Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Study Selection
2.2. Characteristics of the Included Studies
2.3. Quality Assessment and Publication Bias
2.4. Overall Antibiotic Resistance Patterns
2.5. Resistance to First-Line Anti-TB Drugs
2.6. Resistance to Second-Line Anti-TB Drugs
2.7. Drug-Resistance Pattern Based on Treatment History
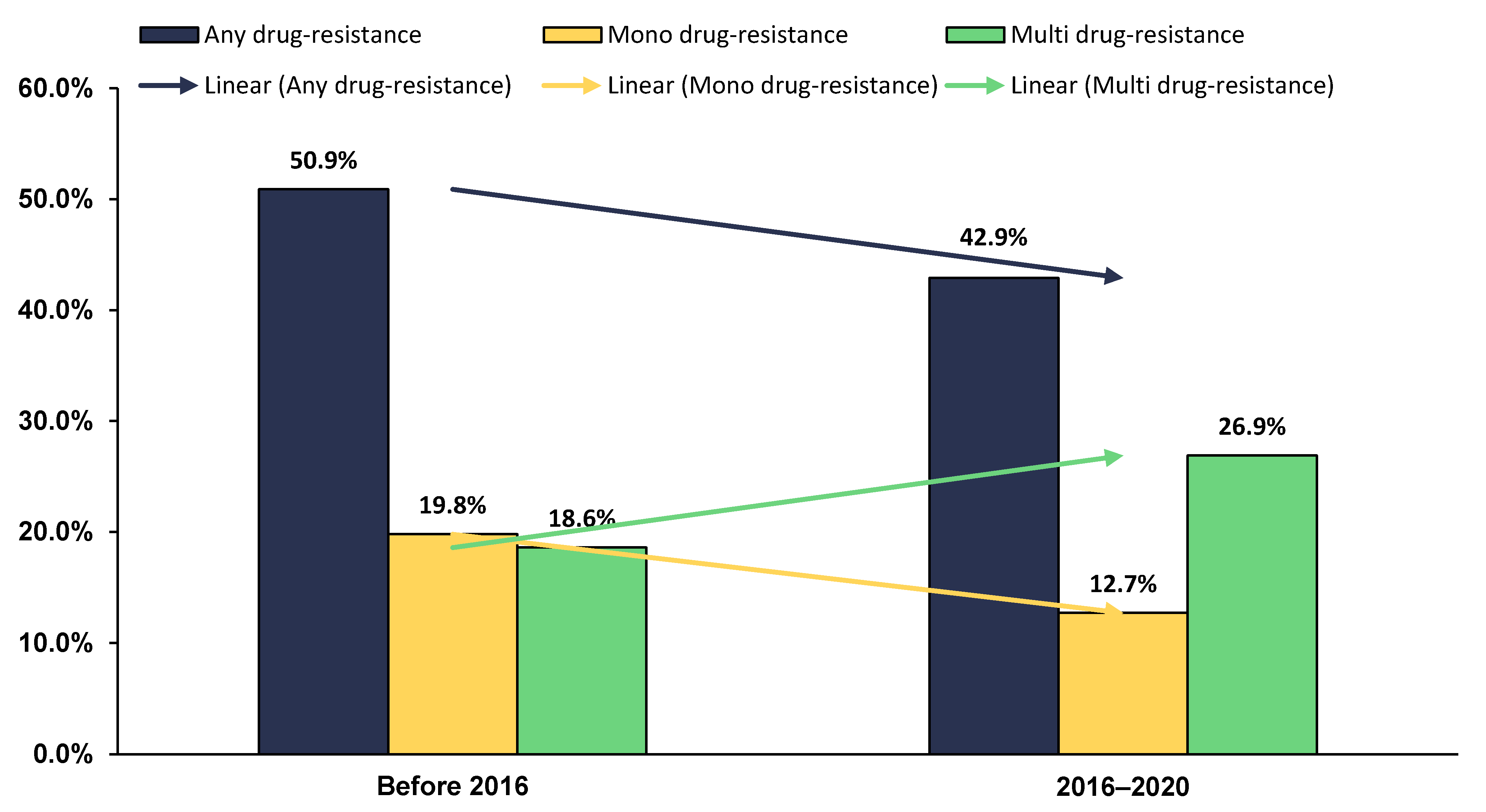
2.8. Time Trend of Anti-TB DR in Sudan
2.9. Sensitivity Analyses
3. Discussion
4. Methods
4.1. Reporting Guideline and Protocol Registration
4.2. Search Strategies
4.3. Selection Criteria
4.4. Data Management and Study Selection
4.5. Operational Definitions
4.6. Data Extrction
4.7. Quality Assessment
4.8. Data Analyses
4.9. Subgroup and Sensitivity Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf (accessed on 1 June 2021).
- Simmons, J.D.; Stein, C.M.; Seshadri, C.; Campo, M.; Alter, G.; Fortune, S.; Schurr, E.; Wallis, R.S.; Churchyard, G.; Mayanja-Kizza, H.; et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 2018, 18, 575–589. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 1 June 2021).
- Trauer, J.M.; Dodd, P.J.; Gomes, M.G.M.; Gomez, G.B.; Houben, R.M.; McBryde, E.S.; Melsew, Y.A.; Menzies, N.A.; Arinaminpathy, N.; Shrestha, S. The importance of heterogeneity to the epidemiology of tuberculosis. Clin. Infect. Dis. 2019, 69, 159–166. [Google Scholar] [CrossRef]
- Lönnroth, K.; Jaramillo, E.; Williams, B.G.; Dye, C.; Raviglione, M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc. Sci. Med. 2009, 68, 2240–2246. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Agumage, I.; Sylvanus, T.D.; Nawaila, I.J.; Ekwere, W.A.; Nasiru, M.; Okon, E.E.; Ekpenyong, A.M.; Lucero-Prisno III, D.E. Burden of tuberculosis and challenges facing its eradication in West Africa. Int. J. Infect. 2019, 6, e92250. [Google Scholar] [CrossRef]
- World Health Organization. Rapid Communication: Key Changes to Treatment of Multidrug- and Rifampicin-Resistant Tuberculosis (MDR/RR-TB). 2018. Available online: https://apps.who.int/iris/handle/10665/275383 (accessed on 1 June 2021).
- Faustini, A.; Hall, A.J.; Perucci, C.A. Risk factors for multidrug resistant tuberculosis in Europe: A systematic review. Thorax 2006, 61, 158–163. [Google Scholar] [CrossRef]
- Saravanan, M.; Niguse, S.; Abdulkader, M.; Tsegay, E.; Hailekiros, H.; Gebrekidan, A.; Araya, T.; Pugazhendhi, A. Review on emergence of drug-resistant tuberculosis (MDR & XDR-TB) and its molecular diagnosis in Ethiopia. Microb. Pathog. 2018, 117, 237–242. [Google Scholar]
- Jassal, M.; Bishai, W.R. Extensively drug-resistant tuberculosis. Lancet Infect. Dis. 2009, 9, 19–30. [Google Scholar] [CrossRef]
- Dookie, N.; Rambaran, S.; Padayatchi, N.; Mahomed, S.; Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 2018, 73, 1138–1151. [Google Scholar] [CrossRef]
- World Health Organization. Guidance for the Surveillance of Drug Resistance in Tuberculosis: Sixth Edition. 2020. Available online: https://www.who.int/publications/i/item/9789240018020 (accessed on 1 June 2021).
- Chisompola, N.K.; Streicher, E.M.; Muchemwa, C.M.K.; Warren, R.M.; Sampson, S.L. Molecular epidemiology of drug resistant Mycobacterium tuberculosis in Africa: A systematic review. BMC Infect. Dis. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Ismail, N.; Omar, S.V.; Ismail, F.; Blows, L.; Koornhof, H.; Onyebujoh, P.C.; Gardee, Y. Drug resistant tuberculosis in Africa: Current status, gaps and opportunities. Afr. J. Lab. Med. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Abdul-Aziz, A.A.; Elhassan, M.M.; Abdulsalam, S.A.; Mohammed, E.O.; Hamid, M.E. Multi-drug resistance tuberculosis (MDR-TB) in Kassala state, eastern Sudan. Trop. Dr. 2013, 43, 66–70. [Google Scholar] [CrossRef]
- Adam, M.A.M.; Ali, H.M.H.; Khalil, E.A.G. First-line drug resistance patterns of Mycobacterium tuberculosis complex isolates from re-treatment patients from Sudan. J. Tuberc. Res. 2016, 4, 98–104. [Google Scholar] [CrossRef][Green Version]
- Adam, M.A.M.; Ali, H.M.H.; Khalil, E.A.G. Initial second-line drug resistance of Mycobacterium tuberculosis isolates from Sudanese retreatment-patients. J. Clin. Tuberc. Other Mycobact. Dis. 2017, 9, 21–23. [Google Scholar] [CrossRef]
- Ali, R.H.; Ibrahim, N.Y.; Elegail, A.M.A.; Eltohami, N.A.M.; Ebraheem, R.S.M.; Ahmed, S.F.M.; Ahmed, H.H.H.; Nour, E.O.M.; Eldirdery, M.M.; Musa, H.H. Evaluation of GeneXpert MTB/RIF and line probe assay for rapid diagnosis of Mycobacterium tuberculosis in Sudanese pulmonary TB patients. Asian Pac. J. Trop. Dis. 2017, 15, 426–429. [Google Scholar] [CrossRef]
- Eldirdery, M.M.; Elrayah, I.E.; Elkarim, M.; Khalid, F.A.; Ma, A.; Elegail, S.; Ibrahim, N.Y.; Nour, E.O.M.; Ali, R.H.; Hamdan, H.M.; et al. Rapid detection of multi drug resistant-tuberculosis using Line Probe Assay (LPA) in Sudan. Eur. Acad. Res. 2016, 3, 10755–10768. [Google Scholar]
- Eldirdery, M.M.; Intisar, E.; Mona, O.; Fatima, A.; Asrar, M.; Nuha, Y. Prevalence of multidrug-resistant tuberculosis among smear positive pulmonary tuberculosis patients in eastern Sudan. Am. J. Microbiol. Res. 2017, 5, 32–36. [Google Scholar]
- Elhassan, M.M.; Saeed, S.M.; Elmekki, M.A.; Al Jarie, A.A.; Hamid, M.E. Detection of multidrug-resistant tuberculosis using PCR compared to the conventional proportional method. Bahrain Med. Bull. 2012, 34, 11–14. [Google Scholar]
- Farah Aldour, M.S.M.; Elhussein, A.R.M.; Elkhidir, I.M.; Tayeib, S.E.; Mohammed Khair, O.; Mohamed, N.S.; Enan, K.A. Detection of Drug Resistant Genes of Mycobacterium tuberculosis in Sudanese Tuberculosis Patients in Khartoum State Using Multiplex PCR. EC Microbiol. 2018, 14, 686–693. [Google Scholar]
- Hassan, S.; Musa, M.; Elsheikh, H.; Eleragi, A.; Saeed, N. Drug resistance in Mycobacterium tuberculosis isolates from northeastern Sudan. J. Adv. Med. Med. Res. 2012, 2, 424–433. [Google Scholar] [CrossRef][Green Version]
- Khalid, F.A.; Hamid, Z.A.; Mukhtar, M. Tuberculosis drug resistance isolates from pulmonary tuberculosis patients, Kassala State, Sudan. Int. J. Mycobacteriol. 2015, 4, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Nour, E.M.; Saeed, E.M.; Zaki, A.; Saeed, N.S. Drug resistance patterns of Mycobacterium tuberculosis isolates from patients with pulmonary tuberculosis in the Sudan. IOSR J. Dent. Med. Sci. 2015, 14, 17–19. [Google Scholar]
- Sabeel, S.; Salih, M.A.; Ali, M.; El-Zaki, S.-E.; Abuzeid, N.; Elgadi, Z.A.M.; Altayb, H.N.; Elegail, A.; Ibrahim, N.Y.; Elamin, B.K. Phenotypic and genotypic analysis of multidrug-resistant Mycobacterium tuberculosis isolates from Sudanese patients. Tuberc. Res. Treat. 2017, 2017, 1–6. [Google Scholar]
- Sharaf-Eldin, G.; Saeed, N.; Hamid, M.; Jordaan, A.; Van der Spuy, G.; Warren, R.; Van Helden, P.; Victor, T. Molecular analysis of clinical isolates of Mycobacterium tuberculosis collected from patients with persistent disease in the Khartoum region of Sudan. J. Infect. 2002, 44, 244–251. [Google Scholar] [CrossRef]
- Eldin, G.S.S.; Fadl-Elmula, I.; Ali, M.S.; Ali, A.B.; Salih, A.L.G.; Mallard, K.; Bottomley, C.; McNerney, R. Tuberculosis in Sudan: A study of Mycobacterium tuberculosis strain genotype and susceptibility to anti-tuberculosis drugs. BMC Infect. Dis. 2011, 11, 1–8. [Google Scholar]
- Shuaib, Y.A.; Khalil, E.A.; Wieler, L.H.; Schaible, U.E.; Bakheit, M.A.; Mohamed-Noor, S.E.; Abdalla, M.A.; Kerubo, G.; Andres, S.; Hillemann, D. Mycobacterium tuberculosis Complex Lineage 3 as Causative Agent of Pulmonary Tuberculosis, Eastern Sudan. Emerg. Infect. Dis. 2020, 26, 427–436. [Google Scholar] [CrossRef]
- Zaki, A.; Ibrahim, N.; Abdelsalam, A.; Osman, M. A study on Prevalence of Drug Resistance in Drug Default Pulmonary Tuberculosis. Sudan J. Med. Sci. 2011, 6, 97–101. [Google Scholar] [CrossRef]
- Kundu, S.; Marzan, M.; Gan, S.H.; Islam, M.A. Prevalence of Antibiotic-Resistant Pulmonary Tuberculosis in Bangladesh: A Systematic Review and Meta-Analysis. Antibiotics 2020, 9, 710. [Google Scholar] [CrossRef]
- Goyal, V.; Kadam, V.; Narang, P.; Singh, V. Prevalence of drug-resistant pulmonary tuberculosis in India: Systematic review and meta-analysis. BMC Public Health 2017, 17, 817. [Google Scholar] [CrossRef]
- Lv, X.-T.; Lu, X.-W.; Shi, X.-Y.; Zhou, L. Prevalence and risk factors of multi-drug resistant tuberculosis in Dalian, China. J. Int. Med. Res. 2017, 45, 1779–1786. [Google Scholar] [CrossRef]
- Saifullah, A.; Mallhi, T.H.; Khan, Y.H.; Iqbal, M.S.; Alotaibi, N.H.; Alzarea, A.I.; Rasheed, M. Evaluation of risk factors associated with the development of MDR-and XDR-TB in a tertiary care hospital: A retrospective cohort study. PeerJ 2021, 9, e10826. [Google Scholar] [CrossRef]
- Balaji, V.; Daley, P.; Anand, A.A.; Sudarsanam, T.; Michael, J.S.; Sahni, R.D.; Chordia, P.; George, I.A.; Thomas, K.; Ganesh, A. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS ONE 2010, 5, e9527. [Google Scholar] [CrossRef] [PubMed]
- Workicho, A.; Kassahun, W.; Alemseged, F. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: A case-control study. Infect. Drug Resist. 2017, 10, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.J.; Dabiri, H.; Darban-Sarokhalil, D.; Rezadehbashi, M.; Zamani, S. Prevalence of drug-resistant tuberculosis in Iran: Systematic review and meta-analysis. Am. J. Infect. Control 2014, 42, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Lohiya, A.; Abdulkader, R.S.; Rath, R.S.; Jacob, O.; Chinnakali, P.; Goel, A.D.; Agrawal, S. Prevalence and patterns of drug resistant pulmonary tuberculosis in India—A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2020, 22, 308–316. [Google Scholar] [CrossRef]
- Onyedum, C.C.; Alobu, I.; Ukwaja, K.N. Prevalence of drug-resistant tuberculosis in Nigeria: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0180996. [Google Scholar] [CrossRef]
- Duan, Q.; Chen, Z.; Chen, C.; Zhang, Z.; Lu, Z.; Yang, Y.; Zhang, L. The prevalence of drug-resistant tuberculosis in mainland China: An updated systematic review and meta-analysis. PLoS ONE 2016, 11, e0148041. [Google Scholar] [CrossRef]
- Sudan National TB Manegement Guideline. 2018. Available online: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/2019/07/Sudan-National-TB-management-Guideline-March.2019-1.pdf (accessed on 20 July 2021).
- Yuen, C.M.; Tolman, A.W.; Cohen, T.; Parr, J.B.; Keshavjee, S.; Becerra, M.C. Isoniazid-resistant tuberculosis in children: A systematic review. Pediatr. Infect. Dis. J. 2013, 32, e217. [Google Scholar] [CrossRef]
- Sinha, P.; Srivastava, G.; Gupta, A.; Anupurba, S. Association of risk factors and drug resistance pattern in tuberculosis patients in North India. J. Glob. Infect. Dis. 2017, 9, 139–145. [Google Scholar]
- Umubyeyi, A.N.; Vandebriel, G.; Gasana, M.; Basinga, P.; Zawadi, J.P.; Gatabazi, J.; Pauwels, P.; Nzabintwali, F.; Nyiramasarabwe, L.; Fissette, K.; et al. Results of a national survey on drug resistance among pulmonary tuberculosis patients in Rwanda. Int. J. Tuberc. Lung Dis. 2007, 11, 189–194. [Google Scholar]
- Girum, T.; Muktar, E.; Lentiro, K.; Wondiye, H.; Shewangizaw, M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: A systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop. Dis. Travel Med. Vaccines 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Eshetie, S.; Gizachew, M.; Dagnew, M.; Kumera, G.; Woldie, H.; Ambaw, F.; Tessema, B.; Moges, F. Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 219. [Google Scholar] [CrossRef]
- Lukoye, D.; Ssengooba, W.; Musisi, K.; Kasule, G.W.; Cobelens, F.G.; Joloba, M.; Gomez, G.B. Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: A systematic review and meta-analysis. BMC Public Health 2015, 15, 291. [Google Scholar] [CrossRef]
- Ding, P.; Li, X.; Jia, Z.; Lu, Z. Multidrug-resistant tuberculosis (MDR-TB) disease burden in China: A systematic review and spatio-temporal analysis. BMC Infect. Dis. 2017, 17, 57. [Google Scholar] [CrossRef]
- Liang, L.; Wu, Q.; Gao, L.; Hao, Y.; Liu, C.; Xie, Y.; Sun, H.; Yan, X.; Li, F.; Li, H.; et al. Factors contributing to the high prevalence of multidrug-resistant tuberculosis: A study from China. Thorax 2012, 67, 632–638. [Google Scholar] [CrossRef]
- Sagonda, T.; Mupfumi, L.; Manzou, R.; Makamure, B.; Tshabalala, M.; Gwanzura, L.; Mason, P.; Mutetwa, R. Prevalence of extensively drug resistant tuberculosis among archived multidrug resistant tuberculosis isolates in Zimbabwe. Tuberc. Res. Treat. 2014, 2014, 349141. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, L.; He, Y.; Pang, Y.; Lu, N.; Liu, J.; Shen, J.; Zhu, D.; Feng, X.; Wang, Y. Prevalence and molecular characterization of second-line drugs resistance among multidrug-resistant Mycobacterium tuberculosis isolates in Southwest of China. Biomed Res. Int. 2017, 2017, 4563826. [Google Scholar] [CrossRef]
- Jugheli, L.; Rigouts, L.; Shamputa, I.C.; Bram de Rijk, W.; Portaels, F. High levels of resistance to second-line anti-tuberculosis drugs among prisoners with pulmonary tuberculosis in Georgia. Int. J. Tuberc. Lung Dis. 2008, 12, 561–566. [Google Scholar]
- Javaid, A.; Hasan, R.; Zafar, A.; Chaudry, M.; Qayyum, S.; Qadeer, E.; Shaheen, Z.; Agha, N.; Rizvi, N.; Afridi, M. Pattern of first-and second-line drug resistance among pulmonary tuberculosis retreatment cases in Pakistan. Int. J. Tuberc. Lung Dis. 2017, 21, 303–308. [Google Scholar] [CrossRef]
- Koch, A.; Cox, H.; Mizrahi, V. Drug-resistant tuberculosis: Challenges and opportunities for diagnosis and treatment. Curr. Opin. Pharmacol. 2018, 42, 7–15. [Google Scholar] [CrossRef]
- Ali, M.H.; Alrasheedy, A.A.; Hassali, M.A.; Kibuule, D.; Godman, B. Predictors of multidrug-resistant tuberculosis (MDR-TB) in Sudan. Antibiotics 2019, 8, 90. [Google Scholar] [CrossRef]
- Fox, G.J.; Schaaf, H.; Mandalakas, A.; Chiappini, E.; Zumla, A.; Marais, B. Preventing the spread of multidrug-resistant tuberculosis and protecting contacts of infectious cases. Clin. Microbiol. Infect. 2017, 23, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Loddenkemper, R.; Sagebiel, D.; Brendel, A. Strategies against multidrug-resistant tuberculosis. Eur. Respir. J. 2002, 20, 66–77. [Google Scholar] [CrossRef]
- Mdivani, N.; Zangaladze, E.; Volkova, N.; Kourbatova, E.; Jibuti, T.; Shubladze, N.; Kutateladze, T.; Khechinashvili, G.; del Rio, C.; Salakaia, A. High prevalence of multidrug-resistant tuberculosis in Georgia. Int. J. Infect. Dis. 2008, 12, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Chesov, D.; Heyckendorf, J.; Leung, C.C.; Udwadia, Z.; Dheda, K. Drug-resistant tuberculosis: An update on disease burden, diagnosis and treatment. Respirology 2018, 23, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Udwadia, Z.; Furin, J. Quality of drug-resistant tuberculosis care: Gaps and solutions. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Mvusi, L.; Nanoo, A.; Dreyer, A.; Omar, S.V.; Babatunde, S.; Molebatsi, T.; Van der Walt, M.; Adelekan, A.; Deyde, V. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: A national and sub-national cross-sectional survey. Lancet Infect. Dis. 2018, 18, 779–787. [Google Scholar] [CrossRef]
- Chia, Y.C.; Islam, M.A.; Hider, P.; Woon, P.Y.; Johan, M.F.; Hassan, R.; Ramli, M. The prevalence of TET2 gene mutations in patients with BCR-ABL-negative myeloproliferative neoplasms (MPN): A systematic review and meta-analysis. Cancers 2021, 13, 3078. [Google Scholar] [CrossRef]
- Islam, M.A.; Kundu, S.; Alam, S.S.; Hossan, T.; Kamal, M.A.; Hassan, R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of 17515 patients. PLoS ONE 2021, 16, e0249788. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Ang, J.-Y.; Islam, M.A.; Chan, H.-K.; Cheah, W.-K.; Gan, S.H. Prevalence of drug-related problems and complementary and alternative medicine use in Malaysia: A systematic review and meta-analysis of 37,249 older adults. Pharmaceuticals 2021, 14, 187. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 1–36. [Google Scholar]
- World Health Organization. Definitions and Reporting Framework for Tuberculosis–2013 Revision: Updated December 2014 and January 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/79199/?sequence=1 (accessed on 20 July 2021).
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Hossan, T.; Kamal, M.A.; Cavestro, C. Prevalence of Headache in Patients With Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of 14,275 Patients. Front. Neurol. 2020, 11, 562634. [Google Scholar] [CrossRef]
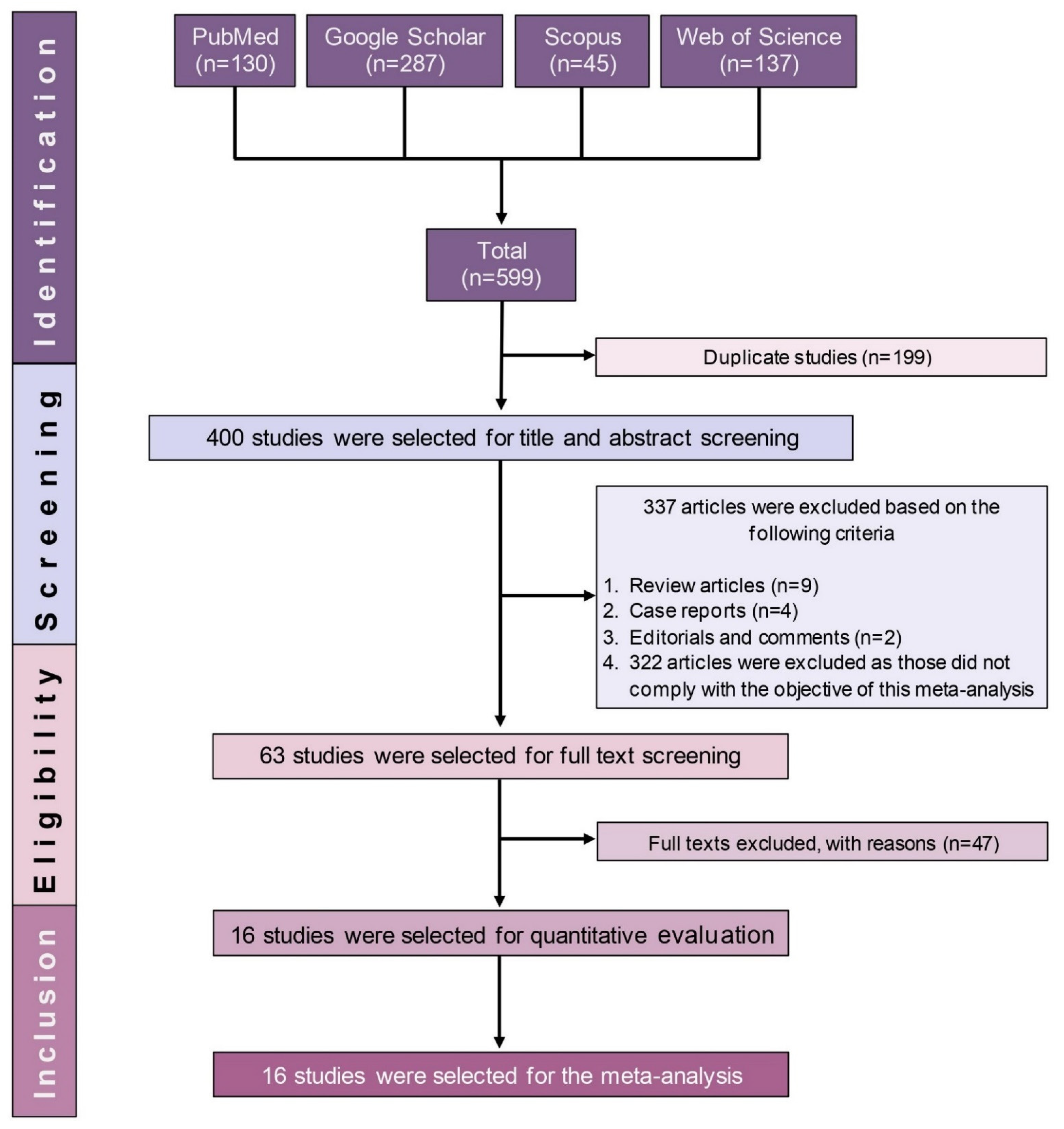
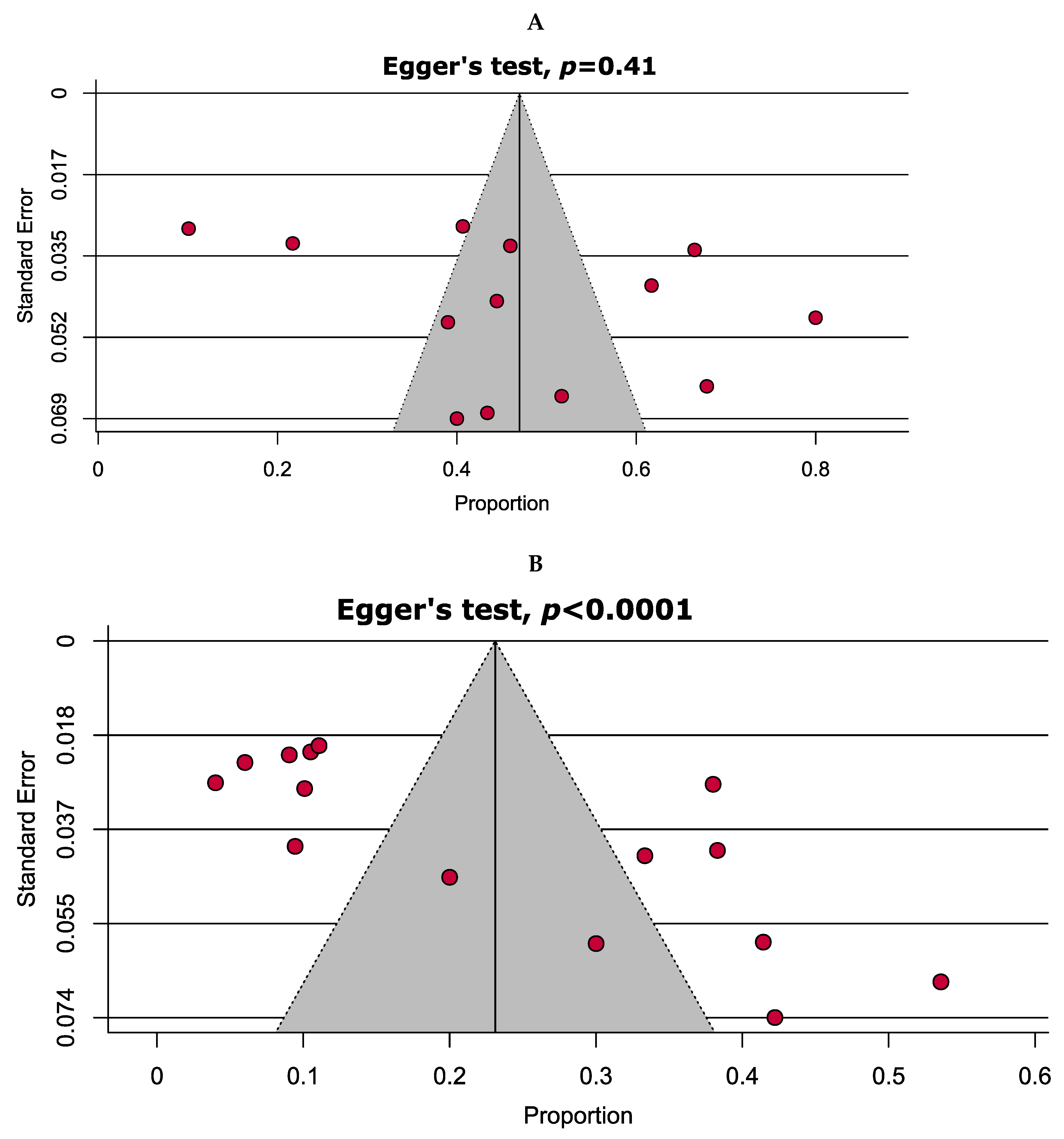
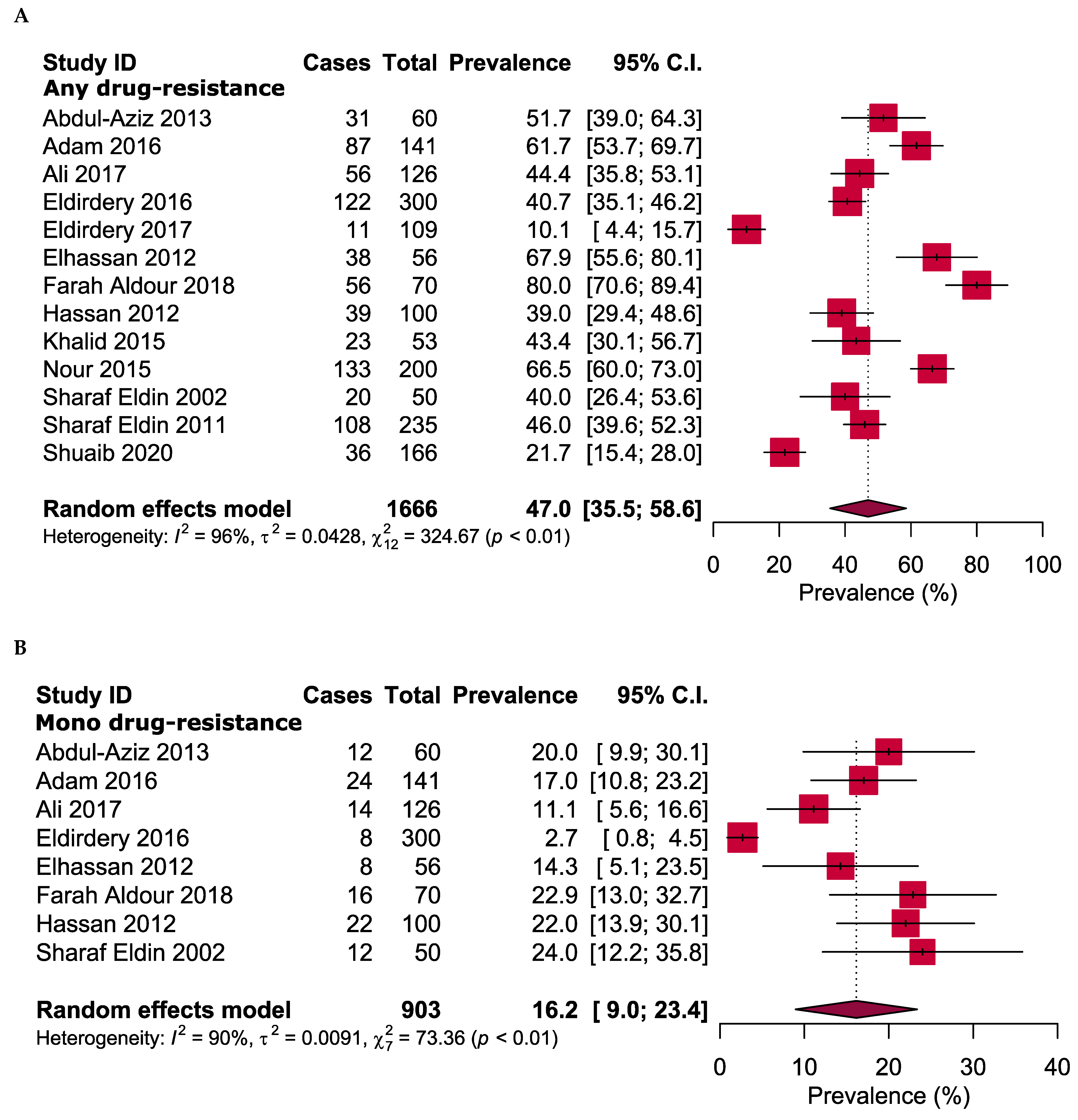
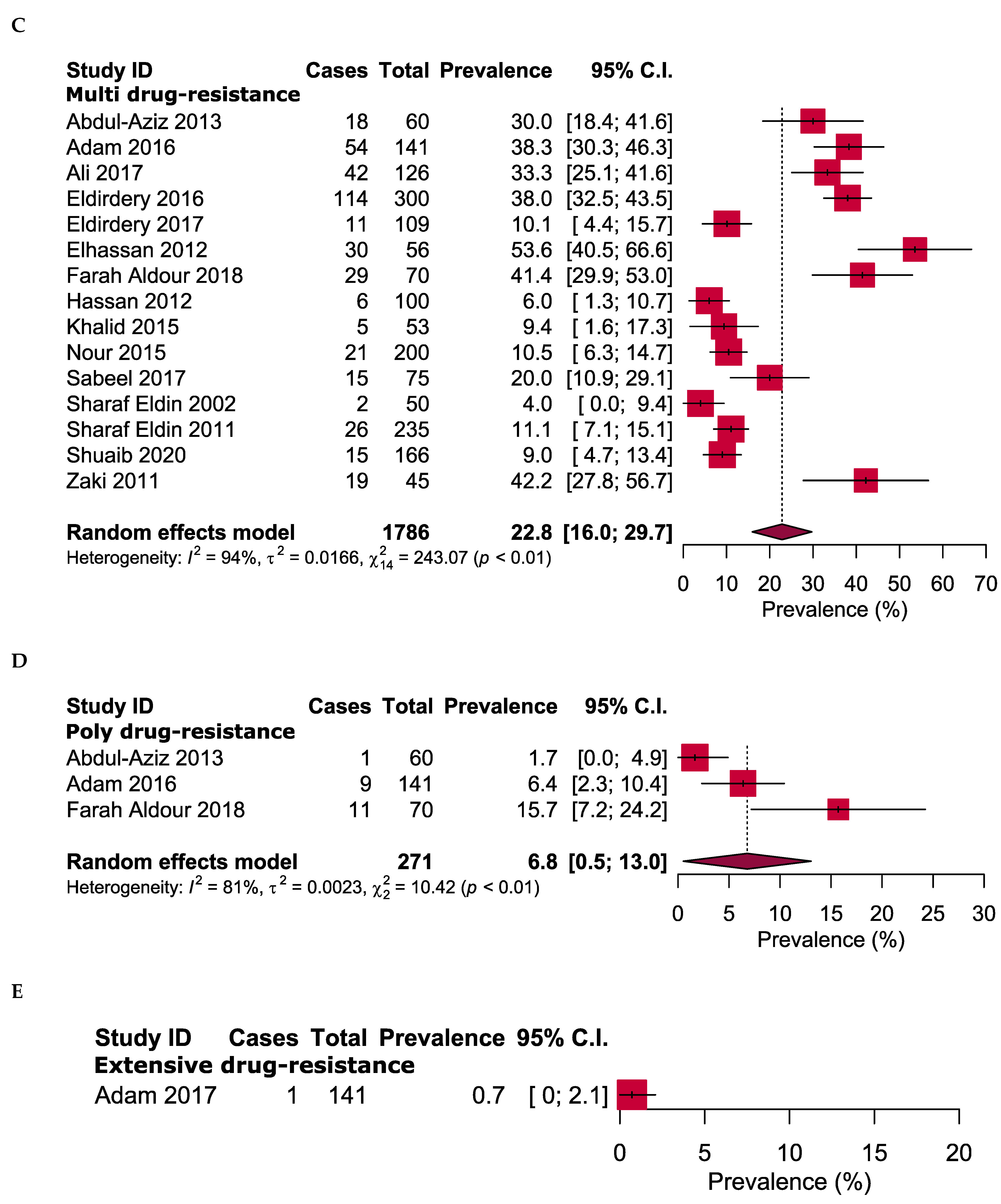
| No. | Study ID [References] | Enrolment Time | Study Area | Sample Size | Gender | Age in Years (Range) | TB-Positive Cases | Drug Susceptibility Tests | Tested Antibiotic | |
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | |||||||||
| 1 | Abdul-Aziz 2013 [15] | 2011 | Kassala | 90 | 54 | 36 | 14–65 | 60 | LJ proportion method and SSCP | STM, RIF, INH, EMB and ETH |
| 2 | Adam 2016 [16] | 2009–2010 | Khartoum | 239 | 175 | 64 | 13–75 | 141 | LJ proportion method | RIF, INH, EMB and STM |
| 3 | Adam 2017 [17] | 2009–2010 | Khartoum | 239 | 175 | 64 | 13–75 | 141 | LJ proportion method and by Hain GenoType MTBDRsl Assay | RIF, INH, STM, EMB, KAN, CAP, OFX and AMK |
| 4 | Ali 2017 [18] | 2011–2015 | Khartoum | 126 | 85 | 41 | 16–30 | 126 | Conventional DST, LPA and GeneXpert assay | RIF and INH |
| 5 | Eldirdery 2016 [19] | NR | Khartoum | 300 | NR | NR | NR | 300 | LJ Proportion method and LPA | INH, RIF, STM and EMB |
| 6 | Eldirdery 2017 [20] | 2011–2012 | Kassala and Geddarif | 109 | 64 | 45 | 13–80 | 109 | LJ Proportion method and LPA | INH, RIF, STM and EMB |
| 7 | Elhassan 2012 [21] | NR | Khartoum | 130 | 82 | 48 | 12–67 | 56 | LJ proportion method and PCR | INH and RIF |
| 8 | Farah Aldour 2018 [22] | 2015 | Omdurman | 70 | NR | NR | 10–80 | 70 | Multiplex PCR | RIF, INH and PZA |
| 9 | Hassan 2012 [23] | 2006–2007 | Port Sudan | 100 | 68 | 32 | ≥18 | 100 | LJ proportion method and MAS-PCR | RIF, INH, STM, EMB and PZA |
| 10 | Khalid 2015 [24] | 2007–2009 | Kassala | 53 | NR | NR | NR | 53 | LJ proportion method | INH, RIF, STM and EMB |
| 11 | Nour 2015 [25] | NR | Khartoum | NR | NR | NR | 5–70 | 200 | LJ proportion method | INH, RIF, STM and EMB |
| 12 | Sabeel 2017 [26] | NR | Khartoum | 100 | NR | NR | NR | 75 | LJ proportion and PCR | INH, RIF, STM and EMB |
| 13 | Sharaf Eldin 2002 [27] | 1998–1999 | Khartoum | 105 | NR | NR | NR | 50 | PCR-based dot-blot method | INH, RIF, STM, PZA and EMB |
| 14 | Sharaf Eldin 2011 [28] | 2005 | Khartoum and Port Sudan | 235 | 175 | 60 | 26–45 | 235 | LJ proportion method | INH, RIF, STM and EMB |
| 15 | Shuaib 2020 [29] | 2014–2016 | Kassala, Port Sudan, and El-Gadarif | 383 | 245 | 138 | 25–45 | 166 | Phenotypic and genotypic DST | RIF, INH, EMB, STM and PZA |
| 16 | Zaki 2011 [30] | 2007–2007 | Khartoum | 111 | 83 | 28 | NR | 45 | LJ proportion method | RIF, INH, STM and EMB |
| Drug-Resistance Patterns | Antibiotics | Number of Analysed Studies | Total Number of Tuberculosis Patients | Prevalence of Antibiotic Resistance [95% CIs] (%) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 | p-Value | ||||||
| Any DR | First-line drugs | Streptomycin | 10 | 1125 | 31.7 [24.6–38.8] | 86% | <0.0001 |
| Isoniazid | 13 | 1624 | 32.3 [23.6–41.1] | 94% | <0.0001 | ||
| Rifampicin | 14 | 1677 | 29.2 [21.4–36.9] | 94% | <0.0001 | ||
| Ethambutol | 9 | 1072 | 15.7 [8.0–23.4] | 95% | <0.0001 | ||
| Pyrazinamide | 3 | 336 | 10.5 [2.8–18.1] | 97% | <0.0001 | ||
| Second-line drugs | Kanamycin | 1 | 141 | 0.7 [0.0–2.1] | NA | NA | |
| Ofloxacin | 1 | 141 | 2.1 [0.0–4.5] | NA | NA | ||
| Mono DR | First-line drugs | Streptomycin | 4 | 351 | 14.0 [9.9–18.1] | 20% | 0.29 |
| Isoniazid | 7 | 833 | 2.8 [1.2–4.5] | 48% | 0.07 | ||
| Rifampicin | 7 | 833 | 0.7 [0.0–1.5] | 16% | 0.38 | ||
| Ethambutol | 3 | 301 | 2.1 [0.5–3.7] | 0% | 0.40 | ||
| Drug-Resistance Patterns | Antibiotics | Number of Analysed Studies | Total Number of Tuberculosis Patients | Prevalence of Antibiotic Resistance [95% CIs] (%) | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| I2 | p-Value | |||||||
| Newly diagnosed tuberculosis patients | ||||||||
| Any DR | First-line drugs | Streptomycin | 4 | 321 | 22.1 [10.7–33.6] | 80% | 0.001 | |
| Isoniazid | 3 | 310 | 15.7 [7.3–24.1] | 74% | 0.02 | |||
| Rifampicin | 4 | 321 | 14.8 [7.5–22.1] | 65% | 0.03 | |||
| Ethambutol | 3 | 310 | 7.9 [3.8–12.1] | 38% | 0.19 | |||
| Pyrazinamide | 1 | 100 | 1.0 [0.0–3.0] | NA | NA | |||
| Mono DR | First-line drugs | Streptomycin | 2 | 146 | 13.5 [4.4–22.6] | 63% | 0.10 | |
| Isoniazid | 2 | 146 | 1.5 [0.0–3.8] | 11% | 0.29 | |||
| Rifampicin | 2 | 146 | 0.2 [0.0–1.5] | 0% | 0.45 | |||
| Ethambutol | 2 | 146 | 3.3 [0.4–6.2] | 0% | 0.69 | |||
| Previously treated tuberculosis patients | ||||||||
| Any DR | First-line drugs | Streptomycin | 3 | 226 | 51.1 [26.1–76.1] | 92% | <0.0001 | |
| Isoniazid | 4 | 296 | 42.8 [37.2–48.4] | 0% | 0.54 | |||
| Rifampicin | 4 | 296 | 39.3 [33.4–45.2] | 8% | 0.35 | |||
| Ethambutol | 3 | 226 | 39.4 [13.0–65.8] | 93% | <0.0001 | |||
| Pyrazinamide | 1 | 70 | 47.1 [35.4–58.8] | NA | NA | |||
| Second-line drugs | Kanamycin | 1 | 141 | 0.7 [0.0–2.1] | NA | NA | ||
| Ofloxacin | 1 | 141 | 2.1 [0.0–4.5] | NA | NA | |||
| Mono DR | First-line drugs | Streptomycin | 2 | 155 | 12.2 [7.1–17.4] | 0% | 0.81 | |
| Isoniazid | 2 | 155 | 2.0 [0.0–4.3] | 0% | 0.80 | |||
| Rifampicin | 2 | 155 | 1.4 [0.0–3.4] | 0% | 0.68 | |||
| Ethambutol | 2 | 155 | 1.5 [0.0–3.5] | 0% | 0.41 | |||
| Strategies of Sensitivity Analyses | Prevalence of Antibiotic Resistance [95% CIs] (%) | Difference of Pooled Prevalence Compared to the Main Result | Number of Studies Analysed | Total Number of TB Patients | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 | p-Value | |||||
| Any drug resistance | ||||||
| Excluding small studies (n < 100) | 41.2 [27.3–55.1] | 6.3% lower | 8 | 1377 | 97% | <0.0001 |
| Excluding low- and moderate-quality studies | 44.9 [31.6–58.1] | 2.6% lower | 8 | 1127 | 96% | <0.0001 |
| Using a fixed-effects model | 42.4 [40.2–44.6] | 5.1% lower | 13 | 1666 | 96% | <0.0001 |
| Mono drug resistance | ||||||
| Excluding small studies (n < 100) | 12.7 [3.5–21.9] | 4.8% lower | 4 | 667 | 93% | <0.0001 |
| Excluding low- and moderate-quality studies | 14.0 [6.4–21.6] | 3.5% lower | 6 | 783 | 91% | <0.0001 |
| Using a fixed-effects model | 6.6 [5.0–8.1] | 10.9% lower | 8 | 903 | 90% | <0.0001 |
| Multidrug resistance | ||||||
| Excluding small studies (n < 100) | 19.2 [10.7–27.7] | 3.0% lower | 8 | 1377 | 95% | <0.0001 |
| Excluding low- and moderate-quality studies | 27.6 [17.9–37.2] | 4.8% higher | 10 | 1247 | 95% | <0.0001 |
| Using a fixed-effects model | 15.7 [14.1–17.3] | 7.1% lower | 15 | 1786 | 94% | <0.0001 |
| Poly drug resistance | ||||||
| Excluding small studies (n < 100) | 6.4 [2.3–10.4] | 0.4% lower | 1 | 141 | NA | NA |
| Excluding low- and moderate-quality studies | 3.9 [0.0–8.5] | 2.9% lower | 2 | 201 | 69% | 0.70 |
| Using a fixed-effects model | 4.5 [2.1–6.9] | 2.3% lower | 3 | 271 | 81% | 0.005 |
| Extensive drug resistance | ||||||
| Excluding small studies (n < 100) | 0.7 [0.0–2.1] | No change | 1 | 141 | NA | NA |
| Excluding low- and moderate-quality studies | 0.7 [0.0–2.1] | No change | 1 | 141 | NA | NA |
| Using a fixed-effects model | 0.7 [0.0–2.1] | No change | 1 | 141 | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajissa, K.; Marzan, M.; Idriss, M.I.; Islam, M.A. Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 932. https://doi.org/10.3390/antibiotics10080932
Hajissa K, Marzan M, Idriss MI, Islam MA. Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis. Antibiotics. 2021; 10(8):932. https://doi.org/10.3390/antibiotics10080932
Chicago/Turabian StyleHajissa, Khalid, Mahfuza Marzan, Mubarak Ibrahim Idriss, and Md Asiful Islam. 2021. "Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis" Antibiotics 10, no. 8: 932. https://doi.org/10.3390/antibiotics10080932
APA StyleHajissa, K., Marzan, M., Idriss, M. I., & Islam, M. A. (2021). Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis. Antibiotics, 10(8), 932. https://doi.org/10.3390/antibiotics10080932







