activity against Carbapenem-Resistant Klebsiella pneumoniae Producing KPC. Antimicrob. Agents Chemother. 2019, 63, e00432-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Shang, X.; Hu, F.; Xingzhen, L.; Xiangdong, G.; Heng, Z.; Wenbing, Y. β-Lactamase inhibitors: An update. Mini Rev. Med. Chem. 2013, 13, 1846–1861. [Google Scholar] [CrossRef]
- González-Bello, C.; Rodríguez, D.; Pernas, M.; Rodríguez, Á.; Colchón, E. β-Lactamase Inhibitors To Restore the Efficacy of Antibiotics against Superbugs. J. Med. Chem. 2020, 63, 1859–1881. [Google Scholar] [CrossRef]
- Davies, D.T.; Everett, M. Designing Inhibitors of β-Lactamase Enzymes to Overcome Carbapenem Resistance in Gram-Negative Bacteria. Acc. Chem. Res. 2021, 54, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Q.; Sun, L.-Y.; Jiang, Z.; Chen, C.; Gao, H.; Chigan, J.-Z.; Ding, H.-H.; Yang, K.-W. Diaryl-substituted thiosemicarbazone: A potent scaffold for the development of New Delhi metallo-β-lactamase-1 inhibitors. Bioorg. Chem. 2021, 107, 140576. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carcione, D.; Siracusa, C.; Sulejmani, A.; Leoni, V.; Intra, J. Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use. Antibiotics 2021, 10, 995. https://doi.org/10.3390/antibiotics10080995
Carcione D, Siracusa C, Sulejmani A, Leoni V, Intra J. Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use. Antibiotics. 2021; 10(8):995. https://doi.org/10.3390/antibiotics10080995
Chicago/Turabian StyleCarcione, Davide, Claudia Siracusa, Adela Sulejmani, Valerio Leoni, and Jari Intra. 2021. "Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use" Antibiotics 10, no. 8: 995. https://doi.org/10.3390/antibiotics10080995
APA StyleCarcione, D., Siracusa, C., Sulejmani, A., Leoni, V., & Intra, J. (2021). Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use. Antibiotics, 10(8), 995. https://doi.org/10.3390/antibiotics10080995





