Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study
Abstract
1. Introduction
2. Results
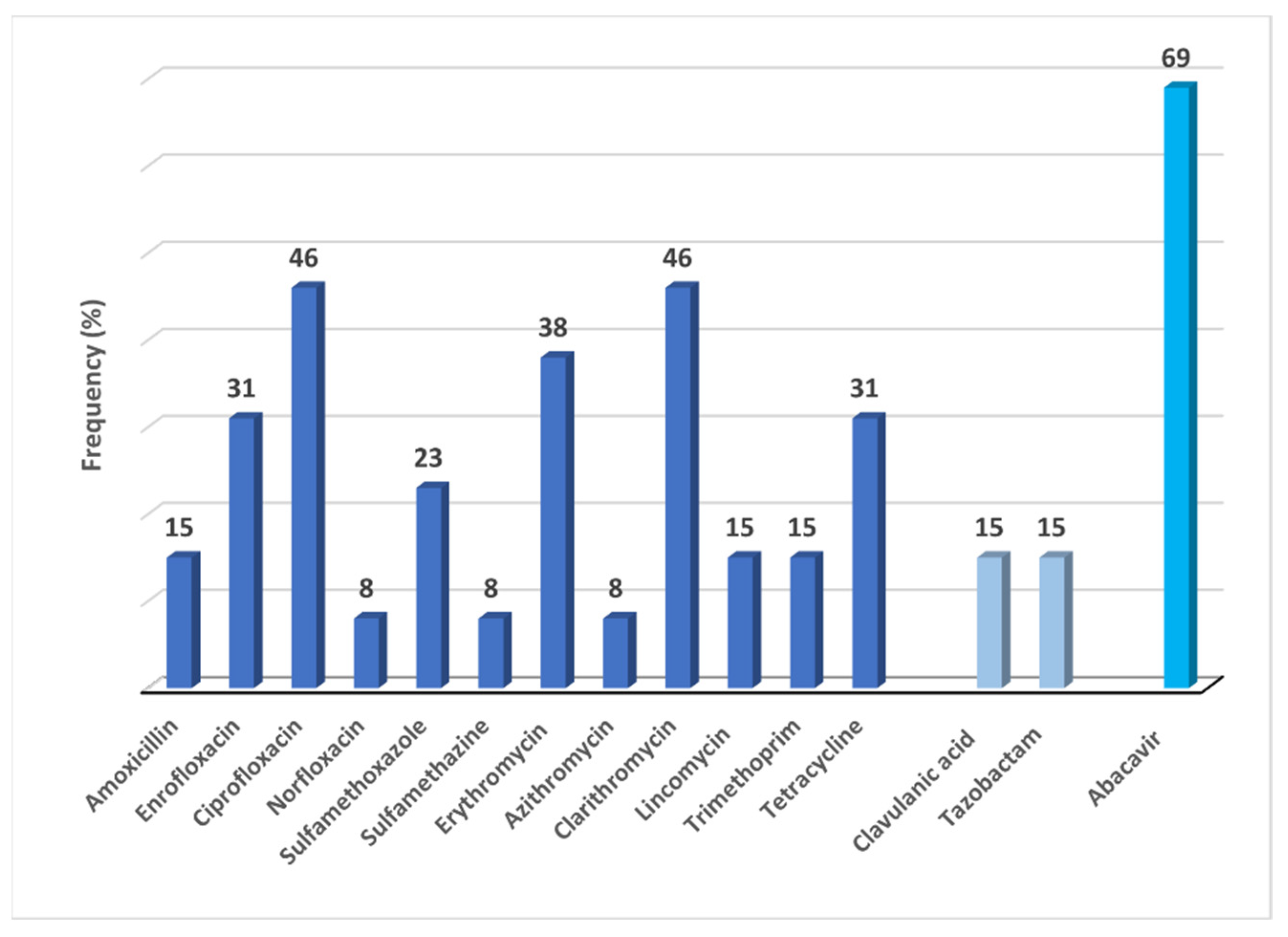
2.1. Frequency of Detections: Antibiotics/Enzyme-Inhibitors and Abacavir in Surface-Groundwater
2.1.1. Antibiotics/Enzyme-Inhibitors and Abacavir in Surface-Water
2.1.2. Antibiotics/Enzyme-Inhibitors and Abacavir in Groundwater
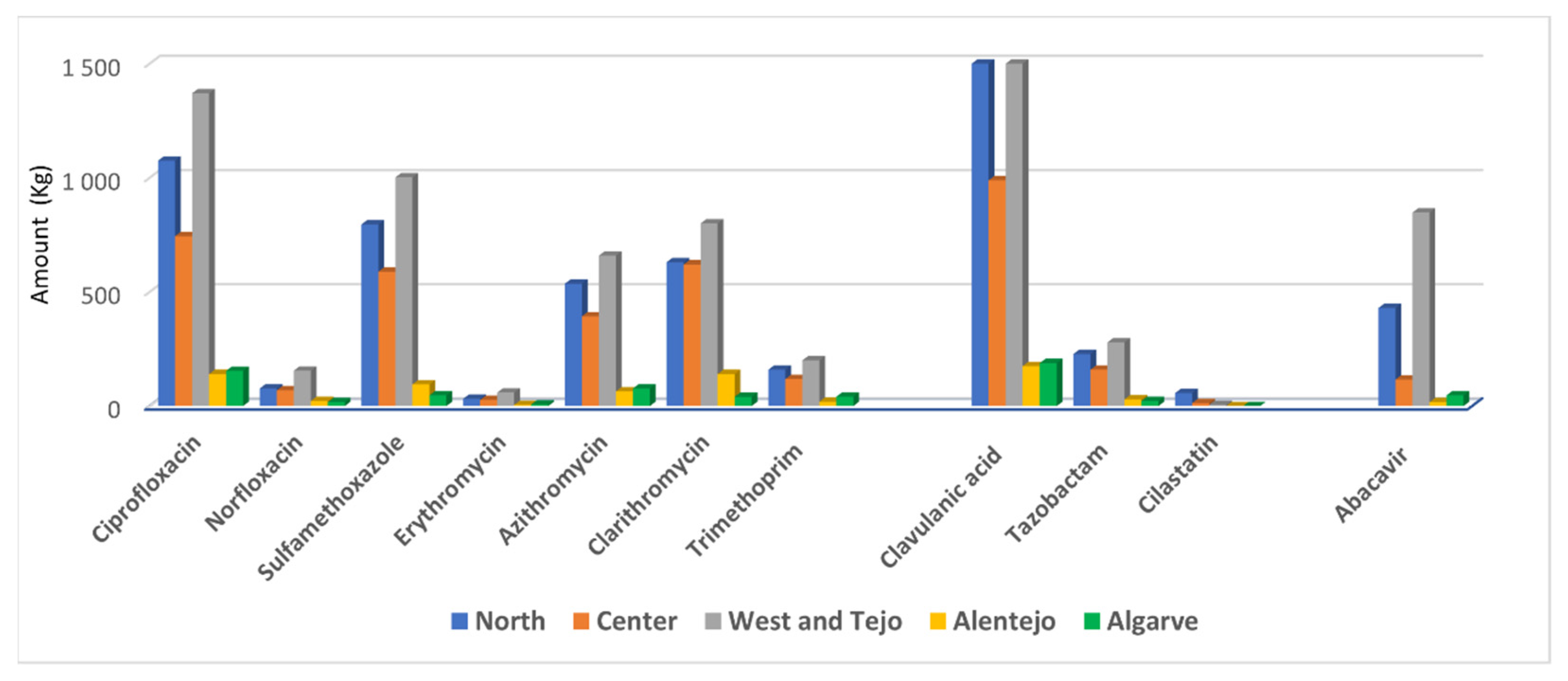
2.2. Consumption of Antibiotics/Enzyme-Inhibitors and Abacavir
2.3. Physicochemical Properties and Key Pharmacokinetic Features of Detected Pharmaceuticals
3. Discussion
The Need for the Ecopharmacovigilance
4. Materials and Methods
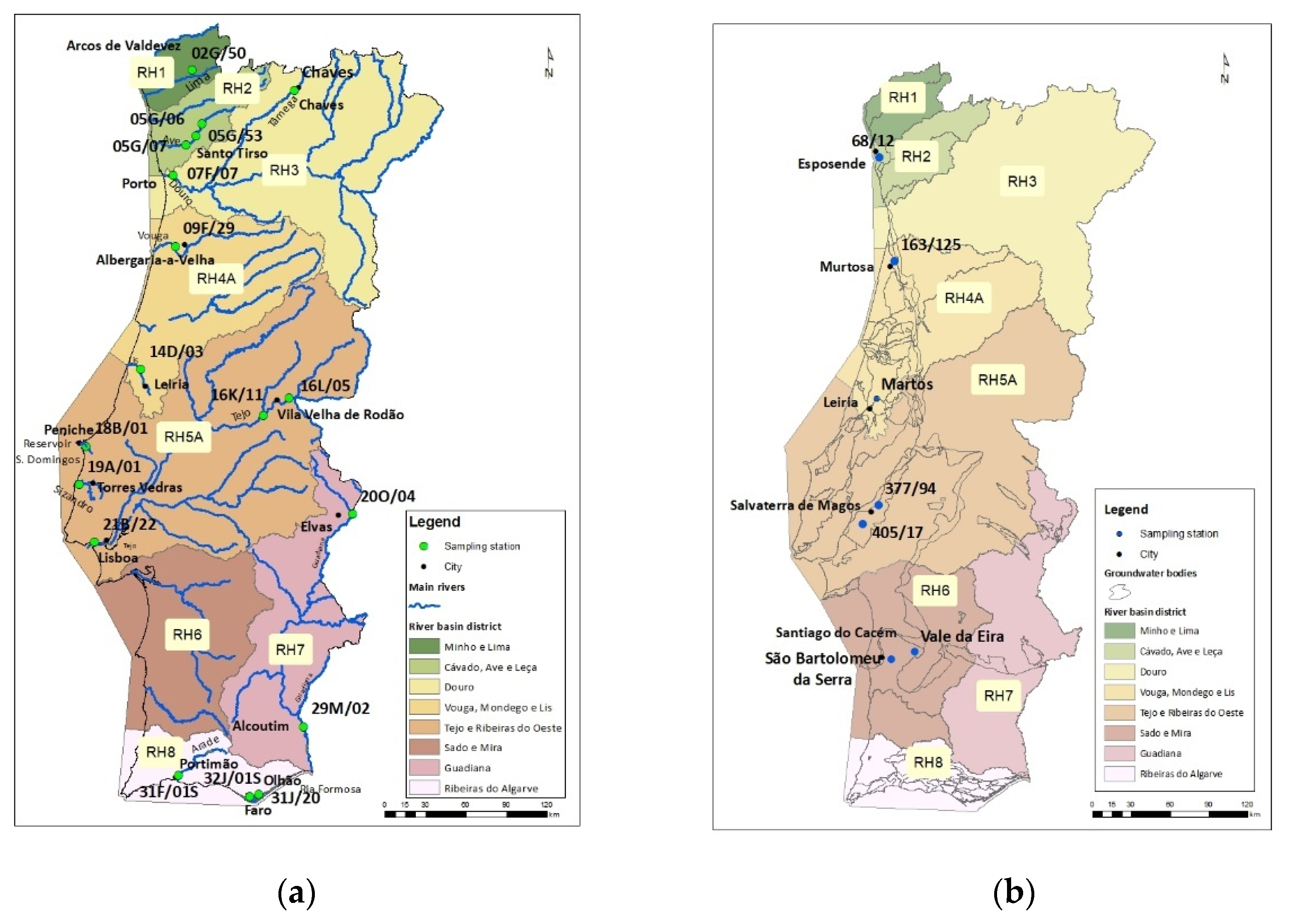
4.1. Study Area/Sampling Stations and Characterisation
4.2. Materials and Chemicals
4.2.1. Passive Sampler Field Deployment
4.2.2. Qualitative Analysis Method Used for the Characterisation of Antibiotics in Surface-Groundwater
Sample Extraction
Ultra-Performance Liquid Chromatography-High Resolution Mass Spectrometry (UHPLC-QqTOF-MS) Analysis
4.2.3. Data Analysis and Validation
4.2.4. Chemicals and Reagents
4.3. Consumption of Detected Antibiotics
4.4. Physicochemical Properties and Key Pharmacokinetic Features of Detected Antibiotics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Cunningham, V.L. Special Characteristics of Pharmaceuticals Related to Environmental Fate. Pharm. Environ. 2004, 13–24. [Google Scholar] [CrossRef]
- Jjemba, P.K. Excretion and Ecotoxicity of Pharmaceutical and Personal Care Products in the Environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef]
- Ding, C.; He, J. Effect of Antibiotics in the Environment on Microbial Populations. Appl. Microbiol. Biotechnol. 2010, 87, 925–941. [Google Scholar] [CrossRef]
- Manaia, C.M.; Rocha, J.; Scaccia, N.; Marano, R.; Radu, E.; Biancullo, F.; Cerqueira, F.; Fortunato, G.; Iakovides, I.C.; Zammit, I.; et al. Antibiotic Resistance in Wastewater Treatment Plants: Tackling the Black Box. Environ. Int. 2018, 115, 312–324. [Google Scholar] [CrossRef]
- Manaia, C.M.; Graham, D.; Topp, E.; Martinez, J.L.; Collignon, P.; Gaze, W.H. Antibiotic Resistance in the Environment: Expert Perspectives. Handb. Environ. Chem. 2020, 91, 1–18. [Google Scholar] [CrossRef]
- Harman, C.; Allan, I.J.; Vermeirssen, E.L.M. Calibration and Use of the Polar Organic Chemical Integrative Sampler-a Critical Review. Environ. Toxicol. Chem. 2012, 31, 2724–2738. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, and the European Economic and Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment; European Commision: Brussels, Blgium, 2019; Volume 128, p. 13. [Google Scholar]
- EC DIRECTIVE 2013/39/EU of the European Parliament and of the Council, Brussels, Belgium. 2013. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 3 May 2021).
- EC DIRECTIVE 2008/105/EC of the European Parliament and of the Council, Brussels, Belgium. 2008. Available online: https://www.ecolex.org/details/legislation/directive-2008105ec-of-the-european-parliament-and-of-the-council-on-environmental-quality-standards-in-the-field-of-water-policy-amending-and-subsequently-repealing-council-directives-82176eec-83513eec-84156eec-84491eec-86280eec-and-amending-directive-200060ec-of-the-european-parliament-and-of-the-council-lex-faoc084568/ (accessed on 3 May 2021).
- Loos, R.; Marinov, D.; Sanseverino, I.; Napierska, D.; Lettieri, T. Review of the 1st Watch List under the Water Framework Directive and Recommendations for the 2nd Watch List; EUR 29173 EN; Publications Office of the European Union: Luxembourg, 2018; p. JRC111198. [Google Scholar] [CrossRef]
- European Commission. Guidance Document No. 19 Guidance on Surface Water Chemical Monitoring under the Water Framework Directive, Brussels, Belgium. 2009. Available online: https://op.europa.eu/en/publication-detail/-/publication/91d313f0-2cc7-4874-b101-a7dba97401b0 (accessed on 3 May 2021).
- Garcia-Rodríguez, A.; Fontàs, C.; Matamoros, V.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Development of a Polymer Inclusion Membrane-Based Passive Sampler for Monitoring of Sulfamethoxazole in Natural Waters. Minimising the Effect of the Flow Pattern of the Aquatic System. Microchem. J. 2016, 124, 175–180. [Google Scholar] [CrossRef]
- EMA. A Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use, EMEA/CHMP/SWP/4447/00 Rev. 1 2018, Amsterdam, The Netherlands. 2018. Available online: https://www.ema.europa.eu/en/environmental-risk-assessment-medicinal-products-human-use (accessed on 30 May 2021).
- Chen, W.; Son, L.; Gan, N.; Li, L. Sorption, Degradation and Mobility of Microcystins in Chinese Agriculture Soils: Risk Assessment for Groundwater Protection. Environ. Pollut. 2006, 144, 752–758. [Google Scholar] [CrossRef]
- Drug Bank Online. Available online: https://go.drugbank.com/ (accessed on 3 May 2021).
- European Chemicals Agency. Available online: https://Echa.Europa.Eu/Registration-Dossier/-/Registered-Dossier/12616/5/5/2 (accessed on 3 May 2021).
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.-F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and Transport of Antibiotic Residues and Antibiotic Resistance Genes Following Land Application of Manure Waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Dong, Y. Hua Effect of Low-Molecular-Weight Organic Acids on the Adsorption of Norfloxacin in Typical Variable Charge Soils of China. J. Hazard. Mater. 2008, 151, 833–839. [Google Scholar] [CrossRef]
- Nguyen Dang Giang, C.; Sebesvari, Z.; Renaud, F.; Rosendahl, I.; Hoang Minh, Q.; Amelung, W. Occurrence and Dissipation of the Antibiotics Sulfamethoxazole, Sulfadiazine, Trimethoprim, and Enrofloxacin in the Mekong Delta, Vietnam. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- ECETOC Environmental Exposure Assessment of Ionisable Organic Compounds—ECETOC Technical Report No. 123; Brussels, Belgium. 2013. Available online: https://www.ecetoc.org/wp-content/uploads/2014/08/ECETOC-TR-123-Environmental-risk-assessment-of-ionisable-compounds.pdf (accessed on 3 May 2021).
- National Library of Medicine. Available online: https://Pubchem.Ncbi.Nlm.Nih.Gov/Compound/Azithromycin#section=ATC-Code (accessed on 3 May 2021).
- National Library of Medicine. Available online: https://Pubchem.Ncbi.Nlm.Nih.Gov/Compound/Clarithromycin (accessed on 3 May 2021).
- Botton, M.J. Veterinary Antibiotics in the Terrestrial Environment. Available online: https://Www.Envchemgroup.Com/Veterinary-Antibiotics-in-the-Terrestrial-Environment.Html (accessed on 3 May 2021).
- CHMP. Committee for Medicinal Products for Human Use (CHMP)—CHMP Assessment Report; Zerbaxa (International Non-Proprietary Name: Ceftolozane/Tazobactam): London, UK, 2015. [Google Scholar]
- Reyns, T.; de Boever, S.; Baert, K.; Croubels, S.; Schauvliege, S.; Gasthuys, F.; de Backer, P. Disposition and Oral Bioavailability of Amoxicillin and Clavulanic Acid in Pigs. J. Vet. Pharmacol. Ther. 2007, 30, 550–555. [Google Scholar] [CrossRef]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.L. Occurrence of Antibiotics in the Aquatic Environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Suarez-Kurtz, G.; Ribeiro, F.M.; Vicente, F.L.; Struchiner, C.J. Development and Validation of Limited-Sampling Strategies for Predicting Amoxicillin Pharmacokinetic and Pharmacodynamic Parameters. Antimicrob. Agents Chemother. 2001, 45, 3029–3036. [Google Scholar] [CrossRef][Green Version]
- INFARMED. Anexo I—Resumo das Características do Medicamento; Amoxicilina Aurobindo: Lisbon, Potugal, 2021. [Google Scholar]
- Slana, M.; Dolenc, M.S. Environmental Risk Assessment of Antimicrobials Applied in Veterinary Medicine-A Field Study and Laboratory Approach. Environ. Toxicol. Pharmacol. 2013, 35, 131–141. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Yue, L.; Sun, Y.; Ding, H.; Liu, Y. Excretion of Enrofloxacin in Pigs and Its Effect on Ecological Environment. Environ. Toxicol. Pharmacol. 2008, 26, 272–277. [Google Scholar] [CrossRef]
- LeBel, M. Ciprofloxacin: Chemistry, Mechanism of Action, Resistance, Antimicrobial Spectrum, Pharmacokinetics, Clinical Trials, and Adverse Reactions. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1988, 8, 3–30. [Google Scholar] [CrossRef]
- EMA. Enrofloxacin Summary Report 1; Committee for Veterinary Medicinal Products: London, UK, 1998; pp. 1–3. [Google Scholar]
- Stein, G.E. Review of the Bioavailability and Pharmacokinetics of Oral Norfloxacin. Am. J. Med. 1987, 82, 18–21. [Google Scholar] [CrossRef]
- Straub, J.O. Aquatic Environmental Risk Assessment for Human Use of the Old Antibiotic Sulfamethoxazole in Europe. Environ. Toxicol. Chem. 2016, 35, 767–779. [Google Scholar] [CrossRef]
- Vree, T.B.; O’Reilly, W.J.; Hekster, Y.A.; Damsma, J.E.; van der Kleijn, E. Determination of the Acetylator Phenotype and Pharmacokinetics of Some Sulphonamides in Man. Clin. Pharmacokinet. 1980, 5, 274–294. [Google Scholar] [CrossRef]
- LeBel, M. Pharmacokinetic Properties of Clarithromycin: A Comparison with Erythromycin and Azithromycin. Can. J. Infect. Dis. 1993, 4, 148–152. [Google Scholar] [CrossRef]
- Sun, H.; Frassetto, L.A.; Huang, Y.; Benet, L.Z. Hepatic Clearance, but Not Gut Availability, of Erythromycin is Altered in Patients with End-Stage Renal Disease. Clin. Pharmacol. Ther. 2010, 87, 465–472. [Google Scholar] [CrossRef]
- Suzuki, A.; Iida, I.; Hirota, M.; Akimoto, M.; Higuchi, S.; Suwa, T.; Chiba, K.; Tani, M.; Ishizaki, T. CYP Isoforms Involved in the Metabolism of Clarithromycin in Vitro: Comparison between the Identification from Disappearance Rate and That from Formation Rate of Metabolites. Drug Metab. Pharmacokinet. 2003, 18, 104–113. [Google Scholar] [CrossRef][Green Version]
- Goldman, J.L.; Leeder, J.S.; van Haandel, L.; Pearce, R.E. In Vitro Hepatic Oxidative Biotransformation of Trimethoprim. Drug Metab. Dispos. 2015, 43, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.; Gyrd-Hansen, N. Oral Bioavailability of Sulphadiazine and Trimethoprim in Fed and Fasted Pigs. Res. Vet. Sci. 1994, 56, 48–52. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and Pharmacodynamics of the Tetracyclines Including Glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.; Gyrd-Hansen, N. Bioavailability of Oxytetracycline, Tetracycline and Chlortetracycline after Oral Administration to Fed and Fasted Pigs. J. Vet. Pharmacol. Ther. 1996, 19, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hornish, R.E.; Gosline, R.E.; Nappier, J.M. Comparative Metabolism of Lincomycin in the Swine, Chicken, and Rat. Drug Metab. Rev. 1987, 18, 177–214. [Google Scholar] [CrossRef] [PubMed]
- EMA. Lincomycin Summary Report 1; Committee for Veterinary Medicinal Products: London, UK, 1998; pp. 1–10. [Google Scholar]
- Halstenson, C.E.; Wong, M.O.; Johnson, C.A.; Zimmerman, S.W.; Onorato, J.J.; Keane, W.F.; Doepner, M.; Sia, L.; Tantillo, K.; Bansal, S.; et al. Pharmacokinetics of Tazobactam M1 Metabolite After Administration of Piperacillin/Tazobactam in Subjects with Renal Impairment. J. Clin. Pharmacol. 1994, 34, 1208–1217. [Google Scholar] [CrossRef]
- Westphal, J.F.; Brogard, J.M.; Caro-Sampara, F.; Adloff, M.; Blicklé, J.F.; Monteil, H.; Jehl, F. Assessment of Biliary Excretion of Piperacillin-Tazobactam in Humans. Antimicrob. Agents Chemother. 1997, 41, 1636–1640. [Google Scholar] [CrossRef]
- EMA. Anexo I-Resumo Das Características Do Medicamento–Zerbaxa. 2010. Available online: https://www.ema.europa.eu/en/documents/product-information/zerbaxa-epar-product-information_es.pdf (accessed on 3 May 2021).
- Adam, D.; de Visser, I.; Koeppe, P. Pharmacokinetics of Amoxicillin and Clavulanic Acid Administered Alone and in Combination. Antimicrob. Agents Chemother. 1982, 22, 353–357. [Google Scholar] [CrossRef]
- Bolton, G.C.; Allen, G.D.; Davies, B.E.; Filer, C.W.; Jeffery, D.J. The Disposition of Clavulanic Acid in Man. Xenobiotica 1986, 16, 853–863. [Google Scholar] [CrossRef]
- Reyns, T.; de Boever, S.; de Baere, S.; de Backer, P.; Croubels, S. Tissue Depletion of Amoxicillin and Its Major Metabolites in Pigs: Influence of the Administration Route and the Simultaneous Dosage of Clavulanic Acid. J. Agric. Food Chem. 2008, 56, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Reyns, T.; de Boever, S.; Schauvliege, S.; Gasthuys, F.; Meissonnier, G.; Oswald, I.; de Backer, P.; Croubels, S. Influence of Administration Route on the Biotransformation of Amoxicillin in the Pig. J. Vet. Pharmacol. Ther. 2009, 32, 241–248. [Google Scholar] [CrossRef]
- EMA. Annex I—Summary of Product Characteristics-Recabrio. 2021. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/recarbrio (accessed on 3 May 2021).
- Reed, M.D.; Stern, R.C.; O’Brien, C.A.; Yamashita, T.S.; Myers, C.M.; Blumer, J.L. Pharmacokinetics of Imipenem and Cilastatin in Patients with Cystic Fibrosis. Antimicrob. Agents Chemother. 1985, 27, 583–588. [Google Scholar] [CrossRef]
- Infomed. Available online: https://Extranet.Infarmed.Pt/INFOMED-Fo/ (accessed on 3 May 2021).
- MedVet. Available online: https://Medvet.Dgav.Pt/ (accessed on 3 May 2021).
- Von der Ohe, P.C.; Dulio, V.; Slobodnik, J.; de Deckere, E.; Kühne, R.; Ebert, R.U.; Ginebreda, A.; de Cooman, W.; Schüürmann, G.; Brack, W. A New Risk Assessment Approach for the Prioritisation of 500 Classical and Emerging Organic Microcontaminants as Potential River Basin Specific Pollutants under the European Water Framework Directive. Sci. Total Environ. 2011, 409, 2064–2077. [Google Scholar] [CrossRef]
- Tsaboula, A.; Papadakis, E.N.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Papadopoulou-Mourkidou, E. Environmental and Human Risk Hierarchy of Pesticides: A Prioritisation Method, Based on Monitoring, Hazard Assessment and Environmental Fate. Environ. Int. 2016, 91, 78–93. [Google Scholar] [CrossRef]
- Van den Brink, P.J.; Boxall, A.B.A.; Maltby, L.; Brooks, B.W.; Rudd, M.A.; Backhaus, T.; Spurgeon, D.; Verougstraete, V.; Ajao, C.; Ankley, G.T.; et al. Toward Sustainable Environmental Quality: Priority Research Questions for Europe. Environ. Toxicol. Chem. 2018, 37, 2281–2295. [Google Scholar] [CrossRef]
- Křesinová, Z.; Petrů, K.; Lhotský, O.; Rodsand, T.; Cajthaml, T. Passive Sampling of Pharmaceuticals and Personal Care Products in Aquatic Environments. Eur. J. Environ. Sci. 2016, 6, 43–56. [Google Scholar] [CrossRef][Green Version]
- Rico, A.; Arenas-Sánchez, A.; Alonso-Alonso, C.; López-Heras, I.; Nozal, L.; Rivas-Tabares, D.; Vighi, M. Identification of Contaminants of Concern in the Upper Tagus River Basin (Central Spain). Part 1: Screening, Quantitative Analysis and Comparison of Sampling Methods. Sci. Total Environ. 2019, 666, 1058–1070. [Google Scholar] [CrossRef]
- Almeida, A.; Duarte, S.; Nunes, R.; Rocha, H.; Pena, A.; Meisel, L. Human and Veterinary Antibiotics Used in Portugal—A Ranking for Ecosurveillance. Toxics 2014, 2, 188–225. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of Hospital Effluents to the Load of Pharmaceuticals in Urban Wastewaters: Identification of Ecologically Relevant Pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; van de Schans, M.G.M. The Persistence of a Broad Range of Antibiotics during Calve, Pig and Broiler Manure Storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Brown, A.K.; Wong, C.S. Distribution and Fate of Pharmaceuticals and Their Metabolite Conjugates in a Municipal Wastewater Treatment Plant. Water Res. 2018, 144, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, L.A.; Knapp, C.W.; Larive, C.K.; Belden, J.B.; Lydy, M.; Graham, D.W. Factors Affecting the Fate of Ciprofloxacin in Aquatic Field Systems. Water Air Soil Pollut. 2005, 161, 383–398. [Google Scholar] [CrossRef]
- Parpounas, A.; Litskas, V.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Assessing the Presence of Enrofloxacin and Ciprofloxacin in Piggery Wastewater and Their Adsorption Behaviour onto Solid Materials, with a Newly Developed Chromatographic Method. Environ. Sci. Pollut. Res. 2017, 24, 23371–23381. [Google Scholar] [CrossRef] [PubMed]
- Speltini, A.; Sturini, M.; Maraschi, F.; Profumo, A.; Albini, A. Analytical Methods for the Determination of Fluoroquinolones in Solid Environmental Matrices. TrAC Trends Anal. Chem. 2011, 30, 1337–1350. [Google Scholar] [CrossRef]
- Boy-Roura, M.; Mas-Pla, J.; Petrovic, M.; Gros, M.; Soler, D.; Brusi, D.; Menció, A. Towards the Understanding of Antibiotic Occurrence and Transport in Groundwater: Findings from the Baix Fluvià Alluvial Aquifer (NE Catalonia, Spain). Sci. Total Environ. 2018, 612, 1387–1406. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Bergamaschi, B.A.; Loftin, K.A.; Meyer, M.T.; Harter, T. Use and Environmental Occurrence of Antibiotics in Freestall Dairy Farms with Manured Forage Fields. Environ. Sci. Technol. 2010, 44, 6591–6600. [Google Scholar] [CrossRef] [PubMed]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the Environment: An Overview on the Occurrence, Fate, Toxicity, Detection, Removal Methods, and Sludge Management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017: Trends from 2010–2017; Ninth ESVAC Report—EMA/294674/2019; European Medicines Agency: Amsterdam, The Nederlands, 2019; p. 106. [Google Scholar]
- Abuin, S.; Codony, R.; Compañó, R.; Granados, M.; Prat, M.D. Analysis of Macrolide Antibiotics in River Water by Solid-Phase Extraction and Liquid Chromatography-Mass Spectrometry. J. Chromatogr. A 2006, 1114, 73–81. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Baumann, M.; Weiss, K.; Maletzki, D.; Schüssler, W.; Schudoma, D.; Kopf, W.; Kühnen, U. Aquatic Toxicity of the Macrolide Antibiotic Clarithromycin and Its Metabolites. Chemosphere 2015, 120, 192–198. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision (EU) 2015 495—Of 20 March 2015—Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/ 105/ EC of the European Parliament and of the Council—(Notified under Document C(2015) 1756); European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of Antibiotic Resistance in China and Its Environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Bonvin, F.; Omlin, J.; Rutler, R.; Schweizer, W.B.; Alaimo, P.J.; Strathmann, T.J.; McNeill, K.; Kohn, T. Direct Photolysis of Human Metabolites of the Antibiotic Sulfamethoxazole: Evidence for Abiotic Back-Transformation. Environ. Sci. Technol. 2013, 47, 6746–6755. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Frömel, T.; Müller, J.; Peschka, M.; Knepper, T.; Díaz-Cruz, S.; Barceló, D. Biodegradation Studies of N 4-Acetylsulfapyridine and N 4-Acetylsulfamethazine in Environmental Water by Applying Mass Spectrometry Techniques. Anal. Bioanal. Chem. 2012, 402, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Zhong, Z.; Guo, C.; Li, L.; He, Y.; Fan, W.; Chen, Y. Degradation of Sulfonamides Antibiotics in Lake Water and Sediment. Environ. Sci. Pollut. Res. 2013, 20, 2372–2380. [Google Scholar] [CrossRef]
- Wegst-Uhrich, S.R.; Navarro, D.A.; Zimmerman, L.; Aga, D.S. Assessing Antibiotic Sorption in Soil: A Literature Review and New Case Studies on Sulfonamides and Macrolides. Chem. Cent. J. 2014, 8. [Google Scholar] [CrossRef]
- Kuchta, S.L.; Cessna, A.J. Lincomycin and Spectinomycin Concentrations in Liquid Swine Manure and Their Persistence during Simulated Manure Storage. Arch. Environ. Contam. Toxicol. 2009, 57, 1–10. [Google Scholar] [CrossRef]
- Kuchta, S.L.; Cessna, A.J.; Elliott, J.A.; Peru, K.M.; Headley, J.V. Transport of Lincomycin to Surface and Ground Water from Manure-Amended Cropland. J. Environ. Qual. 2009, 38, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.P.; Duvenage, C.S.J.; Rohwer, E. The Occurrence of Anti-Retroviral Compounds Used for HIV Treatment in South African Surface Water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, P.; Vyas, R.K.; Pandit, P.; Dalai, A.K. Occurrence and Removal of Antiviral Drugs in Environment: A Review. Water Air Soil Pollut. 2013, 224, 1410. [Google Scholar] [CrossRef]
- Ncube, S.; Madikizela, L.M.; Chimuka, L.; Nindi, M.M. Environmental Fate and Ecotoxicological Effects of Antiretrovirals: A Current Global Status and Future Perspectives. Water Res. 2018, 145, 231–247. [Google Scholar] [CrossRef]
- Fernández, L.P.; Brasca, R.; Goicoechea, H.; Culzoni, M.J. Fluorescence-Kinetic Four-Way Data Generation and Modeling for Abacavir Determination in Water Samples. Microchem. J. 2020, 159. [Google Scholar] [CrossRef]
- Aminot, Y.; Litrico, X.; Chambolle, M.; Arnaud, C.; Pardon, P.; Budzinski, H. Erratum to: Development and Application of a Multi-Residue Method for the Determination of 53 Pharmaceuticals in Water, Sediment, and Suspended Solids Using Liquid Chromatography-Tandem Mass Spectrometry (Analytical and Bioanalytical Chemistry 10.1007/S00216-015-9017-3). Anal. Bioanal. Chem. 2015, 407, 8623. [Google Scholar] [PubMed]
- Prasse, C.; Schlüsener, M.P.; Schulz, R.; Ternes, T.A. Antiviral Drugs in Wastewater and Surface Waters: A New Pharmaceutical Class of Environmental Relevance? Environ. Sci. Technol. 2010, 44, 1728–1735. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, A.; Gómez-Oliván, L.M.; Galar-Martínez, M.; Islas-Flores, H.; Dublán-García, O.; SanJuan-Reyes, N. Amoxicillin in the Aquatic Environment, Its Fate and Environmental Risk. In Environmental Health Risk—Hazardous Factors Living Species; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen Limited: London, UK, 2016. [Google Scholar] [CrossRef]
- Gozlan, I.; Rotstein, A.; Avisar, D. Amoxicillin-Degradation Products Formed under Controlled Environmental Conditions: Identification and Determination in the Aquatic Environment. Chemosphere 2013, 91, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Gozlan, I.; Rotstein, A.; Avisar, D. Investigation of an Amoxicillin Oxidative Degradation Product Formed under Controlled Environmental Conditions. Environ. Chem. 2010, 7, 435–442. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision (EU) 2018/ 840—Of 5 June 2018—Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/ 105/ EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/ 495—(Notified under Document C (2018) 3362); European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Ortiz de García, S.; Pinto Pinto, G.; García Encina, P.; Irusta Mata, R. Consumption and Occurrence of Pharmaceutical and Personal Care Products in the Aquatic Environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotic Resistance in the Environment: A Link to the Clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef]
- Le Page, G.; Gunnarsson, L.; Snape, J.; Tyler, C.R. Integrating Human and Environmental Health in Antibiotic Risk Assessment: A Critical Analysis of Protection Goals, Species Sensitivity and Antimicrobial Resistance. Environ. Int. 2017, 109, 155–169. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Sandegren, L. Selection of Antibiotic Resistance at Very Low Antibiotic Concentrations. Upsala J. Med Sci. 2014, 119, 103–107. [Google Scholar] [CrossRef]
- Khan, S.; Beattie, T.K.; Knapp, C.W. The Use of Minimum Selectable Concentrations (MSCs) for Determining the Selection of Antimicrobial Resistant Bacteria. Ecotoxicology 2017, 26, 283–292. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Holm, G.; Snape, J.R.; Murray-Smith, R.; Talbot, J.; Taylor, D.; Sörme, P. Implementing Ecopharmacovigilance in Practice: Challenges and Potential Opportunities. Drug Saf. 2013, 36, 533–546. [Google Scholar] [CrossRef]
- APA. Final Report—PROJETO POSEUR-03-2013-FC-000001—“Melhoria da Avaliação Ddo Estado das Massas de Água”. Cofinanciado por UNIÃO EUROPEIA, Fundo de Coesão; APA: Lisbon, Potugal, 2019. [Google Scholar]
- APA. Classificação Das Massas de Água. Avaliação Intercalar 2014–2017; APA: Lisbon, Potugal, 2019. [Google Scholar]
- INFARMED. Estatística do Medicamento e Produtos de Saúde 2017. Lisbon, Potugal. 2018. Available online: https://www.infarmed.pt/documents/15786/1229727/Estat%C3%ADstica+do+Medicamento+2017/c759b946-9dcb-4b0a-b10b-6287bf76c114?version=1.0 (accessed on 3 May 2021).
- EMA. European Surveillance of Veterinary Antimicrobial Consumption, 2019. ‘Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017’; (EMA/294674/2019); EMA: London, UK, 2019. [Google Scholar]

| Pharmaceuticals | Regional Amount (Kg) | Σ | ||||
|---|---|---|---|---|---|---|
| North | Centre | West/Tejo | Alentejo | Algarve | ||
| Antibiotics | ||||||
| Amoxicillin | 15,494 | 9664 | 15,669 | 1715 | 1866 | 44,408 |
| Enrofloxacin | NA | NA | NA | NA | NA | 3600 1 |
| Ciprofloxacin | 1075 | 744 | 1370 | 140 | 154 | 3483 |
| Norfloxacin | 77 | 69 | 155 | 22 | 17 | 340 |
| Sulfamethoxazole | 796 | 589 | 1002 | 95 | 47 | 2529 |
| Sulfamethazine | NA | NA | NA | NA | NA | 5800 1,2 |
| Erythromycin | 32 | 27 | 59 | 5 | 7 | 130 |
| Azithromycin | 536 | 393 | 659 | 64 | 77 | 1729 |
| Clarithromycin | 630 | 620 | 800 | 140 | 40 | 2230 |
| Trimethoprim | 159 | 118 | 200 | 19 | 41 | 537 |
| Tetracycline | NA | NA | NA | NA | NA | 45,000 1 |
| Lincomycin | NA | NA | NA | NA | NA | 3000 1 |
| Inhibitors | ||||||
| Clavulanic acid | 1535 | 989 | 1587 | 175 | 188 | 4474 |
| Tazobactam | 228 | 159 | 279 | 29 | 22 | 717 |
| Cilastatin | 57 | 13 | 5 | 0.6 | 0.3 | 76 |
| Antiviral | ||||||
| Abacavir | 430 | 116 | 848 | 17 | 47 | 1458 |
| Active Substances | CAS-N° 1 | MW 1 g/mol | Water Solubility 1 | pKa Acid and Basic 1 | log Kow 1 | log Koc L/kg |
|---|---|---|---|---|---|---|
| Amoxicillin | 26787-78-0 | 365.4 | high | 7.4 | 0.87 | 3.3 2 |
| Enrofloxacin | 93106-60-6 | 359.4 | high | 6.7 | 0.58 | 4.22–5.89 3 |
| Ciprofloxacin | 85721-33-1 | 331.3 | high | 6.1 | 0.28 | 4.78 3 |
| Norfloxacin | 70458-96-7 | 319.3 | high | 5.8 | −1.03 | 4.4 (Kd) 4 |
| 8.7 | ||||||
| Sulfamethoxazole | 723-46-6 | 253.3 | high | 6.2 | 0.89 | 2.3 5 |
| 2 | ||||||
| Sulfamethazine | 57-68-1 | 278.3 | high | 7.6 | 0.89 | 1.78–2.32 3 |
| Erythromycin | 114-07-8 | 733.9 | low | 8.9 | 2.6 | 2.3 6 |
| Azithromycin | 83905-01-5 | 749 | high | 8.5 | 3 | 3.5 7 |
| Clarithromycin | 81103-11-9 | 748 | high | 9 | 3.2 | 2.2 8 |
| Trimethoprim | 738-70-5 | 290.3 | moderate | 7.1 | 0.91 | 2.5 5 |
| Tetracycline | 60-54-8 | 444.4 | low | 3.3 | −1.3 | 4.9 9 |
| Lincomycin | 859-18-7 | 406.5 | high | 7.6 | 0.56 | NA |
| Tazobactam | 89786-04-9 | 300.3 | high | 2.1 | −1.8 | 0.87 10 |
| Clavulanic acid | 58001-44-8 | 199.2 | high | 2.7 | −2.3 | NA |
| Cilastatin | 82009-34-5 | 358.5 | moderate | 9.5 | 0.29 | NA |
| 2.5 | ||||||
| Abacavir | 136470-78-5 | 286.3 | high | 15.4 | 1.2 | 3.0 6 |
| 5.8 |
| Active Substance | Therapeutical Use Dosage Forms 1,2—Target Species | Bioavailability 1,2 | Excretion | References | |
|---|---|---|---|---|---|
| Unchanged Form | Metabolites (%) | ||||
| Amoxicillin | Human use Capsule; tablets; Powder for oral suspension; Powder for solution for injection or infusion. Veterinary use Premix for medicated feed—pigs; Powder in drinking water—poultry, pigs; Injectable suspension—cattle, sheep, pigs; Palatable tablets—dogs and cats. | 70%; 23% 3 | Renal: 80–90% Faecal: 5–10% | Amoxicilloic acid; Piperazine-2,5-dione (diketopiperazine). (Both 10–20%) | [26,27,28,29] |
| Enrofloxacin | Veterinary use Oral solution—cats, pigs, broilers, rabbits Solution for drinking water—chickens, turkeys, rabbits; Solution for injection—dogs, cats, cattle, pigs; Tablets—dogs and cats. | 73–101% 3 | Renal: 21% 3 Faecal: 19% 3 | Ciprofloxacin (20–50%); Active dealkylated and hydroxylated enrofloxacin (<10%). | [30,31] |
| Ciprofloxacin | Human use Tablets. | 64–85% | Renal: 30–50% Faecal: 15–62% | Oxociprofloxacin; Desethylene ciprofloxacin; Formylciprofloxacin. | [32,33] |
| Norfloxacin | Human use Tablets; Eye drops solution. | 30–50% | Renal: 30–70% Faecal: 30% | 3-oxo-1-piperazinyl metabolite (<20%). | [34] |
Sulfamethoxazole | Human use 5 Tablets; Syrup; Solution for injection. Veterinary use 4 Solution for drinking water—pigs. | NA | Renal: 10–40% | N4-acetyl- sulfamethoxazole (30–70%); Sulfamethoxazole glucuronide. | [35] |
| Sulfamethazine (sulfadimidine) | NA | NA | Renal: <12% | N4-acetyl-sulfamethazine (61–81%); Conjugated hydroxylated Metabolites (10–20%). | [36] |
| Erythromycin 5 | Human use Tablets; Granules for oral suspension; Powder for solution for injection; Topical skin solutions. Veterinary use Injectable solution—cattle, pigs and sheep. | 25% | Renal: 2–5% | N-desmethyl-erythromycin; Anhydroerythromycin. | [37,38] |
| Azithromycin | Human use Powder for oral suspension; Powder for solution for infusion; Tablets; Eye drops solution. | 37% | Renal: 20% | N-desmethyl derivatives. | [37,38] |
| Clarithromycin | Human use Tablets; Granules for oral suspension; Powder for solution for injection. | 55% | Renal: 30–40% | 14-(R)-hydroxy-clarithromycin (active metabolite); N-demethyl-clarithromycin. | [37,38,39] |
| Trimethoprim | Human use 6 Tablets; Syrup; Solution for injection; Veterinary use 6 Premix for medicated feed—pigs and sheep; Solution for drinking water (pigs, broilers, calves, lambs, rabbits); Solution for injection—cattle, horses, sheep; Oral paste and oral powder—horses. | 90% 3 | Renal: ±80% | 3-Desmethyl-trimethoprim (65%); 4-Desmethyl-trimethoprim (25%); N-oxides (≤5%). | [40,41] |
| Tetracycline | Human use Capsule; Ophthalmic ointment; Veterinary use Premix for medicated feed—fish farm; pigs. Powder for oral solution—calves, lambs, pigs, rabbits and poultry; Solution for injection—cattle, pigs, horses, sheep, dogs and cats. | 5% 3 | Renal: 30% Faecal: 20–60% | D-epitetracycline (5%). | [42,43] |
| Lincomycin | Veterinary use Premix for medicated feed—pigs; Powder for drinking water—pigs, chickens; Injectable solution—cattle, sheep, goats, swine, chickens, turkeys, cats; Intramammary solution for lactating cows. | 20–50% 3 | Renal: 14–21% 3 Faecal: 79–86% 3 | Lincomycin sulphoxide. | [44,45] |
| Tazobactam | Human use 7 Powder for solution for injection or infusion. | NA | Renal: 60–80% | M1-hydrolyzed metabolite (20–26%). | [46,47,48] |
| Clavulanic Acid 4 | Human use 8 Tablets; Powder for oral suspension; Powder for solution for injection or infusion. Veterinary use Powder for drinking water—pigs; Powder for oral suspension—dogs and cats; Injectable—Cattle, Canines, Felines, Pigs; Palatable tablets—dogs and cats; Intramammary suspension for lactating cattle. | 45% 3 | Renal: 40–73% | 2,5-dihydro-4-(2- hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid (15.6%). 1-amino-4-hydroxy-butan-2-one (<10%). | [26,49,50,51,52] |
| Cilastatin | Human use 9 Powder for solution for infusion. | NA | Renal: 78% | N-acetyl metabolite (10%). | [53,54] |
| Abacavir 5 | Human use Film-coated tablet 10; oral solution. | 83% | Renal: 1% Feacal: 16% | 5′-carboxylic-acid metabolite (30%); 5′-glucuronide metabolite (36%); Minor metabolites (15%). | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, P.; Meisel, L.; Lopes, A.; de Jesus, R.; Sarmento, G.; Duarte, S.; Sepodes, B.; Fernandes, A.; dos Santos, M.M.C.; Almeida, A.; et al. Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study. Antibiotics 2021, 10, 888. https://doi.org/10.3390/antibiotics10080888
Viana P, Meisel L, Lopes A, de Jesus R, Sarmento G, Duarte S, Sepodes B, Fernandes A, dos Santos MMC, Almeida A, et al. Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study. Antibiotics. 2021; 10(8):888. https://doi.org/10.3390/antibiotics10080888
Chicago/Turabian StyleViana, Paula, Leonor Meisel, Ana Lopes, Rosário de Jesus, Georgina Sarmento, Sofia Duarte, Bruno Sepodes, Ana Fernandes, Margarida M. Correia dos Santos, Anabela Almeida, and et al. 2021. "Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study" Antibiotics 10, no. 8: 888. https://doi.org/10.3390/antibiotics10080888
APA StyleViana, P., Meisel, L., Lopes, A., de Jesus, R., Sarmento, G., Duarte, S., Sepodes, B., Fernandes, A., dos Santos, M. M. C., Almeida, A., & Oliveira, M. C. (2021). Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study. Antibiotics, 10(8), 888. https://doi.org/10.3390/antibiotics10080888






