Pharmacokinetic/Pharmacodynamic Adequacy of Novel β-Lactam/β-Lactamase Inhibitors against Gram-Negative Bacterial in Critically Ill Patients
Abstract
:1. Introduction
2. Results
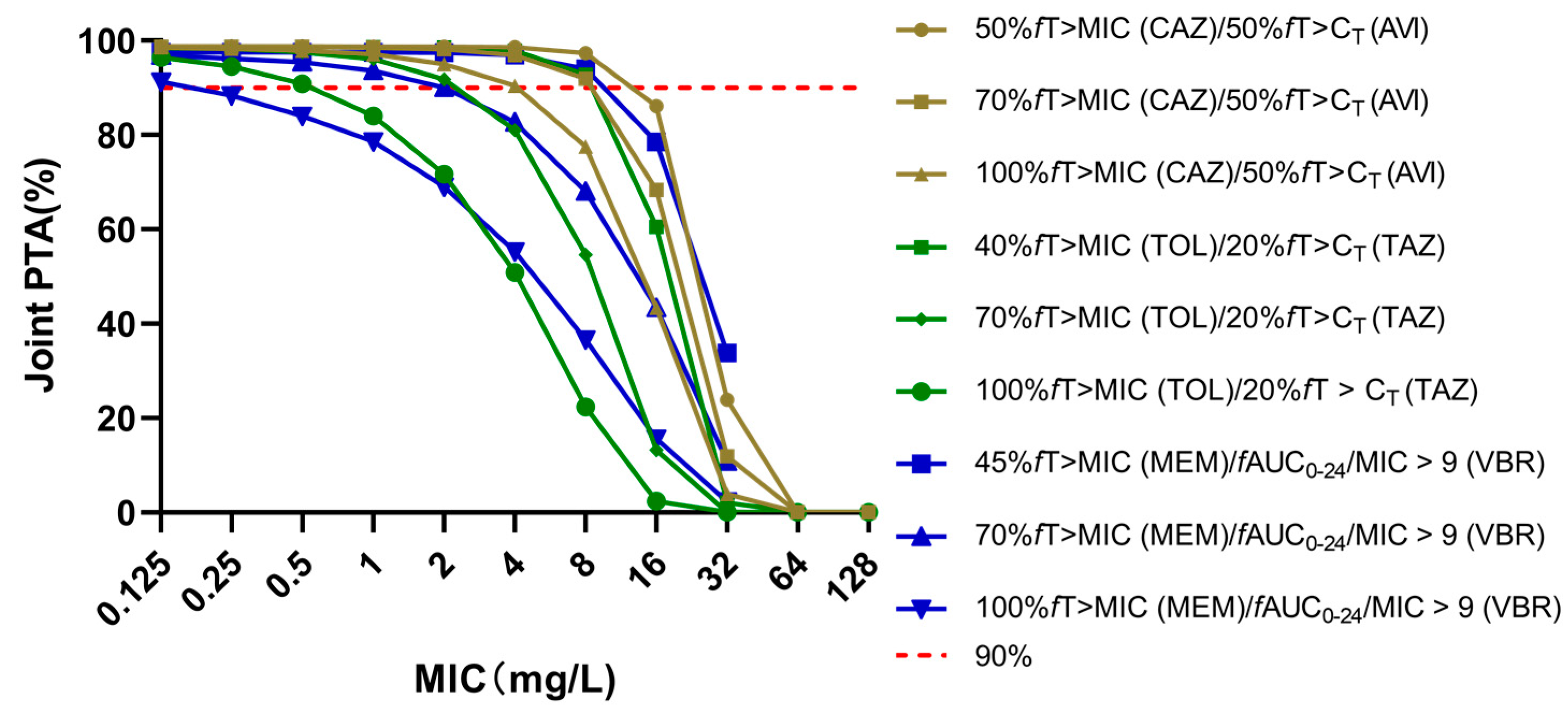
2.1. Probability of Target Attainments of Three Novel BLBLIs
2.2. Cumulative Fraction of Responses
3. Discussion
4. Materials and Methods
4.1. PK Parameters
4.2. PD Data
4.3. PK/PD Targets
4.4. Monte Carlo Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venkataraman, R.; Divatia, J.V.; Ramakrishnan, N.; Chawla, R.; Amin, P.; Gopal, P.; Chaudhry, D.; Zirpe, K.; Abraham, B. Multicenter Observational Study to Evaluate Epidemiology and Resistance Patterns of Common Intensive Care Unit-Infections. Indian J. Crit Care Med. 2018, 22, 20–26. [Google Scholar] [CrossRef]
- Tumbarello, M.; Viale, P.; Viscoli, C.; Trecarichi, E.M.; Tumietto, F.; Marchese, A.; Spanu, T.; Ambretti, S.; Ginocchio, F.; Cristini, F.; et al. Predictors of Mortality in Bloodstream Infections Caused by Klebsiella Pneumoniae Carbapenemase-Producing K. Pneumoniae: Importance of Combination Therapy. Clin. Infect. Dis. 2012, 55, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Wilson, G.M.; Fitzpatrick, M.; Walding, K.; Gonzalez, B.; Schweizer, M.L.; Suda, K.J.; Evans, C.T. Meta-Analysis of Clinical Outcomes Using Ceftazidime/Avibactam, Ceftolozane/Tazobactam, and Meropenem/Vaborbactam for the Treatment of Multidrug-Resistant Gram-Negative Infections. Open Forum Infect. Dis. 2021, 8, ofaa651. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The Effect of Pathophysiology on Pharmacokinetics in the Critically Ill Patient--Concepts Appraised by the Example of Antimicrobial Agents. Adv. Drug Deliv Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Hayashi, Y.; Lipman, J.; Udy, A.A.; Ng, M.; McWhinney, B.; Ungerer, J.; Lust, K.; Roberts, J.A. β-Lactam Therapeutic Drug Monitoring in the Critically Ill: Optimising Drug Exposure in Patients with Fluctuating Renal Function and Hypoalbuminaemia. Int. J. Antimicrob. Agents 2013, 41, 162–166. [Google Scholar] [CrossRef]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The Influence of Inadequate Antimicrobial Treatment of Bloodstream Infections on Patient Outcomes in the ICU Setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic Resistance--What’s Dosing Got to Do with It? Crit Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Alsultan, A.; Abouelkheir, M.; Alqahtani, S.; Aljabri, A.; Somily, A.M.; Alsubaie, S.; Alrabiaah, A.; Bukhari, E.; Alzamil, F. Optimizing Vancomycin Monitoring in Pediatric Patients. Pediatric Infect. Dis. J. 2018, 37, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Castaldo, N.; Cattelan, A.; Mussini, C.; Righi, E.; Tascini, C.; Menichetti, F.; Mastroianni, C.M.; Tumbarello, M.; Grossi, P.; et al. Ceftolozane/Tazobactam for the Treatment of Serious Pseudomonas Aeruginosa Infections: A Multicentre Nationwide Clinical Experience. Int. J. Antimicrob Agents 2019, 53, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; McCreary, E.K.; Marini, R.; Kline, E.G.; Jones, C.E.; Hao, B.; Chen, L.; Kreiswirth, B.N.; Doi, Y.; Clancy, C.J.; et al. Early Experience With Meropenem-Vaborbactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Infect. Dis 2020, 71, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Potoski, B.A.; Haidar, G.; Hao, B.; Doi, Y.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Infect. Dis 2016, 63, 1615–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of Pharmacokinetic/Pharmacodynamic (PK/PD) Terminology for Anti-Infective Drugs: An Update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensman, T.J.; Wang, J.; Jayne, J.; Fukushima, L.; Rao, A.P.; D’Argenio, D.Z.; Beringer, P.M. Pharmacokinetic-Pharmacodynamic Target Attainment Analyses to Determine Optimal Dosing of Ceftazidime-Avibactam for the Treatment of Acute Pulmonary Exacerbations in Patients with Cystic Fibrosis. Antimicrob. Agents Chemother. 2017, 61, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, W.A. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis 1998, 26, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; Stone, G.G.; Newell, P.; Broadhurst, H.; Wardman, A.; MacPherson, M.; Yates, K.; Riccobene, T.; Critchley, I.A.; Das, S. Ceftazidime-Avibactam Susceptibility Breakpoints against Enterobacteriaceae and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.G.; Newell, P.; Gasink, L.B.; Broadhurst, H.; Wardman, A.; Yates, K.; Chen, Z.; Song, J.; Chow, J.W. Clinical Activity of Ceftazidime/Avibactam against MDR Enterobacteriaceae and Pseudomonas Aeruginosa: Pooled Data from the Ceftazidime/Avibactam Phase III Clinical Trial Programme. J. Antimicrob. Chemother. 2018, 73, 2519–2523. [Google Scholar] [CrossRef]
- Winkler, M.L.; Papp-Wallace, K.M.; Hujer, A.M.; Domitrovic, T.N.; Hujer, K.M.; Hurless, K.N.; Tuohy, M.; Hall, G.; Bonomo, R.A. Unexpected Challenges in Treating Multidrug-Resistant Gram-Negative Bacteria: Resistance to Ceftazidime-Avibactam in Archived Isolates of Pseudomonas Aeruginosa. Antimicrob Agents Chemother 2015, 59, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Shi, Q.; Hu, H.; Hong, B.; Wu, X.; Du, X.; Akova, M.; Yu, Y. Emergence of Ceftazidime/Avibactam Resistance in Carbapenem-Resistant Klebsiella Pneumoniae in China. Clin. Microbiol Infect. 2020, 26, 124.e1–124.e4. [Google Scholar] [CrossRef] [Green Version]
- Xiao, A.J.; Caro, L.; Popejoy, M.W.; Huntington, J.A.; Kullar, R. PK/PD Target Attainment With Ceftolozane/Tazobactam Using Monte Carlo Simulation in Patients With Various Degrees of Renal Function, Including Augmented Renal Clearance and End-Stage Renal Disease. Infect. Dis. 2017, 6, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakara, M.; Larson, K.; Feng, H.P.; Shiomi, M.; Yoshitsugu, H.; Rizk, M.L. Population Pharmacokinetics of Tazobactam/Ceftolozane in Japanese Patients with Complicated Urinary Tract Infection and Complicated Intra-Abdominal Infection. J. Infect. Chemother. 2019, 25, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sader, H.S.; Carvalhaes, C.G.; Streit, J.M.; Doyle, T.B.; Castanheira, M. Antimicrobial Activity of Ceftazidime-Avibactam, Ceftolozane-Tazobactam and Comparators Tested against Pseudomonas Aeruginosa and Klebsiella Pneumoniae Isolates from United States Medical Centers in 2016-2018. Microb. Drug Resist. 2021, 27, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam-β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34. [Google Scholar] [CrossRef]
- Novelli, A.; del Giacomo, P.; Rossolini, G.M.; Tumbarello, M. Meropenem/Vaborbactam: A next Generation β-Lactam β-Lactamase Inhibitor Combination. Expert Rev. Anti Infect. 2020, 18, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Teng, M.; Zhang, Y.; Zhang, T.; Wang, T.; Chen, J.; Li, S.; Yang, B.; Shi, Y.; Dong, Y.; et al. Choosing Optimal Antibiotics for the Treatment of Patients Infected With Enterobacteriaceae: A Network Meta-Analysis and Cost-Effectiveness Analysis. Front. Pharmacol. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment Options for Carbapenem-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef] [Green Version]
- Jaruratanasirikul, S.; Thengyai, S.; Wongpoowarak, W.; Wattanavijitkul, T.; Tangkitwanitjaroen, K.; Sukarnjanaset, W.; Jullangkoon, M.; Samaeng, M. Population Pharmacokinetics and Monte Carlo Dosing Simulations of Meropenem during the Early Phase of Severe Sepsis and Septic Shock in Critically Ill Patients in Intensive Care Units. Antimicrob. Agents Chemother. 2015, 59, 2995–3001. [Google Scholar] [CrossRef] [Green Version]
- Stein, G.E.; Smith, C.L.; Scharmen, A.; Kidd, J.M.; Cooper, C.; Kuti, J.; Mitra, S.; Nicolau, D.P.; Havlichek, D.H. Pharmacokinetic and Pharmacodynamic Analysis of Ceftazidime/Avibactam in Critically Ill Patients. Surg Infect. (Larchmt) 2019, 20, 55–61. [Google Scholar] [CrossRef]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Population Pharmacokinetics of Unbound Ceftolozane and Tazobactam in Critically Ill Patients without Renal Dysfunction. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Burgos, R.M.; Biagi, M.J.; Rodvold, K.A.; Danziger, L.H. Pharmacokinetic Evaluation of Meropenem and Vaborbactam for the Treatment of Urinary Tract Infection. Expert Opin Drug Metab. Toxicol. 2018, 14, 1007–1021. [Google Scholar] [CrossRef]
- VABOMERE-Meropenem-Vaborbactam Injection, Powder, for Solution Melinta Therapeutics [package insert]. The Medicines Company. 2017.
- Castanheira, M.; Huband, M.D.; Mendes, R.E.; Flamm, R.K. Meropenem-Vaborbactam Tested against Contemporary Gram-Negative Isolates Collected Worldwide during 2014, Including Carbapenem-Resistant, KPC-Producing, Multidrug-Resistant, and Extensively Drug-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Ferrada, A.; Salavert, M.; Gordon, M.; Villarreal, E.; Castellanos-Ortega, Á.; Ramirez, P. Ceftolozane/Tazobactam Dosing Requirements Against Pseudomonas Aeruginosa Bacteremia. Dose Response 2020, 18, 1559325819885790. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.; Yu, Y.; Wei, X.; Florian, J.; Jang, S.H.; Reynolds, K.S.; Wang, Y. Evaluation of Hemodialysis Effect on Pharmacokinetics of Meropenem/Vaborbactam in End-Stage Renal Disease Patients Using Modeling and Simulation. J. Clin. Pharm. 2020, 60, 1011–1021. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Griffith, D.C.; Sabet, M.; Tarazi, Z.; Lomovskaya, O.; Dudley, M.N. Pharmacokinetics/Pharmacodynamics of Vaborbactam, a Novel Beta-Lactamase Inhibitor, in Combination with Meropenem. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lovern, M.; Green, M.L.; Chiu, J.; Zhou, D.; Comisar, C.; Xiong, Y.; Hing, J.; MacPherson, M.; Wright, J.G.; et al. Ceftazidime-Avibactam Population Pharmacokinetic Modeling and Pharmacodynamic Target Attainment Across Adult Indications and Patient Subgroups. Clin. Transl. Sci. 2019, 12, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, B.; Ni, Y.; Kuti, J.L.; Chen, B.; Chen, M.; Nicolau, D.P. Pharmacodynamic Target Attainment of Seven Antimicrobials against Gram-Negative Bacteria Collected from China in 2003 and 2004. Int. J. Antimicrob. Agents 2007, 30, 452–457. [Google Scholar] [CrossRef] [PubMed]
| Dose (mg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | 3 h | 4 h | Continuous Infusion | 2 h | 3 h | 4 h | Continuous Infusion | 2 h | 3 h | 4 h | Continuous Infusion | |
| Escherichia coli | ||||||||||||
| 2000/500 q8h | 90.31% | 92.65% | 93.07% | 93.11% | 88.65% | 92.05% | 92.64% | 92.93% | 84.54% | 89.94% | 90.90% | 92.35% |
| 2500/625 q8h | 91.51% | 93.08% | 93.25% | 93.25% | 90.11% | 92.68% | 92.97% | 93.16% | 86.42% | 90.96% | 91.64% | 92.78% |
| Klebsiella pneumoniae | ||||||||||||
| 2000/500 q8h | 84.58% | 86.60% | 87.00% | 87.06% | 82.53% | 86.04% | 86.64% | 86.96% | 77.63% | 83.71% | 84.73% | 86.45% |
| 2000/500 q6h | 86.95% | 87.09% | 87.12% | 87.10% | 86.73% | 86.95% | 87.03% | 87.05% | 85.72% | 86.19% | 86.48% | 86.85% |
| 2500/625 q8h | 85.70% | 86.95% | 87.12% | 87.12% | 84.10% | 86.60% | 86.89% | 87.08% | 79.79% | 84.74% | 85.51% | 86.80% |
| 2500/625 q6h | 87.06% | 87.12% | 87.14% | 87.13% | 86.92% | 87.04% | 87.11% | 87.12% | 86.16% | 86.5% | 86.81% | 87.01% |
| 3000/750 q8h | 86.27% | 87.05% | 87.13% | 87.14% | 85.09% | 86.78% | 86.99% | 87.12% | 81.57% | 85.26% | 85.90% | 87.10% |
| 3000/750 q6h | 87.09% | 87.14% | 87.14% | 87.14% | 87.01% | 87.11% | 87.13% | 87.13% | 86.43% | 86.74% | 86.93% | 87.11% |
| 3500/875 q8h | 86.58% | 87.08% | 87.14% | 87.14% | 85.71% | 86.87% | 87.07% | 87.14% | 82.65% | 85.58% | 86.26% | 87.04% |
| 3500/875 q6h | 87.13% | 87.15% | 87.15% | 87.16% | 87.06% | 87.12% | 87.14% | 87.15% | 86.55% | 86.87% | 87.00% | 87.12% |
| 4000/1000 q8h | 86.74% | 87.12% | 87.16% | 87.14% | 86.01% | 86.95% | 87.07% | 87.14% | 83.33% | 85.90% | 86.31% | 87.09% |
| 4000/1000 q6h | 87.15% | 87.17% | 87.17% | 87.15% | 87.09% | 87.12% | 87.14% | 87.14% | 86.71% | 86.88% | 87.01% | 87.13% |
| Pseudomonas aeruginosa | ||||||||||||
| 2000/500 q8h | 80.28% | 87.4% | 87.73% | 87.60% | 77.07% | 85.13% | 85.89% | 86.49% | 70.58% | 80.42% | 81.73% | 84.23% |
| 2000/500 q6h | 88.86% | 88.86% | 88.82% | 88.62% | 87.20% | 87.48% | 87.64% | 87.63% | 84.16% | 84.88% | 85.21% | 86.07% |
| 2500/625 q8h | 82.80% | 89.47% | 89.64% | 89.47% | 79.77% | 87.35% | 87.71% | 88.40% | 73.52% | 82.90% | 83.92% | 86.23% |
| 2500/625 q6h | 90.76% | 90.73% | 90.65% | 90.41% | 89.13% | 89.44% | 89.47% | 89.50% | 86.25% | 86.8% | 87.3% | 87.85% |
| 3000/750 q8h | 84.73% | 90.91% | 91.08% | 90.97% | 81.90% | 88.90% | 89.29% | 89.94% | 76.23% | 84.73% | 85.77% | 88.86% |
| 3000/750 q6h | 92.09% | 92.05% | 91.99% | 91.69% | 90.67% | 90.82% | 90.89% | 90.84% | 87.94% | 88.4% | 88.92% | 89.24% |
| 3500/875 q8h | 85.59% | 92.05% | 92.22% | 92.05% | 83.39% | 90.08% | 90.56% | 91.09% | 78.22% | 86.15% | 87.26% | 89.16% |
| 3500/875 q6h | 93.19% | 93.10% | 93.08% | 92.84% | 91.78% | 91.91% | 92.06% | 91.99% | 89.10% | 89.61% | 90.11% | 90.56% |
| 4000/1000 q8h | 87.43% | 93.04% | 93.21% | 93.03% | 84.96% | 91.11% | 91.47% | 92.10% | 79.84% | 87.44% | 88.23% | 90.12% |
| 4000/1000 q6h | 94.24% | 94.11% | 93.96% | 93.70% | 92.79% | 92.89% | 92.93% | 92.98% | 90.34% | 90.65% | 91.00% | 91.50% |
| Dose (mg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 3 h | 4 h | Continuous Infusion | 1 h | 3 h | 4 h | Continuous Infusion | 1 h | 3 h | 4 h | Continuous Infusion | |
| Escherichia coli | ||||||||||||
| 1000/500 q8h | 98.72% | 98.99% | 99.02% | 99.00% | 97.18% | 98.21% | 98.47% | 98.78% | 92.21% | 95.11% | 96.49% | 98.35% |
| 1250/625 q8h | 98.93% | 99.16% | 99.17% | 99.16% | 97.61% | 98.54% | 98.69% | 98.95% | 93.13% | 96.10% | 97.02% | 98.60% |
| Klebsiella pneumoniae | ||||||||||||
| 1000/500 q8h | 80.78% | 81.28% | 81.38% | 81.26% | 76.76% | 78.40% | 78.99% | 80.04% | 70.09% | 73.37% | 75.12% | 78.45% |
| 1000/500 q6h | 82.06% | 82.11% | 81.98% | 81.66% | 79.39% | 80.25% | 80.45% | 80.53% | 75.91% | 77.86% | 78.47% | 79.22% |
| 1250/625 q8h | 81.97% | 82.48% | 82.50% | 82.44% | 77.87% | 79.50% | 79.95% | 81.01% | 71.63% | 75.00% | 76.17% | 79.35% |
| 1250/625 q6h | 83.59% | 83.44% | 83.25% | 83.12% | 80.46% | 81.25% | 81.37% | 81.79% | 77.10% | 78.91% | 79.44% | 80.32% |
| 1500/750 q8h | 83.27% | 83.67% | 83.75% | 83.62% | 78.83% | 80.30% | 80.77% | 81.95% | 73.04% | 75.90% | 77.09% | 80.12% |
| 1500/750 q6h | 85.03% | 85.04% | 84.82% | 84.33% | 81.45% | 82.21% | 82.51% | 82.70% | 78.17% | 79.75% | 80.31% | 81.12% |
| 1750/875 q8h | 84.47% | 85.02% | 84.98% | 84.84% | 79.71% | 81.16% | 81.60% | 82.93% | 73.97% | 76.87% | 77.84% | 80.82% |
| 1750/875 q6h | 86.30% | 86.35% | 86.27% | 85.83% | 82.31% | 83.28% | 83.61% | 83.89% | 78.84% | 80.48% | 81.13% | 81.98% |
| 2000/1000 q8h | 85.55% | 85.83% | 86.26% | 86.00% | 80.51% | 81.87% | 82.41% | 83.75% | 74.96% | 77.66% | 78.65% | 81.50% |
| 2000/1000 q6h | 87.7% | 87.53% | 87.26% | 86.96% | 83.40% | 84.31% | 84.39% | 84.87% | 79.69% | 81.28% | 81.72% | 82.70% |
| Pseudomonas aeruginosa | ||||||||||||
| 1000/500 q8h | 86.19% | 86.73% | 86.79% | 86.74% | 80.56% | 83.64% | 84.82% | 86.16% | 68.29% | 74.13% | 77.55% | 84.42% |
| 1000/500 q6h | 83.02% | 80.57% | 77.64% | 71.51% | 80.84% | 79.62% | 77.02% | 71.15% | 75.39% | 76.51% | 74.90% | 70.35% |
| 1250/625 q8h | 86.72% | 87.16% | 87.17% | 87.15% | 82.05% | 84.92% | 85.64% | 86.63% | 71.35% | 77.11% | 79.27% | 85.46% |
| 1250/625 q6h | 86.62% | 86.10% | 83.44% | 82.80% | 84.79% | 85.26% | 82.82% | 82.40% | 79.91% | 82.68% | 81.22% | 81.72% |
| 1500/750 q8h | 87.18% | 87.50% | 87.54% | 87.50% | 83.17% | 85.52% | 86.18% | 86.98% | 73.53% | 78.61% | 80.76% | 86.03% |
| 1500/750 q6h | 87.67% | 87.73% | 86.85% | 85.6% | 86.02% | 86.83% | 86.18% | 85.14% | 81.90% | 84.69% | 84.8% | 84.55% |
| 1750/875 q8h | 87.65% | 87.91% | 87.87% | 87.83% | 83.95% | 86.06% | 86.56% | 87.29% | 75.08% | 79.96% | 81.74% | 86.44% |
| 1750/875 q6h | 88.32% | 88.26% | 88.12% | 88.16% | 86.48% | 87.28% | 87.31% | 87.07% | 82.62% | 85.29% | 86.08% | 86.42% |
| 2000/1000 q8h | 88.14% | 88.10% | 88.37% | 88.25% | 84.67% | 86.45% | 86.92% | 87.53% | 76.45% | 81.03% | 82.76% | 86.75% |
| 2000/1000 q6h | 89.24% | 88.95% | 88.81% | 88.43% | 87.02% | 87.67% | 87.73% | 87.58% | 83.54% | 85.87% | 86.55% | 86.92% |
| Dose (mg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 h | 4 h | 5 h | 3 h | 4 h | 5 h | 3 h | 4 h | 5 h | |
| Escherichia coli | |||||||||
| 2000/2000 q8h | 97.48% | 97.49% | 97.49% | 97.35% | 97.47% | 97.48% | 95.37% | 96.52% | 97.10% |
| 2500/2500 q8h | 99.24% | 99.24% | 99.24% | 99.14% | 99.23% | 99.23% | 97.64% | 98.23% | 98.95% |
| Klebsiella pneumoniae | |||||||||
| 2000/2000 q8h | 96.44% | 96.60% | 96.65% | 95.49% | 95.86% | 96.06% | 91.98% | 93.78% | 94.70% |
| 2500/2500 q8h | 98.52% | 98.64% | 98.78% | 97.54% | 97.82% | 98.07% | 94.66% | 95.61% | 96.83% |
| Pseudomonas aeruginosa | |||||||||
| 2000/2000 q8h | 94.04% | 94.98% | 95.30% | 87.62% | 90.16% | 91.92% | 75.11% | 79.50% | 82.86% |
| 2000/2000 q6h | 95.87% | 95.84% | 96.08% | 92.64% | 93.62% | 94.76% | 86.40% | 88.47% | 91.71% |
| 2500/2500 q8h | 97.04% | 97.65% | 98.09% | 91.18% | 93.12% | 95.03% | 79.63% | 82.70% | 86.67% |
| 2500/2500 q6h | 98.38% | 98.61% | 98.52% | 95.55% | 96.76% | 97.56% | 89.14% | 91.86% | 94.88% |
| 3000/3000 q8h | 98.10% | 98.68% | 99.02% | 92.73% | 94.72% | 96.24% | 81.49% | 84.78% | 88.23% |
| 3000/3000 q6h | 99.24% | 99.43% | 99.45% | 96.95% | 97.96% | 98.84% | 90.86% | 93.54% | 96.58% |
| 3500/3500 q8h | 98.60% | 99.20% | 99.50% | 93.31% | 95.61% | 97.28% | 82.30% | 86.11% | 89.69% |
| 3500/3500 q6h | 99.66% | 99.78% | 99.83% | 97.73% | 98.67% | 99.44% | 92.37% | 94.51% | 97.48% |
| 4000/4000 q8h | 99.16% | 99.53% | 99.72% | 94.72% | 96.26% | 97.78% | 84.76% | 86.98% | 90.59% |
| 4000/4000 q6h | 99.80% | 99.87% | 99.92% | 98.19% | 98.96% | 99.66% | 93.03% | 95.14% | 98.18% |
| MIC | ≤0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ceftazidime/avibactam | ||||||||||||||
| E. coli | - | - | 10 | 29 | 25 | 23 | 23 | 32 | 16 | 6 | 3 | 0 | 2 | 10 |
| KP | - | - | 3 | 8 | 17 | 16 | 31 | 26 | 11 | 9 | 1 | 0 | 0 | 18 |
| PA | - | - | 0 | 0 | 1 | 20 | 96 | 82 | 42 | 20 | 22 | 17 | 16 | 8 |
| ceftolozane/tazobactam | ||||||||||||||
| E. coli | - | 1 | 80 | 1995 | 1820 | 691 | 343 | 119 | 70 | 45 | 27 | 27 | 17 | - |
| KP | - | - | 28 | 576 | 866 | 447 | 292 | 175 | 116 | 80 | 98 | 175 | 400 | 6 a |
| PA | - | - | - | 8 | 150 | 1738 | 1528 | 737 | 533 | 225 | 68 | 82 | 645 | 10 a |
| meropenem/vaborbactam | ||||||||||||||
| E. coli | 3916 | 274 | 32 | 2 | 4 | 3 | 4 | 1 | 1 | 0 | 0 | 1 b | ||
| KP | 819 | 928 | 32 | 41 | 50 | 47 | 28 | 9 | 12 | 6 | 7 | 31 b | ||
| PA | 41 | 78 | 245 | 442 | 424 | 438 | 234 | 159 | 185 | 138 | 125 | 95 b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Sun, D.; Li, S.; Chen, J.; Teng, M.; Yang, B.; Dong, Y.; Wang, T. Pharmacokinetic/Pharmacodynamic Adequacy of Novel β-Lactam/β-Lactamase Inhibitors against Gram-Negative Bacterial in Critically Ill Patients. Antibiotics 2021, 10, 993. https://doi.org/10.3390/antibiotics10080993
Han R, Sun D, Li S, Chen J, Teng M, Yang B, Dong Y, Wang T. Pharmacokinetic/Pharmacodynamic Adequacy of Novel β-Lactam/β-Lactamase Inhibitors against Gram-Negative Bacterial in Critically Ill Patients. Antibiotics. 2021; 10(8):993. https://doi.org/10.3390/antibiotics10080993
Chicago/Turabian StyleHan, Ruiying, Dan Sun, Sihan Li, Jiaojiao Chen, Mengmeng Teng, Bo Yang, Yalin Dong, and Taotao Wang. 2021. "Pharmacokinetic/Pharmacodynamic Adequacy of Novel β-Lactam/β-Lactamase Inhibitors against Gram-Negative Bacterial in Critically Ill Patients" Antibiotics 10, no. 8: 993. https://doi.org/10.3390/antibiotics10080993
APA StyleHan, R., Sun, D., Li, S., Chen, J., Teng, M., Yang, B., Dong, Y., & Wang, T. (2021). Pharmacokinetic/Pharmacodynamic Adequacy of Novel β-Lactam/β-Lactamase Inhibitors against Gram-Negative Bacterial in Critically Ill Patients. Antibiotics, 10(8), 993. https://doi.org/10.3390/antibiotics10080993





