Abstract
Overuse of antibiotics during the Coronavirus Disease 2019 (COVID-19) pandemic could increase the selection of extensively resistant bacteria (XDR). However, it is unknown what impact they could have on the evolution of patients, particularly critically ill patients. This study aimed to evaluate the characteristics and impact of ICU-acquired infections in patients with COVID-19. A retrospective cohort study was conducted, evaluating all patients with critical COVID-19 admitted to the intensive care unit (ICU) of a hospital in Southern Peru from 28 March 2020 to 1 March 2021. Of the 124 patients evaluated, 50 (40.32%) developed a healthcare-associated infection (HAI), which occurred at a median of 8 days (IQR 6–17) after ICU admission. The proportion of patients with HAI that required ceftriaxone was significantly higher; the same was true for the use of dexamethasone. Forty bacteria isolations (80%) were classified as XDR to antibiotics, with the most common organisms being Acinetobacter baumannii (54%) and Pseudomonas aeruginosa (22%); 33% (41/124) died at the ICU during the follow-up. In the adjusted analysis, healthcare-associated infection was associated with an increased risk of mortality (aHR= 2.7; 95% CI: 1.33–5.60) and of developing acute renal failure (aRR = 3.1; 95% CI: 1.42–6.72). The incidence of healthcare infection mainly by XDR pathogens is high in critically ill patients with COVID-19 and is associated with an increased risk of complications or death.
1. Introduction
The Coronavirus Disease 2019 (COVID-19) outbreak was declared a pandemic in early 2020. Since then, its etiological agent, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has spread worldwide. This pandemic has created an unprecedented challenge for healthcare systems [1], mainly because of the large number of patients requiring intensive care unit (ICU) management, despite the low proportion of severe patients [2]. It should be remembered that as of 31 July 2021, more than 197.5 million cases of COVID-19 have been diagnosed (and 4.21 million deaths recorded).
A large number of patients with COVID-19 admitted to the ICU require invasive measures, including mechanical ventilation, central venous catheters, and foley catheters, among others, which may increase the risk of developing healthcare-associated infections [3]. These secondary infections have represented a significant cause of morbidity and mortality in other pandemics such as influenza [4]. However, the impact of these infections on mortality in patients with COVID-19 and its association with the use of antibiotics, especially in Peru and Latin America, are still uncertain.
In addition, recent studies have reported an unwarranted increase in the use of antibiotics in patients with COVID-19, especially in Latin America and particularly in Peru, where it is reported that up to 99% of patients received antibiotics at hospitalization [5,6]. Moreover, antibiotics are easily sold without a medical prescription, facilitating their inappropriate use and consequences. Therefore, the impact of overuse of these therapies on the spread of bacterial resistance is a matter of concern, which may be worse during the COVID-19 pandemic, where multiple antimicrobials (e.g., azithromycin) have been repurposed, without evidence supporting its clinical benefit as antiviral in the SARS-CoV-2 infection. Indeed, the correct use of antibiotics in COVID-19 is critical for secondary infections due to bacteria, especially in the respiratory tract. Therefore, the primary objective of this study was to assess the impact of healthcare-associated infection on mortality in patients with COVID-19 admitted to the ICU of a referral hospital in Southern Peru, one of the countries with the highest COVID-19 mortality rate in the world [7] in 2021.
2. Results
2.1. Patients
During the study period, 129 patients with critical COVID-19 required invasive mechanical ventilation and were admitted to the ICU. Five patients were excluded (three were still in the ICU at the end of the study, and two did not have all the laboratory tests). The flow diagram of the study population is shown in Figure 1. A total of 124 adults hospitalized for critical COVID-19 were analyzed. From the total, 82.26% (n = 102) were male, with a mean age of 54.47 years (standard deviation, SD ± 12.03); their median hospital and ICU stay durations were 19 days (interquartile range, IQR 14–27.5) and ten days (IQR 6–16.5), respectively. The most frequent comorbidities were obesity (61.29%), diabetes (28.23%), and hypertension (28.23%).
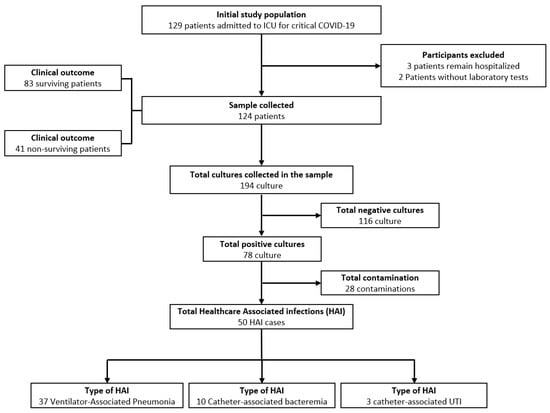
Figure 1.
Sample selection flow chart.
During the initial emergency care, the patients’ median oxygen saturation and PaO2/FiO2 were 87% (IQR 80–89) and 249.5 (IQR 175–294), respectively. Antibiotics were administered to 95.16% (n = 118) of patients, the most frequent being ceftriaxone (74.19%) and azithromycin (33.06%). On admission to the ICU, median leukocytes, C-reactive protein, and procalcitonin were 10,100 cells/mm3 (IQR 7000–13,000), 12.52 mg/dL (IQR 6.62–17.43), and 0.12 ng/mL (IQR 0.03–0.385), respectively (Table 1).

Table 1.
Clinical and laboratory characteristics of the study population and comparison between survivors and non-survivors.
Mortality during hospitalization was 33.06% (n = 41), with a mortality rate of 1.45 deaths per 100 people/days at risk. On admission to the ICU, the most frequent complications were acute respiratory distress syndrome (ARDS) (100%), sepsis (66.94%), and healthcare-associated infection (40.32%). Likewise, antibiotics were administered to 99.19% (n = 123) of the patients, with piperacillin/tazobactam (44.35%) and vancomycin (34.68%) being the most frequently used. In addition, 98.37% (n = 121) of patients received corticosteroid treatment, with methylprednisolone 100 mg being the most commonly used treatment in 54.03% (n = 67), followed by dexamethasone 6 mg with 37.10% (n = 46). Of the group, 48.39% (n = 60) received vasopressor therapy and 12.10% (n = 15) required renal replacement therapy (Table 2).

Table 2.
Complications and treatment received during ICU stay in the study population and comparison between survivors and non-survivors.
2.2. Bivariate Analysis According to Mortality in the Study Population
During the hospital stay, statistically significant differences were observed concerning mortality in patients over 60 years of age and in those with comorbidities such as hypertension, heart failure, asthma, chronic kidney disease (CKD), and immunosuppression. PaO2/FiO2 was lower in the group of patients who died (p = 0.002). It was also observed that the increases in procalcitonin, LDH, total CPK, and creatinine were higher in patients who died. The most frequent complications in the group of patients who died were bacterial superinfection, especially when the pathogen was XDR, sepsis, septic shock, acute kidney failure (AKI), gastrointestinal bleeding, arrhythmias, and in-hospital cardiac arrest (Table 2).
2.3. Source of Infection and Microbiological Characteristics
Of 50 (40.32%) episodes of healthcare-associated infection, ventilator-associated pneumonia was the most frequent, occurring in 37 patients, followed by catheter-associated bacteremia in 10 patients. The median time from ICU admission to the onset of healthcare-associated infection was eight days (IQR 6–17). (Table 2) The most frequent pathogen in ventilator-associated pneumonia (VAP) was Acinetobacter baumannii, in 25 (67.57%) patients, followed by Pseudomonas aeruginosa in seven patients. The most frequent cause of catheter-associated bacteremia was Staphylococcus epidermidis, in three patients, followed by Acinetobacter baumannii in two patients (Supplementary Table S1).
Of all the isolated bacteria, 40 (80%) were classified as XDR, the most frequent being Acinetobacter baumannii in 25 (62.5%) cultures and Pseudomonas aeruginosa in 10 (25%) cultures. In the same way, six (12%) bacterial isolates were classified as MDR, the most frequent being Staphylococcus epidermidis in two (33%) cultures (Supplementary Tables S2 and S3). During ICU stay, the most frequent characteristics among patients who developed healthcare-associated infection were: age over 60 years, chronic kidney disease, and prolonged hospital stay. Moreover, leukocytes, lymphocytes, procalcitonin, LDH, and total CPK were higher in this group. Conversely, a lower proportion of patients that did not develop infection required ceftriaxone as an empirical antibiotic and 6 mg dexamethasone as a corticosteroid (Table 3).

Table 3.
Clinical and laboratory characteristics of the study population and comparison between those who did and did not develop a healthcare-associated infection.
2.4. Survival Estimated by Kaplan–Meier Curves
Patients who did not present healthcare-associated infection during hospitalization showed a better survival curve, the difference being statistically significant (p = 0.034) (Figure 2).
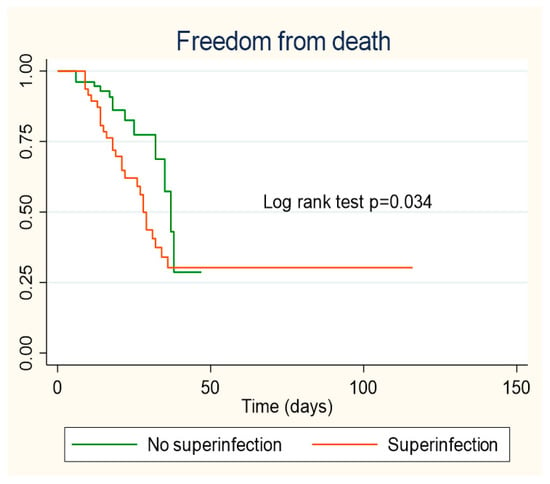
Figure 2.
Kaplan–Meier survival curves according to healthcare-associated infection.
2.5. Healthcare Infection Associated with Mortality and Acute Renal Failure
In the crude Cox regression analysis, healthcare-associated infection was associated with an increased risk of mortality (cHR = 2.216; 95% CI 1.12–4.39). In the multivariate analysis, after adjusting for age, sex, comorbidities, and PaO2/FiO2 levels, healthcare-associated infection was independently associated with hospital mortality (aHR = 2.733; 95% CI 1.33–5.60). Likewise, PaO2/FiO2 values between 200 and 300 (aHR = 4.256; 95% CI 1.14–15.80), 100 and 200 (aHR = 3.796; 95% CI 1.01–14.47), or less than 100 (aHR = 5.503; 95% CI 1.34–22.52) were independently associated with in-hospital mortality (Table 4).

Table 4.
Cox regression analysis to evaluate predictors of mortality in patients hospitalized for critical COVID-19.
In the second analysis using crude robust variance Poisson regression, healthcare-associated infection was associated with an increased risk of acute renal failure (cRR = 3.594; 95% CI 1.60–8.05), while in the multivariate model, after adjusting for age, sex, comorbidities, and PaO2/FiO2 grades, healthcare-associated infection was independently associated with an increased risk of acute renal failure (aRR = 3.093; 95% CI 1.42–6.72) (Table 5).

Table 5.
Poisson regression analysis to evaluate risk factors for acute kidney injury in patients hospitalized for critical COVID-19.
3. Discussion
In the present retrospective cohort study in critically ill COVID-19 patients, we found a high incidence of healthcare-associated infections, primarily due to extremely antibiotic-resistant pathogens; these infections were associated with an increased risk of developing acute renal failure and significantly impacted in-hospital mortality, representing the leading cause of death in 70% of patients who died in the ICU.
The incidence of healthcare-associated infections observed in this study was high compared to that reported in other studies, where the proportion of patients with secondary infection ranged between 5% and 30% [8,9,10,11], probably due to the variability of the diagnostic methods used as well as the treatments indicated. However, when these results are compared with those of studies that only evaluated patients hospitalized in the ICU, more similar results are observed, with incidences of in-hospital infections ranging between 40% and 58% [3,12,13,14]. This high incidence, especially in patients in the ICU, could be explained by different reasons: (a) SARS-CoV-2 virulence factors could compromise the innate immune response at several levels, resulting in increased bacterial adhesion, growth, and dissemination [15]; (b) the possible anti-inflammatory or immunosuppressive effect developed by the use of steroids and biological agents (anti-IL-6 receptor monoclonal antibodies) [16]; (c) invasive procedures cause a breakdown of the body’s first defense barrier; (d) inflammatory syndromes, hypercatabolism, and physical immobilization, typical of critical patients, predispose them to nutritional alterations, which would generate a greater risk of developing secondary infections [17].
The mortality found in this study (33%) is similar to that reported for ICU patients in other hospitals [12,18,19,20]. Although most of these reports are from high-income countries, patients with critical COVID-19 in our hospital were governed by constantly updated protocols as new evidence appeared, which probably impacted mortality.
The study by Bardi, T. et al. [12] used a similar methodological structure to our study. Although there are apparent differences in the populations, treatments, and local bacterial resistance, they also found that healthcare-associated infection was independently associated with an increased risk of hospital mortality (OR 2.7; 95% CI: 1.2–5.9). Likewise, in the systematic review and meta-analysis by Musuuza, J. et al. [21], in which they included patients with superinfection or bacterial coinfection associated with CODVID-19, although 35% were case series and included studies performed outside the ICU, they found a higher probability of death in superinfected patients compared to those with only SARS-CoV-2 infection (PR 3.31; 95% CI: 1.82–5.99).
The most frequently isolated bacterial microorganisms in our cohort were similar to those reported in other studies, where the three main bacteria were Acinetobacter spp. (22.0%), Pseudomonas (10.8%), and Escherichia coli (6.9%) [21], although with a higher prevalence in our cohort of non-fermenting bacteria, mainly Acinetobacter and Pseudomonas in respiratory infections, which differs from that reported by other authors, where Pseudomonas aeruginosa (21. 1%), Klebsiella spp. (17.2%), and Staphylococcus aureus (13.5%) were the most commonly isolated microorganisms in respiratory samples [10]. This difference is particularly relevant due to the high rate of carbapenem resistance of A. baumannii and Pseudomonas aeruginosa in this cohort, which was 96% and 100%. This high proportion of multidrug-resistant microorganisms could be due to the almost universal consumption of antibiotics in our cohort (99%), well above that described by other authors (64–72%) [22,23,24], and to the deviation from adequate infection prevention and control practices, probably related to the collapse of the Peruvian health system [20].
The role of procalcitonin (PCT) in guiding antibiotic therapy in critically ill COVID-19 patients is still uncertain. Pink et al. [25] reported that those patients with COVID-19 and secondary bacterial infection during hospitalization had higher PCT values (0.4 vs. 0.1 ng/mL; p = 0.016) and a cut-off point of >0.55 ng/mL, which managed to discriminate those patients with secondary bacterial infection with a sensitivity and specificity of 91% and 81%. In our study, higher PCT values were also observed in those with healthcare-associated infection, although with a slightly lower cut-off point. However, in some other studies, the procalcitonin value did not show statistically significant differences in patients with bacterial superinfections [26,27]. This phenomenon is probably because, usually, the level of procalcitonin increases in the presence of cytokines, caused by a bacterial infection (IL-1b, TNF-a, and IL-6), and its production is regulated by the signal transducer and activator of transcription 3 (STAT-3). In contrast, increased IFN-gamma, often associated with viral infections, inhibits PCT production. Surprisingly, the SARS-CoV-2 virus inhibits STAT-1 and IFN function, leading to the compensatory activation of STAT3, which would be responsible for some phenomena of severe COVID-19, such as coagulopathy, thrombosis, proinflammatory state, T-cell lymphopenia, increased ACE2 expression (STAT-3 alpha), and also increased procalcitonin, which could falsely be interpreted as bacterial superinfection [28].
In order to avoid the inappropriate use of antibiotics, Foschi C. et al. [29] performed a study in which the performance of multiplex PCR (FilmArray® pneumonia Plus panel) compared to traditional culture for the detection of respiratory pathogens in patients with COVID-19 hospitalized in ICU, which showed a sensitivity and specificity of 89.6% and 98.3%, respectively, and a significant decrease in the turn-around time of at least 48 h, with a high negative predictive value that could be useful for the appropriate initiation of antibiotics, ruling out bacterial superinfections and reducing unnecessary antibiotic consumption, which is why its implementation should be considered in antimicrobial stewardship programs, at least in patients with critical COVID-19.
We observed that healthcare-associated infection (HAI) was associated with an increased risk of developing acute renal failure (AKI), probably because a significant proportion of over-infected patients developed sepsis and septic shock, which would increase the hyperinflammatory state observed in patients with critical COVID-19 [30], causing circulatory collapse and reducing renal perfusion. Several studies have supported this hypothesis by examining renal biopsies, demonstrating that acute tubular injury is the most common pathological finding in patients with COVID-19 and AKI [31,32,33,34]. In addition, an acute tubular injury may occur in the context of prolonged volume depletion and in hemodynamic states that reduce renal perfusion, as is the case in over-infected patients and hyperinflammatory states [30]. This study compared those that developed HAI with those that did not, as well as also assessing the prevalence of chronic kidney disease and the hospital stay length, time at ICU, and mechanical ventilation, among other variables. As expected, the median number of leukocytes was also higher among them. Regarding the laboratory findings, procalcitonin, LDH, and total CPK were significantly higher too. Those infected required significantly more ceftriaxone, dexamethasone (6 mg), dialysis, and vasopressor therapies.
This study has some limitations. First, the study design was retrospective. Second, the number of patients evaluated was small, which reduces the possibility of controlling for various confounding and data collection and inclusion of more variables (for example, drugs administered) within the regression model. On the other hand, for the diagnosis of healthcare-associated infection, only those patients with positive conventional cultures were evaluated; therefore, it is possible that those patients with healthcare-associated infections with negative cultures due to the use of antibiotics at the time that the samples were obtained, or low bacterial load, were not included. Likewise, the laboratory data were obtained at hospital admission, and these could vary over time, especially in those with superinfection.
Furthermore, since practically all the patients received antibiotics at hospital admission, we could not evaluate their influence on superinfection and the proposed outcomes. Finally, this study was conducted in only one health institution, which has its local epidemiology of antimicrobial resistance, limiting the generalizability of these results. Therefore, prospective, multicenter studies are needed, including a more significant number of patients and a more significant amount of information about them.
4. Materials and Methods
4.1. Study Design and Setting
A retrospective cohort study was designed using data collected from a level III hospital with 110 inpatient beds and 24 intensive care beds, the Daniel Alcides Carrión Hospital. Tacna, Peru. The study period was from 28 March 2020 to 1 March 2021. The study was devised following the STROBE recommendations for reporting observational studies [35].
4.2. Population and Sample
The study population included all adult patients (≥18 years) who were hospitalized in the ICU for infection secondary to SARS-CoV-2, confirmed by positive nasopharyngeal swab real-time polymerase chain reaction (RT-PCR), and who also presented critical COVID-19, defined according to World Health Organization criteria by the presence of acute respiratory distress syndrome (ARDS) or sepsis or septic shock [36]. Patients were excluded when they were still in hospitalization during data collection, when they did not have all the laboratory tests, and when the diagnosis of COVID-19 could not be confirmed.
The study by Bardi, T. et al. [12] was used to find the statistical impact and the independent variable (exposure) of bacterial superinfection. In their study, 54.3% of patients with healthcare-associated infection died during follow-up, compared to 24% mortality in those without healthcare-associated infection. Likewise, an unexposed/exposed ratio of 1.45 (83/57) was reported. With these parameters, at a confidence level of 95% and 124 participants in the sample of this study, the statistical power of 93.3% was calculated. Due to the limited number of ICU beds available in the hospital (n = 24), we projected a priori that there would be a limited number of patients, so we decided to evaluate all those in the study period who met the eligibility criteria.
4.3. Data Collection and Variable Definition
Information was collected from the electronic medical records of the patients at the time of admission to emergency (oxygen saturation and PaFiO2). From when they were hospitalized in the ICU (clinical characteristics, complications and treatment) until the clinical outcome (discharge or death), the data were collected by two investigators, a double entry of data was performed (by different investigators), and a third investigator was in charge of quality control. When some variation was found, the information was contrasted with the digital medical record. In addition, cases of healthcare-associated infection (ventilator-associated pneumonia or intravascular catheter-related bacteremia or catheter-associated urinary tract infection) were reviewed by a specialist in infectious and tropical diseases to determine the presence of a true healthcare-associated infection and its origin.
4.3.1. Result Variables
Hospital Mortality
Mortality in patients with a diagnosis of COVID-19 was assessed as an outcome variable. This was collected according to the outcome recorded in the medical record until the end of the follow-up (1 March 2021).
Acute Kidney Failure
Acute kidney injury (AKI) was assessed as a complication during ICU stay, diagnosed according to the Kidney Disease Improving Global Outcome (KDIGO) criteria clinical practice guidelines [37].
4.3.2. Exposure Variables
Ventilator-Associated Pneumonia
Ventilator-associated pneumonia (VAP) was defined as a new or changing chest X-ray infiltrate or infiltrates that occurred more than 48 h after the initiation of invasive mechanical ventilation, in addition to three of the following criteria: new onset of fever (body temperature ≥ 38 °C), hypothermia (body temperature ≤ 35 °C), leukocytosis (total peripheral white blood cell count ≥ 10,000 cell/μL), leukopenia (total leukocyte count ≤ 4500 cell/μL), or left shift (>15% immature neutrophils); new respiratory aspirated secretions or need for acute changes in the ventilator support system to improve oxygenation; and positive culture for bacterial respiratory pathogens [38].
Intravascular Catheter-Related Bacteremia
Intravascular catheter-related bacteremia was diagnosed according to the recommendations of the clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection of the Infectious Diseases Society of America (IDSA) [39].
Catheter-Associated Urinary Tract Infection
Catheter-associated urinary tract infection (UTI) was diagnosed if there was a positive culture with more than 103 colony-forming units/mL of uropathogenic bacteria in the presence of signs or symptoms compatible with UTI in a patient with a urinary catheter [40].
Bacterial Isolation, Susceptibility, and Resistance
Clinical samples were obtained during routine evaluations in the ICU and were sent to the hospital microbiology laboratory. The DL-BT64 automated detection system processed blood cultures, and when these were positive, as well as respiratory samples (endotracheal aspirate, bronchoalveolar lavage, or bronchial brushing), they were incubated for 24–48 h on blood agar or MacConkey agar; bacterial colonies were recovered and reconstituted in a 0.5 McFarland suspension (in 0.45% NaCl) and then inoculated in MicroScan Panel negative combo type 66 and Panel positive combo type 42 on the corresponding panels. Susceptibility testing and identification of bacterial isolates were performed using the Microscan Walkaway 96S® plus system according to the manufacturer’s instructions.
Microorganisms were defined as multidrug-resistant (MDR) when they were resistant to at least one agent in three or more antimicrobial categories, extensively resistant (XDR) when they were resistant to at least one agent in all but two or fewer antimicrobial categories, and neither MDR nor XDR when they did not satisfy any of the above criteria [41].
Clinical Characteristics
The clinical characteristics were: age (grouped as 18–49 years old, 50–64, and ≥65 years), sex (female/male), comorbidities (obesity, arterial hypertension, heart failure, type 2 diabetes mellitus, asthma, chronic kidney disease (CKD), cancer, and immunosuppression), a variable was generated to group the number of comorbidities (≤2/≥3). We also estimated the total length of stay in the hospital, in mechanic ventilation (MV) and ICU (days).
Auxiliary Reference Tests
The following laboratory tests were considered: leukocytes (cells/mm3), percentage of lymphocytes (%), platelets (cells/mm3), C-reactive protein (CRP; mg/dL), procalcitonin (ng/mL), lactate dehydrogenase (LDH; U/L), total and myocardial creatinine phosphokinase (U/L), aspartate aminotransferase (GOT; U/L), alanine aminotransferase (GPT; U/L), total bilirubin (mg/dL), and creatinine (mg/dL). Oxygen saturation (SatO2; ≤80/81–84/85–89/≥90%) and the correlation between oxygen pressure over inspired oxygen fraction (PaO2/FiO2; <100/100–200/200–300/>300) were evaluated at hospital admission.
Complications during ICU Stay
During ICU stay, the complications were: sepsis, septic shock, ARDS, barotrauma, stroke, digestive bleeding, malignant cardiac arrhythmias, and in-hospital cardiac arrest. All were diagnosed according to international criteria [42,43,44,45,46,47,48].
Treatment Received
The treatments administered to patients on hospital admission included: antibiotics (ceftriaxone, azithromycin, piperacillin-tazobactam, meropenem, vancomycin, linezolid), corticosteroids (dexamethasone, methylprednisolone), colchicine, tocilizumab, vasopressors, and dialysis.
4.4. Statistical Analysis
Categorical variables were described as absolute frequencies and percentages; numerical variables were summarized as mean and standard deviation (SD) or median and interquartile range (IQR) according to whether their distribution was symmetrical or not. The comparison of proportions between categorical variables and the result was performed using the chi2 test or Fisher’s exact test as appropriate. In contrast, for numerical variables, the Student’s T-test was used if there was a standard distribution or, failing that, the Mann–Whitney U test.
To test the hypothesis that healthcare-associated infection was associated with increased hospital mortality in patients with critical COVID-19, Cox proportional hazard models were used to find hazard ratios (HR) and their respective 95% confidence intervals (95% CI). First, clinical variables were tested for associations with outcomes in crude Cox regression models. Then, factors potentially associated with mortality in the crude regression (p < 0.2) were included in an adjusted Cox regression model, and the following confounders were added: sex, age, and comorbidities. Compliance with proportionality assumptions was verified using Schoenfeld residuals, and collinearity relationships in the fitted models were assessed. In addition, a secondary analysis was performed to test the hypothesis that healthcare-associated infection was associated with an increased risk of developing acute renal failure, using Poisson regression models with robust variance and finding the relative risks (RR) and their 95% CIs. In the case of AKI, not all medical records recorded the initial date of AKI (less 35%). This is why the Poisson regression with robust variance was used, since it does not use the variable time until the event. The same methodology was used for the crude and adjusted regression as in the Cox model. The linearity of the model was evaluated with each independent variable as well as multicollinearity.
Finally, the survival of patients with bacterial superinfection was described using the Kaplan–Meier method, with death as the event of interest. The log-rank test was used to establish differences in survival. All statistical analyses were performed using Stata® v16 software (StataCorp., College Station, TX, USA).
4.5. Ethics
This study was conducted following the Helsinki Declaration of 1975. The institutional research ethics committee approved the Faculty of Health Sciences protocol of the Universidad Privada de Tacna (identification code: 049-FACSA-UI). However, informed consent was not requested due to the retrospective, observational nature of the study.
5. Conclusions
There is an overuse of antibiotics from the onset of illness and a high incidence of healthcare-associated infection mainly by XDR pathogens in critically ill patients. Superinfection occurred after one week of ICU stay. It was associated with an increased risk of developing complications such as acute renal failure and significantly raising the risk of death. In this sense, measures should be implemented to promote the rational use of antibiotics in patients with COVID-19, especially those who are critically ill. More studies are needed to evaluate biomarkers that help to guide the indication of antibiotics.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10080959/s1, Tables S1: Pathogens found in the cultures of the study population.; Table S2: Description of the antibiotic resistance profile of Acinetobacter Baumannii present in the study cultures (n = 27).; Table S3: Description of the antibiotic resistance profile of Pseudomonas aeruginosa present in the study cultures (n = 11).
Author Contributions
Conceptualization, C.C.-C. and M.H.-Z.; methodology, C.C.-C., M.H.-Z. and V.A.B.-Z.; formal analysis, V.A.B.-Z.; writing—original draft preparation, V.A.B.-Z. and A.J.R.-M.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Universidad Privada de Tacna (identification code: 049-FACSA-UI, 1 April 2021).
Informed Consent Statement
Patient consent was waived as the study was retrospective.
Data Availability Statement
Available upon reasonable request.
Acknowledgments
Acknowledgment to the COVID critical care staff of Hospital III Daniel Alcides Carrion: Eyner Cordova Tejada, Rodrigo Flores Palacios, Juan Mendoza Laredo, Yessica Reynoso Rejas, Jonathan Huanacuni Ramos, Andres Gutierrez Avila and Fabrizzio Guevara Velez.
Conflicts of Interest
Rodriguez-Morales is a medical advisor at Abbott Diagnostics for Latin America, outside the submitted work. The rest of the authors declare no conflict of interest.
References
- Zu, Z.Y.; Jiang, M.D.; Xu, P.P.; Chen, W.; Ni, Q.Q.; Lu, G.M.; Zhang, L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020, 296, E15–E25. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Giacobbe, D.R.; Battaglini, D.; Enrile, E.M.; Dentone, C.; Vena, A.; Robba, C.; Ball, L.; Bartoletti, M.; Coloretti, I.; Di Bella, S.; et al. Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study. J. Clin. Med. 2021, 10, 555. [Google Scholar] [CrossRef]
- Martinez-Guerra, B.A.; Gonzalez-Lara, M.F.; de-Leon-Cividanes, N.A.; Tamez-Torres, K.M.; Roman-Montes, C.M.; Rajme-Lopez, S.; Villalobos-Zapata, G.I.; Lopez-Garcia, N.I.; Martínez-Gamboa, A.; Sifuentes-Osornio, J.; et al. Antimicrobial Resistance Patterns and Antibiotic Use during Hospital Conversion in the COVID-19 Pandemic. Antibiot. Basel 2021, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Moreno, C.; Valderrama-Beltrán, S.; Rodriguez-Morales, A.J. Implications of Antibiotic Use during the COVID-19 Pandemic: The Example of Associated Antimicrobial Resistance in Latin America. Antibiotics 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vélez, C.; Urrunaga-Pastor, D.; Romero-Cerdán, A.; Peña-Sánchez, E.; Fernández, J.L.; Cossio, J.D.; Marreros Ascoy, G.C.; Benites-Zapata, V.A. Risk factors for mortality in hospitalized patients with COVID-19 from three hospitals in Peru: A retrospective cohort study. F1000Research 2021, 10, 224. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Response Fund. 4 June 2021. Available online: https//covid19.who.int/region/amro/country/pe (accessed on 12 June 2021).
- Baskaran, V.; Lawrence, H.; Lansbury, L.E.; Webb, K.; Safavi, S.; Zainuddin, N.I.; Huq, T.; Eggleston, C.; Ellis, J.; Thakker, C.; et al. Coinfection in critically ill patients with COVID-19: An observational cohort study from England. J. Med. Microbiol. 2021, 70, 001350. [Google Scholar] [CrossRef]
- Szakmany, T.; Tuckwell, W.; Harte, E.; Wetherall, N.; Ramachandran, S.; Price, S.; Breen, H.; Killick, C.; Cheema, Y.; King, C.; et al. Differences in Inflammatory Marker Kinetics between the First and Second Wave of COVID-19 Patients Admitted to the ICU: A Retrospective, Single-Center Study. J. Clin. Med. 2021, 10, 3290. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.K.; Ananthakrishnan Ramani Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, D.; Fu, S.; Zhang, J.; Yang, X.; Xu, L.; Xu, J.; Wu, Y.; Huang, C.; Ouyang, Y.; et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit. Care 2020, 24, 219. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- He, S.; Liu, W.; Jiang, M.; Huang, P.; Xiang, Z.; Deng, D.; Chen, P.; Xie, L. Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: A multi-center study. PLoS ONE 2021, 16, e0249668. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eu. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Bamford, C.G. SARS-CoV-2, bacterial coinfections, and AMR: The deadly trio in COVID-19? EMBO Mol. Med. 2020, 12, e12560. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Nicolini, L.A.; Signori, A.; Di Biagio, A.; Sepulcri, C.; Russo, C.; Dettori, S.; Berruti, M.; Sormani, M.P.; Giacobbe, D.R.; et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS ONE 2020, 15, e0237831. [Google Scholar] [CrossRef]
- Thibault, R.; Seguin, P.; Tamion, F.; Pichard, C.; Singer, P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): A practical guidance. Crit. Care 2020, 24, 447. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- Hueda Zavaleta, M.; Copaja-Corzo, C.; Bardales-Silva, F.; Flores-Palacios, R.; BarretoRocchetti, L.; Benites Zapata, V.A. Factores asociados a la muerte por COVID-19 en pacientes admitidos en un hospital público en Tacna, Perú. Rev. Peru. Med. Exp. Salud Publica 2021, 38. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of coinfection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 58, 711–712. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 20, 1–9. [Google Scholar] [CrossRef]
- Buehler, P.K.; Zinkernagel, A.S.; Hofmaenner, D.A.; Garcia, P.D.W.; Acevedo, C.T.; Gómez-Mejia, A.; Shambat, S.M.; Andreoni, F.; Maibach, M.A.; Bartussek, J.; et al. Bacterial pulmonary superinfections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Rep. Med. 2021, 2, 100229. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. COVID-BioB study group. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Lugito, N.P.H. Is procalcitonin a part of human immunological response to SARS-CoV-2 infection or “just” a marker of bacterial coinfection? Curr. Res. Transl. Med. 2021, 69, 103289. [Google Scholar] [CrossRef] [PubMed]
- Foschi, C.; Zignoli, A.; Gaibani, P.; Vocale, C.; Rossini, G.; Lafratta, S.; Liberatore, A.; Turello, G.; Lazzarotto, T.; Ambretti, S. Respiratory bacterial coinfections in intensive care unit-hospitalized COVID-19 patients: Conventional culture vs BioFire FilmArray pneumonia Plus panel. J. Microbiol. Methods 2021, 186, 106259. [Google Scholar] [CrossRef]
- Ng, J.H.; Bijol, V.; Sparks, M.A.; Sise, M.E.; Izzedine, H.; Jhaveri, K.D. Pathophysiology and Pathology of Acute Kidney Injury in Patients with COVID-19. Adv. Chronic Kidney Dis. 2020, 27, 365–376. [Google Scholar] [CrossRef]
- Sharma, P.; Uppal, N.N.; Wanchoo, R.; Shah, H.H.; Yang, Y.; Parikh, R.; Khanin, Y.; Madireddy, V.; Larsen, C.P.; Jhaveri, K.D.; et al. COVID-19-Associated Kidney Injury: A Case Series of Kidney Biopsy Findings. J. Am. Soc. Nephrol. 2020, 31, 1948–1958. [Google Scholar] [CrossRef]
- Kudose, S.; Batal, I.; Santoriello, D.; Xu, K.; Barasch, J.; Peleg, Y.; Canetta, P.; Ratner, L.E.; Marasa, M.; Gharavi, A.G.; et al. Kidney Biopsy Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Golmai, P.; Larsen, C.P.; DeVita, M.V.; Wahl, S.J.; Weins, A.; Rennke, H.G.; Bijol, V.; Rosenstock, J.L. Histopathologic and Ultrastructural Findings in Postmortem Kidney Biopsy Material in 12 Patients with AKI and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1944–1947. [Google Scholar] [CrossRef]
- Santoriello, D.; Khairallah, P.; Bomback, A.S.; Xu, K.; Kudose, S.; Batal, I.; Barasch, J.; Radhakrishnan, J.; D’Agati, V.; Markowitz, G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. COVID-19 Clinical Management: Living Guidance. 25 January 2021. Available online: https//www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed on 6 June 2021).
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Campogiani, L.; Tejada, S.; Ferreira-Coimbra, J.; Restrepo, M.I.; Rello, J. Evidence supporting recommendations from international guidelines on treatment, diagnosis, and prevention of HAP and VAP in adults. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 483–491. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Housman, B.; Jacobi, A.; Carollo, A.; Nobel, T.; Eber, C.; Acquah, S.; Powell, C.; Kaufman, A.; Lee, D.S.; Nicastri, D.; et al. COVID-19 ventilator barotrauma management: Less is more. Ann. Transl. Med. 2020, 8, 1575. [Google Scholar] [CrossRef] [PubMed]
- Morassi, M.; Bagatto, D.; Cobelli, M.; D′Agostini, S.; Gigli, G.L.; Bnà, C.; Vogrig, A. Stroke in patients with SARS-CoV-2 infection: Case series. J. Neurol. 2020, 267, 2185–2192. [Google Scholar] [CrossRef]
- Wilkins, T.; Wheeler, B.; Carpenter, M. Upper Gastrointestinal Bleeding in Adults: Evaluation and Management. Am. Fam. Physician 2020, 101, 294–300. [Google Scholar] [PubMed]
- Turagam, M.K.; Musikantow, D.; Goldman, M.E.; Bassily-Marcus, A.; Chu, E.; Shivamurthy, P.; Lampert, J.; Kawamura, I.; Bokhari, M.; Whang, W.; et al. Malignant Arrhythmias in Patients With COVID-19: Incidence, Mechanisms, and Outcomes. Circ. Arrhythm. Electrophysiol. 2020, 13, e008920. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-Hospital Cardiac Arrest: A Review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).