Identification of Coinfections by Viral and Bacterial Pathogens in COVID-19 Hospitalized Patients in Peru: Molecular Diagnosis and Clinical Characteristics
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Definitions
4.3. Sampling and Nucleic Acids Extraction
4.4. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) for the Analysis of Respiratory Viruses
4.5. Conventional Polymerase Chain Reaction (PCR) for Atypical Bacteria Mycoplasma pneumoniae and Chlamydia pneumoniae
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia, P.J.; Alarcón, A.; Bayer, A.; Buss, P.; Guerra, G.; Ribeiro, H.; Rojas, K.; Saenz, R.; Salgado de Snyder, N.; Solimano, G.; et al. COVID-19 Response in Latin America. Am. J. Trop. Med. Hyg. 2020, 103, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. Latin America is the World’s Most Unequal Region. Here’s How to Fix it; World Economic Forum: Geneva, Switzerland, 2016; Available online: https://www.weforum.org/agenda/2016/01/inequality-is-getting-worse-in-latin-america-here-s-how-to- (accessed on 14 March 2021).
- Giraldo, E.B. COVID-19 in Peru. Indian J. Psychiatry 2020, 62 (Suppl. 3), S498–S501. [Google Scholar] [CrossRef]
- The Lancet. COVID-19: Learning from experience. Lancet 2020, 395, 1011. [Google Scholar] [CrossRef]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef] [Green Version]
- Yue, H.; Zhang, M.; Xing, L.; Wang, K.; Rao, X.; Liu, H.; Tian, J.; Zhou, P.; Deng, Y.; Shang, J. The epidemiology and clinical characteristics of co-infection of SARS-CoV-2 and influenza viruses in patients during COVID-19 outbreak. J. Med. Virol. 2020, 92, 2870–2873. [Google Scholar] [CrossRef]
- Khaddour, K.; Sikora, A.; Tahir, N.; Nepomuceno, D.; Huang, T. Case Report: The Importance of Novel Coronavirus Disease (COVID-19) and Coinfection with Other Respiratory Pathogens in the Current Pandemic. Am. J. Trop. Med. Hyg. 2020, 102, 1208. [Google Scholar] [CrossRef]
- Chen, H.R.; Zou, H.; Xue, M.; Chen, Z.B.; Chen, W.X. A Case of Childhood COVID-19 Infection with Pleural Effusion Complicated by Possible Secondary Mycoplasma Pneumoniae Infection. Pediatr. Infect. Dis. J. 2020, 39, e135–e137. [Google Scholar] [CrossRef]
- Azekawa, S.; Namkoong, H.; Mitamura, K.; Kawaoka, Y.; Saito, F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases 2020, 20, e00775. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Rubio-Gomez, H.; Roa, A.A.; Miller, N.; Eckardt, P.A. Co-Infection with SARS-COV-2 and Parainfluenza in a young adult patient with pneumonia: Case Report. IDCases 2020, 20, e00762. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Guo, Y.; Dai, Y.; Xu, Y.; Cai, Y.; et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020, 63, 606–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.; Rothrock, A.N.; Swetland, S.; Andris, H.; Davis, P.; Rothrock, S.G. Viral and atypical respiratory co-infections in COVID-19: A systematic review and meta-analysis. J. Am. Coll. Emerg. Physicians Open 2020, 1, 533–548. [Google Scholar] [CrossRef]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef]
- Vial, M.R.; Peters, A.; Pérez, I.; Spencer-Sandino, M.; Barbé, M.; Porte, L.; Weitzel, T.; Aylwin, M.; Vial, P.; Araos, R.; et al. Covid-19 in South America: Clinical and epidemiological characteristics among 381 patients during the early phase of the pandemic in Santiago, Chile. BMC Infect Dis. 2020, 20, 955. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S. Metagenome of SARS-Cov2 from a Patient in Brazil Shows a Wide Range of Bacterial Species—Lautropia, Prevotella, Haemophilus—Overshadowing Viral Reads, Which Does Not Even Add up to a Full Genome, Explaining False Negatives [Internet]. OSF Preprints. 2020. Available online: osf.io/2xt3w (accessed on 21 April 2021).
- Orozco-Hernández, J.P.; Montoya-Martínez, J.J.; Pacheco-Gallego, M.C.; Céspedes-Roncancio, M.; Porras-Hurtado, G.L. Coinfección por SARS-CoV-2 y rinovirus-enterovirus en una paciente adulta joven críticamente enferma en Colombia. Biomedica 2020, 40, 34–43. [Google Scholar] [CrossRef]
- Loens, K.; Ieven, M. Mycoplasma pneumoniae: Current knowledge on nucleic acid amplification techniques and serological diagnostics. Front. Microbiol. 2016, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Beersma, M.F.C.; Dirven, K.; Van Dam, A.P.; Templeton, K.E.; Claas, E.C.J.; Goossens, H. Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard”. J. Clin. Microbiol. 2005, 43, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Gayam, V.; Konala, V.M.; Naramala, S.; Garlapati, P.R.; Merghani, M.A.; Regmi, N.; Balla, M.; Adapa, S. Presenting characteristics, comorbidities and outcomes of patients coinfected with COVID-19 and Mycoplasma pneumoniae in the USA. J. Med. Virol. 2020, 92, 2181–2187. [Google Scholar] [CrossRef]
- Amin, D.; McKitish, K.; Shah, P.S. Association of mortality and recent Mycoplasma pneumoniae infection in COVID-19 patients. J. Med. Virol. 2021, 93, 1180–1183. [Google Scholar] [CrossRef]
- Oliva, A.; Siccardi, G.; Migliarini, A.; Cancelli, F.; Carnevalini, M.; D’Andria, M.; Attilia, I.; Danese, V.C.; Cecchetti, V.; Romiti, R.; et al. Co-infection of SARS-CoV-2 with Chlamydia or Mycoplasma pneumoniae: A case series and review of the literature. Infection 2020, 48, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahariadis, G.; Gooley, T.A.; Ryall, P.; Hutchinson, C.; Latchford, M.I.; Fearon, M.A.; Jamieson, F.B.; Richardson, S.; Kuschak, T.; Mederski, B. Risk of ruling out severe acute respiratory syndrome by ruling in another diagnosis: Variable incidence of atypical bacteria coinfection based on diagnostic assays. Can. Respir. J. 2006, 13, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Deeb, A.M.; Al-Hameed, F.; Mandourah, Y.; Almekhlafi, G.A.; Sindi, A.A.; Al-Omari, A.; Shalhoub, S.; Mady, A.; Alraddadi, B.; et al. Saudi Critical Care Trials group. Macrolides in critically ill patients with Middle East Respiratory Syndrome. Int. J. Infect. Dis. 2019, 81, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, N.; Matsushima, T.; Oka, M. The JRS guidelines for the management of community-acquired pneumonia in adults: An update and new recommendations. Intern. Med. 2006, 45, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, N.; Kawai, Y.; Yamaguchi, T.; Ouchi, K.; Oka, M.; Maruyama, T.; Taguchi, O.; Nakahama, C.; Yoneyama, H.; Nakamura, J.; et al. Clinical potential of diagnostic methods for the rapid diagnosis of Mycoplasma pneumoniae pneumonia in adults. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 439–446. [Google Scholar] [CrossRef]
- Miyashita, N.; Ouchi, K.; Kawasaki, K.; Oda, K.; Kawai, Y.; Shimizu, H.; Kobashi, Y.; Oka, M. Mycoplasma pneumoniae pneumonia in the elderly. Med. Sci. Monit. 2008, 14, CR387–CR391. [Google Scholar] [PubMed]
- Fan, B.E.; Lim, K.G.E.; Chong, V.C.L.; Chan, S.S.W.; Ong, K.H.; Kuperan, P. COVID-19 and mycoplasma pneumoniae coinfection. Am. J. Hematol. 2020, 95, 723–724. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA-J. Am. Med. Assoc. 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- WHO/PAHO/ Regional Update: Influenza & Other Respiratory Viruses. 2020. Available online: https://docs.bvsalud.org/biblioref/2020/11/1128699/2020-phe-influenza-report-44.pdf (accessed on 9 December 2020).
- Ampuero, J.S.; Ocaña, V.; Gómez, J.; Gamero, M.E.; Garcia, J.; Halsey, E.S.; Laguna-Torres, V.A. Adenovirus Respiratory Tract Infections in Peru. PLoS ONE 2012, 7, e46898. [Google Scholar] [CrossRef]
- Belongia, E.A.; Osterholm, M.T. COVID-19 and flu, a perfect storm. Science 2020, 368, 1163. [Google Scholar] [CrossRef]
- Vargas-Ponce, K.G.; Salas-López, J.A.; Llanos-Tejada, F.K.; Morales-Avalos, A. Coinfección de COVID-19 e influenza: Reporte de cinco casos en un hospital peruano. Rev. Fac. Med. Hum. 2020, 20, 738–742. [Google Scholar] [CrossRef]
- Corvalán, L.P.; Arias, B.G.; Morales, S.P.; González, M.R.; Inostroza, S.J.; Fuenzalida, I.L. Inmunofluorescencia indirecta versus reacción de polimerasa en cadena para el diagnóstico de virus respiratorios en niños ingresados en un hospital de la Región Metropolita. Rev. Chil. De Infectología 2019, 36, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Iacobucci, G. Covid-19: Risk of death more than doubled in people who also had flu, English data show. BMJ 2020, 370, m3720. [Google Scholar] [CrossRef]
- Laguna-Torres, V.; Gómez, J.; Hernández, H.; Francia-Romero, J.; Bisso-Andrade, A.; Guerreros, A.; Cerna-Barco, J.; Sanchez-Vergaray, E.; Gotuzzo, E. Vigilancia, prevención y control del virus de la influenza en Perú. Revista Peruana de Medicina. Rev. Peru. De Med. Exp. Y Salud Publica 2019, 36, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Karakike, E.; Giamarellos-Bourboulis, E.; Kyprianou, M.; Fleischmann-Struzek, C.; Pletz, M.W.; Netea, M.G.; Reinhart, K.; Kyriazopoulou, E. Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Sheng, Y.; Zhang, L.; Shen, Z.; Chen, Z. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1034–1038. [Google Scholar] [CrossRef] [Green Version]
- Sieswerda, E.; de Boer, M.G.J.; Bonten, M.M.J.; Boersma, W.G.; Jonkers, R.E.; Aleva, R.M.; Kullberg, B.J.; Schouten, J.A.; van de Garde, E.M.; Verheij, T.J.; et al. Recommendations for antibacterial therapy in adults with COVID-19—an evidence based guideline. Clin. Microbiol. Infect. 2021, 27, 61–66. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Rapid Guideline: Antibiotics for Pneumonia in Adults in Hospital. London: National Institute for Health and Care Excellence (UK); 2020 Oct 9. (NICE Guideline, No. 173). Available online: https://www.ncbi.nlm.nih.gov/books/NBK566162/ (accessed on 11 April 2021).
- Butler, C.; Dorward, J.; Yu, L.; Gbinigie, O.; Hayward, G.; Saville, B.R.; Van Hecke, O.; Berry, N.; Detry, M.; Saunders, C.; et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1063–1074. [Google Scholar] [CrossRef]
- Del Valle-Mendoza, J.; Orellana-Peralta, F.; Marcelo-Rodríguez, A.; Verne, E.; Esquivel-Vizcarra, M.; Silva-Caso, W.; Aguilar-Luis, M.A.; Weilg, P.; Casabona-Oré, V.; Ugarte, C.; et al. High prevalence of mycoplasma pneumoniae and chlamydia pneumoniae in children with acute respiratory infections from Lima, Peru. PLoS ONE 2017, 12, e0170787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, J.D.; Siccha, S.M.; Egoavil, M.; Chaparro, E.; Hernandez, R.; Silva, W.; Águila, O.D.; Saenz, A.; Campos, F.; Reyes, I.; et al. Resistencia antibiótica y distribución de serotipos en cepas neumocócicas invasivas en adultos hospitalizados en Lima, Perú. Rev. Peru. De Med. Exp. Y Salud Pública 2017, 34, 633–641. [Google Scholar] [CrossRef] [Green Version]
- IMAI District Clinician Manual. Hospital Care for Adolescents and Adults; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.T.; Leung, S.T.; Chin, T.W.Y.; Lo, C.S.Y.; Lui, M.M.S.; Lee, J.C.Y.; Chiu, K.W.H.; Chung, T.W.H.; et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 2020, 296, E72–E78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, M.J.; Gunson, R.; Maclean, A.; Coughlan, S.; Fitzgerald, M.; Scully, M.; O’Herlihy, B.; Ryan, J.; O’Flanagan, D.; Connell, J.; et al. Development of a real-time RT-PCR for the detection of swine-lineage influenza A (H1N1) virus infections. J. Clin. Virol. 2009, 45, 196–199. [Google Scholar] [CrossRef]
- Selvaraju, S.B.; Selvarangan, R. Evaluation of three influenza A and B real-time reverse transcription-PCR assays and a new 2009 H1N1 assay for detection of influenza viruses. J. Clin. Microbiol. 2010, 48, 3870–3875. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, D.; Tan, W.; Xu, D.; Qiu, S.; Zeng, Z.; Li, X.; Zhou, R. Epidemiology and clinical presentations of respiratory syncytial virus subgroups a and B detected with multiplex real-time PCR. PLoS ONE 2016, 11, e0165108. [Google Scholar] [CrossRef] [Green Version]
- Heim, A.; Ebnet, C.; Harste, G.; Pring-Akerblom, P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 2003, 70, 228–239. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 295) | SARS-CoV-2 All Coinfections Evaluated (n = 154) | SARS-CoV-2 Monoinfection (n = 141) | SARS-CoV-2 + Adenovirus (n = 5) | SARS-CoV-2 + Mycoplasma pneumoniae (n = 83) | SARS-CoV-2 + Chlamydia pneumoniae (n = 26) | SARS-CoV-2 + Mycoplasma pneumoniae+ Chlamydia pneumoniae (n = 34) | Others (n = 6) | |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 209 (70.9%) [65.3–75.9%] | 112 (72.2%) [64.9–79.5%] | 97 (68.7%) [60.5–76.3%] | 5 (100.0%) | 60 (72.3%) [61.4–81.5%] | 16 (61.5%) [40.6–79.7] | 26 (76.5%) [58.8–89.2%] | 5 (83.3%) [35.9–99.6%] |
| Female | 86 (29.1%) [24.0–34.6%] | 42 (27.8) [20.4–35.0%] | 44 (31.2%) [23.7–39.5%] | 0 (0.0%) | 23 (27.7%) [18.4–38.6%] | 10 (38.5%) [20.2–59.4%] | 8 (23.5%) [10.7–41.1] | 1 (16.6%) [0.4–64.1%] |
| Age | ||||||||
| Mean/SD | 58.0 ± 14.0 | 58.3 (13.8) | 57.7 ± 14.3 | 59.6 ± 10.0 | 60.0 ± 13.7 | 55.8 ± 13.0 | 55.7 ± 15.6 | 59.67 ± 10.44 |
| Comorbidities | ||||||||
| Hypertension | 79 (26.8%) [21.8–32.2%] | 48 (31.1%) [23.9–39.1%] | 31 (22.0%) [15.4–29.7%] | 2 (40.0%) [5.3–85.3%] | 26 (31.3) [21.6–42.4%] | 7 (27.0%) [11.5–47.7%] | 11 (32.3%) [17.4–50.5%] | 2 (33.3%) [4.3–77.7] |
| Diabetes | 66 (22.4%) [17.7–27.5%] | 36 (23.4%) [16.9–30.8%] | 30 (21.3%) [14.8–28.9%] | 2 (40.0%) [5.3–85.3%] | 22 (26.5%) [17.4–37.3%] | 6 (23.1%) [8.9–43.6%] | 6 (17.7%) [6.7–34.5%] | 0 (0.0%) |
| Obesity | 55 (18.6%) [14.4–23.6%] | 24 (15.6%) [10.2–22.2%] | 31 (22.0%) [15.4–29.7%] | 0 (0.0%) | 11 (13.3%) [6.8–22.5%] | 5 (19.2%) [6.6–39.4%] | 7 (20.6%) [87.0–37.9%] | 1 (16.7%) [0.4–64.1%] |
| Asthma | 12 (4.0%) [2.1–6.9%] | 7 (4.5%) [1.8–9.1%] | 5 (3.6%) [1.1–8.1%] | 1 (20.0%) [0.5–71.6%] | 2 (2.4%) [0.3–8.4%] | 3 (11.5%) {24.4–30.2%] | 1 (2.9%) [0.1–15.3%] | 0 (0.0%) |
| Coronary artery disease | 12 (4.1%) [2.1–6.9%] | 4 (2.6%) [0.7–6.5%] | 8 (5.7%) {2.4–10.8%] | 0 (0.0%) | 3 (3.6%) [0.7–10.2%] | 1 (3.9%) [0.1–19.6%] | 0 (0.0%) | 0 (0.0%) |
| Cancer | 7 (2.4%) [0.9–4.8%] | 4 (2.6%) [0.7–6.5%] | 3 (2.1%) [0.4–6.1%] | 0 (0.0%) | 4 (4.8%) [1.3–11.8] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| CKD* | 4 (1.4%) [0.4–3.4%] | 4 (2.6%) [0.7–6.5%] | 0 (0.0%) | 1 (20.0%) [0.5–71.6%] | 0 (0.0%) | 0 (0.0%) | 2 (5.9%) [0.7–19.7%] | 0 (0.0%) |
| Others | 56 (19.0%) [14.7–23.9%] | 28 (18.1%) [12.4–25.2%] | 28 (19.9%) [13.6–27.4%] | 0 (0.0%) | 16 (19.3%) [11.4–29.4%] | 4 (15.4%) [4.4–34.8%] | 7 (20.6%) [8.7–37.9%] | 1 (16.6%) [0.4–64.1%] |
| Symptoms | ||||||||
| Cough | 215 (72.9%) [67.4–77.8%] | 107 (69.5%) [61.5–76.6%] | 108 (76.6%) [68.7–83.3%] | 4 (80.0%) [28.3–99.4%] | 57 (68.7%) [57.5–78.4%] | 17 (65.3%) [44.3–82.7%] | 24 (70.6%) [52.5–84.9%] | 5 (833%) [35.9–99.6%] |
| Dyspnea | 206 (69.8%) [64.2–75.0%] | 105 (68.2%) [60.2–75.4%] | 101 (71.6%) [63.4–78.9%] | 3 (60.0%) [14.7–94.7%] | 61 (73.5%) [62.7–82.6%] | 15 (57.7%) [37.9–76.6%] | 22 (64.7%) [46.5–80.2%] | 4 (66.7%) [22.3–95.7%] |
| Fever | 180 (61.0%) [55.2–66.6%] | 95 (61.7%) [53.5–69.3%] | 85 (60.3%) [51.7–68.4%] | 4 (80.0%) [28.3–99.4%] | 48 (57.8%) [46.5–68.5%] | 17 (65.4%) [44.3–82.7%] | 23 (67.7%) [49.5–82.6%] | 3 (50.0%) [11.8–88.1%] |
| Fatigue | 148 (50.2%) [44.3–56.0%] | 74 (48.1%) [39.9–56.2%] | 74 (52.5%) [43.9–60.9%] | 4 (80.0%) [28.3–99.4%] | 39 (47.0%) [35.9–58.2%] | 13 (50.0%) [29.9–70.0%] | 14 (41.2%) [24.6–59.3%] | 4 (66.7%) [22.3–95.7%] |
| Odynophagia | 39 (13.2%) [9.6–17.6%] | 19 (12.3%) [7.6–18.5%] | 20 (14.2%) [8.8–21.1%] | 0 (0.0%) | 11 (13.3%) [6.8–22.4%] | 3 (11.5%) [2.4–30.1%] | 4 (11.8%) [3.3–27.4%] | 1 (16.7%) [0.4–64.1%] |
| Headache | 35 (11.9%) [8.4–16.1%] | 17 (11.0%) [6.5–17.0%] | 18 (12.8%) [7.7–19.4%] | 1 (20.0%) [0.5–71.6%] | 5 (6.0%) [1.9–13.5%] | 4 (15.4%) [4.4–34.8%] | 6 (17.7%) [6.7–34.5%] | 1 (16.7%) [0.4–64.1%] |
| Nausea/vomiting | 18 (6.1%) [3.6–9.5%] | 12 (7.8%) [4.1–13.2%] | 6 (4.3%) [1.5–9.0%] | 0 (0.0%) | 8 (9.6%) [4.3–18.1%] | 1 (3.9%) [0.1–19.6%] | 2 (5.9%) [0.7–19.6%] | 1 (16.7%) [0.4–64.1%] |
| Diarrhea | 20 (6.8%) [04.2–10.2%] | 11 (7.1%) [3.6–12.4%] | 9 (6.4%) [2.9–11.7%] | 0 (0.0%) | 6 (7.2%) [2.7–15.0%] | 2 (7.7%) [0.9–25.1%] | 2 (5.9%) [0.7–19.6%] | 1 (16.7%) [0.4–64.1%] |
| Expectoration | 27 (9.1%) [6.1–13.0%] | 9 (5.8%) [2.7–10.8%] | 18 (12.8%) [7.7–19.4%] | 0 (0.0%) | 6 (7.2%) [2.7–15.0%] | 1 (3.9%) [0.1–19.6%] | 1 (2.9%) [0.1–15.3%] | 1 (16.7%) [0.4–64.1%] |
| Anosmia | 11 (3.7%) [1.8–6.6%] | 5 (3.3%) [1.1–7.4%] | 6 (4.3%) [1.5–9.0%] | 0 (0.0%) | 3 (3.6%) [0.7–10.2%] | 1 (3.9%) [0.1–19.6%] | 1 (2.9%) [0.1–15.3%] | 0 (0.0%) |
| Days since symptom onset * | 7 (5–10) | 7 (5–10) | 7 (6–10) | 6 (3–9) | 7 (4–10) | 7 (6–9) | 7 (5–13) | 7 (6–12) |
| CURB 65 * | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–3) | 1 (0–2) | 0 (0–1) | 1 (0–1) | 1 (0–2) |
| Total (n = 295) | SARS-CoV-2 All Coinfections Evaluated (n = 154) | SARS-CoV-2 Monoinfection (n = 141) | SARS-CoV-2 + Adenovirus (n = 5) | SARS-CoV-2 + Mycoplasma pneumoniae (n = 83) | SARS-CoV-2 + Chlamydia pneumoniae (n = 26) | SARS-CoV-2 + Mycoplasma pneumoniae+ Chlamydia pneumoniae (n = 34) | Others (n = 6) | |
|---|---|---|---|---|---|---|---|---|
| Laboratory parameters * | ||||||||
| Hemoglobin (g/dl) | 14.20 (13.1–15.4) | 14.5 (13.2–15.4) | 14 (12.9–15.5) | 14 (12.2–16.3) | 14.5 (13.1–15.4) | 14.1 (13.1–14.8) | 14.45 (13.3–15.6) | 14.65 (13.2–16.2) |
| Leucocytes (× 109 mL) | 9.1 (7.9–12.3) | 8.85 (7–11.9) | 9.2 (7–12.3) | 10.4 (5.25–14.05) | 8.3 (6.4–11.4) | 8.65 (7.3–11.5) | 10.1 (7.3–12.8) | 9.4 (8.1–13) |
| Lymphocytes (Absolute count) | 820 (504–1290) | 797 (518–1242) | 847 (497.5–1325.5) | 828 (445.5–1866) | 888 (615–1348) | 837 (495–1442) | 653.5 (468–1020) | 736 (486–872) |
| Platelets (× 109 mL) | 270 (202–350) | 270.5 (204–342.5) | 265 (192.5–355.5) | 213 (148.5–312.5) | 267 (201–340) | 295 (218–333) | 289 (225–394) | 215 (197–232) |
| ALT (U/L) | 49 (26.5–88) | 50 (26–88) | 45 (27–87) | 45 (15.5–193) | 50 (25–93) | 47 (31.5–63) | 56 (26–103) | 60 (52–70) |
| Creatinine (mg/dL) | 0.7 (0.6–0.9) | 0.7 (0.6–0.9) | 0.7 (0.6–0.9) | 1 (0.7–1.2) | 0.7 (0.6–0.8) | 0.65 (0.5–0.9) | 0.75 (0.6–0.9) | 0.75 (0.5–1) |
| C-reactive protein (mg/L) | 90 (56–210) | 90 (58–191) | 90 (54.2–235.1) | 277.4 (NA) | 89 (58.8–174) | 72.7 (43–226) | 90 (62.7–201.75) | 181 (NA) |
| LDH (U/L) | 298 (242.5–378.5) | 307 (251–376) | 281.5 (233–381) | 428 (NA) | 299 (243.5–364) | 331.5 (24.5–366) | 291 (244–387) | 368 (333.5–433) |
| Procalcitonin (ng/mL) | 0.09 (0.06–0.25) | 0.14 (0.07–0.27) | 0.09 (0.04–0.18) | 0.16 (NA) | 0.09 (0.075–0.64) | 0.13 (0.065–0.22) | 0.23 (0.11–1.16) | 0.1 (NA) |
| D-Dimer (µg/mL) | 0.66 (0.39–1.2) | 0.7 (0.3–1.2) | 0.6 (0.39–1.22) | 0.87 (NA) | 0.8 (0.45–0.98) | 0.675 (0.24–1.105) | 0.465 (0.35–1.915) | 0.725 (0.36–1.39) |
| Troponin (ng/mL) | 0.006 (0.001–0.10) | 0.006 (0.001–0.01) | 0.006 (0.003–0.01) | 0.011 (NA) | 0.006 (0.001–0.01) | 0.019 (0.008–0.149) | 0.004 (0.001–0.1) | 0.006 (NA) |
| Ferritin (ng/mL) | 664.5 (346–1220) | 639 (346–1127) | 712 (344–1238.5) | 1260 (NA) | 620.5 (330–1066.5) | 455 (184–821) | 748.5 (510–1387) | 817.5 (239–1759) |
| CPK (U/L) | 55 (33–88) | 42 (31–78) | 49 (34.5–90) | 40 (NA) | 40.5 (34–165) | 42 (18–70) | 45 (NA) | 49 (NA) |
| PT (s) | 10.9 (10.4–11.5) | 10.8 (10.4–11.3) | 11 (10.4–11.6) | 10.8 (10.3–12.2) | 10.9 (10.4–11.25) | 10.7 (10.4–11.9) | 10.9 (10.6–11.2) | 10.25 (10.1–10.8) |
| Radiological score | ||||||||
| Mean /SD | 5.92 ± 1.55 | 5.90 ± 1.15 | 5.92 ± 1.86 | 6 ± 2 | 5.82 ± 1.90 | 5.46 ± 2.00 | 6.53 ± 1.58 | 5.67 ± 2.58 |
| Treatments | ||||||||
| Hydroxychloroquine | 3 (1.0%) [0.2–29.4%] | 1 (0.7%) [0.1–3.5%] | 2 (1.4%) [0.2–5.0%] | 0 (0.0%) | 1 (1.2%) [0.1–6.5%] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Ivermectin | 24 (8.1%) [5.2–11.9%] | 9 (5.8%) [2.7–10.8%] | 15 (10.6%) [6.1–16.9%] | 0 (0.0%) | 7 (8.4%) [3.5–16.6%] | 0 (0.0%) | 1 (2.9%) [0.1–15.3%] | 1 (16.7%) [0.4–64.1%] |
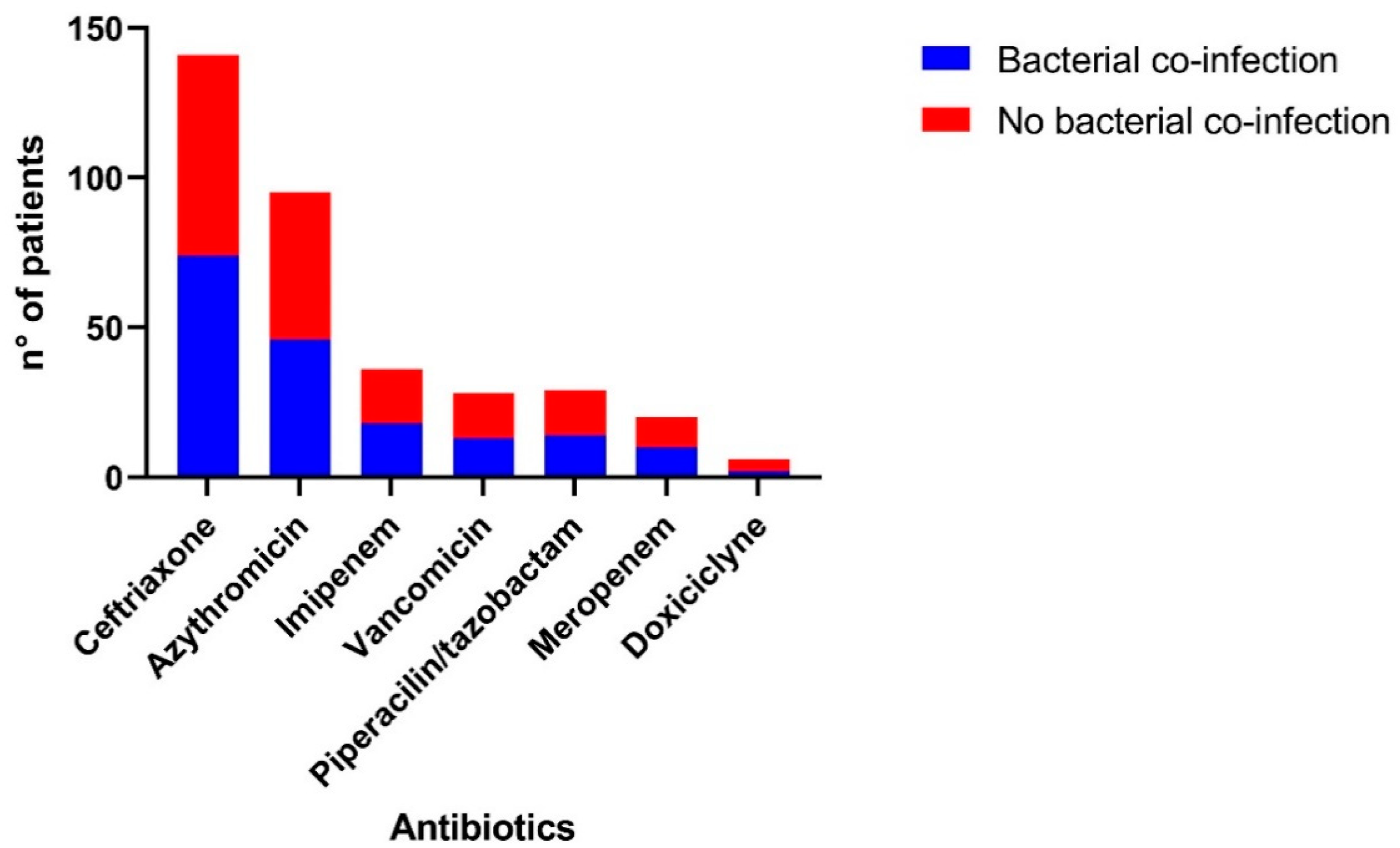
| Antibiotics | 205 (69.5%) [63.8–74.6%] | 110 (71.4%) [63.6–78.4%] | 95 (67.4%) [58.9–75.0%] | 4 (80.0%) [28.3–99.4%] | 59 (71.1%) [60.1–80.5%] | 18 (69.2%) [48.2–85.6%] | 26 (76.5%) [58.8–89.2%] | 3 (50.0%) [11.8–88.1%] |
| Dexamethasone | 250 (84.7%) [80.1–88.7%] | 137 (89.0%) [82.9–93.4%] | 113 (80.1%) [72.6–86.4%] | 5 (100.0%) | 71 (85.5%) [76.1–92.3%] | 22 (84.6%) [65.1–95.6%] | 33 (97.1%) [84.7–99.9%] | 6 (100.0%) |
| Methyilprednisolone | 1 (0.3%) [0.1–18.7%] | 0 (0.0%) | 1 (0.7%) [0.1–3.9%] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Hydrocortisone | 2 (0.7%) [0.1–24.3%] | 1 (0.7%) [0.1–3.5%] | 1 (0.7%) [0.1–3.9%] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) [0.1–15.3%] | 0 (0.0%) |
| Binasal cannula | 161 (54.6%) [48.7–60.4%] | 81 (52.6%) [44.4–60.6%] | 80 (56.7%) [48.1–65.0%] | 2 (40.0%) [5.3–85.3%] | 44 (53.1%) [41.7–64.0%] | 12 (46.2%) [26.6–66.6%] | 19 (55.9%) [37.9–72.8%] | 4 (66.7%) [22.3–95.7%] |
| Reservoir bag | 111 (37.6%) [32.1–43.4%] | 63 (40.9%) [33.1–49.1%] | 48 (34.0%) [26.3–42.5%] | 1 (20.0%) [0.5–71.6%] | 33 (39.8%) [0.8–10.2%] | 12 (46.2%) [26.6–66.6%] | 15 (44.1%) [27.2–62.1%] | 2 (33.3%) [4.3–77.7%] |
| High-flow nasal cannula | 20 (6.8%) [4.2–10.3%] | 12 (7.8%) [4.1–13.2%] | 8 (5.7%) [2.5–10.9%] | 2 (40.0%) [5.3–85.3%] | 6 (7.2%) [2.7–15.0%] | 1 (3.9%) [0.1–19.6%] | 2 (5.9%) [0.7–19.6%] | 1 (16.7%) [0.4–64.1%] |
| Mechanical ventilation | 20 (6.8%) [4.2–10.3%] | 10 (6.5%) [3.2–11.6%] | 10 (7.1%) [3.5–12.6%] | 0 (0.0%) | 5 (6.0%) [1.9–13.5%] | 3 (11.5%) [2.4–30.2%] | 1 (2.9%) [0.1–15.3%] | 1 (16.7%) [0.4–64.1%] |
| Norepinephrine | 21 (7.1%) [4.5–10.7%] | 8 (5.2%) [2.3–99.8%] | 13 (9.3%) [5.0–15.52%] | 1 (20.0%) [0.5–71.6%] | 4 (4.8%) [1.3–11.8%] | 2 (7.7%) [0.9–25.1%] | 1 (2.9%) [0.1–15.3%] | 0 (0.0%) |
| Epinephrine | 3 (1.0%) [2.1–29.4%] | 2 (1.3%) [0.2–4.6%] | 1 (0.7%) [0.1–3.9%] | 1 (20.0%) [0.5–71.6%] | 1 (1.2%) [0.1–6.5%] | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Hemodialysis | 3 (1.0%) [2.1–29.4%] | 1 (0.7%) [0.1–3.5%] | 2 (1.4%) [0.2–5.0%] | 0 (0.0%) | 0 (0.0%) | 1 (3.0%) [0.1–19.6%] | 0 (0.0%) | 0 (0.0%) |
| Clinical Outcomes | Total (n = 295) | SARS-CoV-2 All Coinfections Evaluated (n = 154) | SARS-CoV-2 Monoinfection (n = 141) | SARS-CoV-2 + Adenovirus (n = 5) | SARS-CoV-2 + Mycoplasma pneumoniae (n = 83) | SARS-CoV-2 + Chlamydia pneumoniae (n = 26) | SARS-CoV-2 + Mycoplasma pneumoniae + Chlamydia pneumionie (n = 34) | Others (n = 6) |
|---|---|---|---|---|---|---|---|---|
| Sepsis | 80 (27.1%) [22.1–32.6%] | 51 (33.1%) [25.7–41.1%] | 29 (20.6%) [14.2–28.2%] | 2 (40.0%) [5.3–85.3%] | 31 (37.4%) [26.9–48.6%] | 6 (23.1%) [8.9-43.6%] | 9 (26.5%) [12.8–44.3%] | 3 (50.0%) [11.8–88.1%] |
| ARDS | 60 (20.3%) [15.9–25.4%] | 35 (22.7%) [16.4–30.2%] | 25 (17.7%) [11.8–25.1%] | 2 (40.0%) [5.3–85.3%] | 13 (15.7%) [8.6–25.3%] | 9 (34.6%) [17.2–55.6%] | 8 (23.5%) [10.7–41.1%] | 3 (50.0%) [11.8–88.1%] |
| Heart failure | 25 (8.5%) [5.6–12.3%] | 17 (11.0%) [6.6–17.1%] | 8 (5.7%) [2.4%-10.9%] | 0 (0.0%) | 7 (8.4%) [3.5–16.6%] | 6 (23.1%) [8.9–43.6%] | 3 (8.8%) [1.9–23.6%] | 1 (16.7%) [0.4–64.1%] |
| Septic shock | 24 (8.1%) [5.3–11.8%] | 11 (7.1%) [3.6–12.4%] | 13 (9.2%) [5.0–15.3%] | 2 (40.0%) [5.3–85.3%] | 5 (6.0%) [1.9–13.5%] | 3 (11.5%) [2.4–30.2%] | 1 (2.9%) [0.1–15.3%] | 0 (0.0%) |
| Coagulopathy | 17 (5,8%) [3.4–9.1%] | 10 (6.5%) [3.2–11.6%] | 7 (5.0%) [2.0–9.9%] | 1 (20.0%) [0.5–71.6%] | 4 (4.8%) [1.3–11.8%] | 4 (15.4%) [4.4–34.8%] | 1 (2.9%) [0.1–15.3%] | 0 (0.0%) |
| Acute myocardial injury | 12 (4.1%) [2.1–6.9%] | 4 (2.6%) [0.7–6.5%] | 8 (5.7%) [2.5–10.8%] | 0 (0.0%) | 3 (3.6%) [0.8–10.2%] | 1 (3.9%) [0.1–19.6%] | 0 (0.0%) | 0 (0.0%) |
| Superinfection | 15 (5.1%) [2.8–8.5] | 10 (6.5%) [3.2–11.6%] | 5 (3.6%) [1.2–8.1%] | 1 (20.0%) [0.5–71.6%] | 5 (6.0%) [1.9–13.5%] | 3 (11.5%) [2.4–30.2%] | 1 (2.9%) [0.01–15.3%] | 1 (16.7%) [0.4–64.1%] |
| Acute kidney injury | 30 (10.2%) [6.9–14.1%] | 16 (10.4%) [6.1–16.3%] | 14 (9.9%) [5.5–16.1%] | 1 (20.0%) [0.5–71.6%] | 6 (7.2%) [2.7–15.1%] | 4 (15.4%) [4.4–34.8%] | 4 (11.8%) [3.3–27.4%] | 1 (16.67) (0.4–64.1%) |
| Respiratory acidosis | 28 (9.5%) [6.4–13.4%] | 13 (8.4%) [4.6–14.0%] | 15 (10.6%) [6.1–16.9%] | 1 (20.0%) [0.5–71.6%] | 8 (9.6%) [4.3–18.1%] | 2 (7.7%) [0.9–25.1%] | 1 (2.9%) [0.1–15.3%] | 1 (16.7%) [0.4–64.1%] |
| ICU Admission | 29 (9.8%) [6.6–13.8%] | 17 (11.4%) [6.6–17.1%] | 12 (8.5%) [4.5–14.4%] | 1 (20.0%) [0.5–71.6%] | 10 (12.5%) [5.9–21.0%] | 3 (11.5%) [2.4–30.2%] | 2 (5.9%) [0.7–19.7] | 1 (16.7%) [0.4–64.1%] |
| Days in ICU | 11 (6–21) | 16 (6–19) | 8 (5.5–21) | 17 (NA) | 13.5 (6–25) | 18 (NA) | 35.5 (NA) | 1 (NA) |
| Days in mechanical ventilation | 11 (1–19.5) | 16 (1–19) | 9 (7–20) | 17 (NA) | 11 (1–21) | 18 (NA) | 25 (NA) | 1 (NA) |
| Hospitalization days | 10 (7–15) | 10 (7–15) | 10 (7–15) | 7 (5.5–17.5) | 11 (7–15) | 10.5 (6–21) | 9.5 (7–15) | 8 (7–15) |
| Death | 59 (20.0%) [15.5–25.0%] | 32 (20.8%) [14.7–28.0%] | 27 (19.2%) [13.0–26.6%] | 2 (40.0%) [5.3–85.3%] | 15 (18.1%) [10.5–28.0%] | 6 (23.1%) [8.9–43.6%] | 6 (17.7%) [6.7–34.5%] | 3 (50.0%) [11.8–88.1%] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Lazo, G.; Silva-Caso, W.; del Valle-Mendoza, J.; Morales-Moreno, A.; Ballena-López, J.; Soto-Febres, F.; Martins-Luna, J.; Carrillo-Ng, H.; del Valle, L.J.; Kym, S.; et al. Identification of Coinfections by Viral and Bacterial Pathogens in COVID-19 Hospitalized Patients in Peru: Molecular Diagnosis and Clinical Characteristics. Antibiotics 2021, 10, 1358. https://doi.org/10.3390/antibiotics10111358
Pérez-Lazo G, Silva-Caso W, del Valle-Mendoza J, Morales-Moreno A, Ballena-López J, Soto-Febres F, Martins-Luna J, Carrillo-Ng H, del Valle LJ, Kym S, et al. Identification of Coinfections by Viral and Bacterial Pathogens in COVID-19 Hospitalized Patients in Peru: Molecular Diagnosis and Clinical Characteristics. Antibiotics. 2021; 10(11):1358. https://doi.org/10.3390/antibiotics10111358
Chicago/Turabian StylePérez-Lazo, Giancarlo, Wilmer Silva-Caso, Juana del Valle-Mendoza, Adriana Morales-Moreno, José Ballena-López, Fernando Soto-Febres, Johanna Martins-Luna, Hugo Carrillo-Ng, Luís J. del Valle, Sungmin Kym, and et al. 2021. "Identification of Coinfections by Viral and Bacterial Pathogens in COVID-19 Hospitalized Patients in Peru: Molecular Diagnosis and Clinical Characteristics" Antibiotics 10, no. 11: 1358. https://doi.org/10.3390/antibiotics10111358
APA StylePérez-Lazo, G., Silva-Caso, W., del Valle-Mendoza, J., Morales-Moreno, A., Ballena-López, J., Soto-Febres, F., Martins-Luna, J., Carrillo-Ng, H., del Valle, L. J., Kym, S., Aguilar-Luis, M. A., Peña-Tuesta, I., Tinco-Valdez, C., & Illescas, L. R. (2021). Identification of Coinfections by Viral and Bacterial Pathogens in COVID-19 Hospitalized Patients in Peru: Molecular Diagnosis and Clinical Characteristics. Antibiotics, 10(11), 1358. https://doi.org/10.3390/antibiotics10111358






