High Prevalence of ESBL and Plasmid-Mediated Quinolone Resistance Genes in Salmonella enterica Isolated from Retail Meats and Slaughterhouses in Egypt
Abstract
:1. Introduction
2. Results
2.1. Prevalence of MDR and ESBL-Producing S. enterica Isolated from Retail Meat and Beef Carcasses
2.2. Prevalence of β-Lactamase-Encoding Genes in S. enterica Isolated from Retail Meat and Beef Carcasses in Egypt
2.3. Prevalence of Plasmid-Mediated Quinolone Resistance Genes in S. enterica Isolated from Retail Meats and Beef Carcasses in Egypt
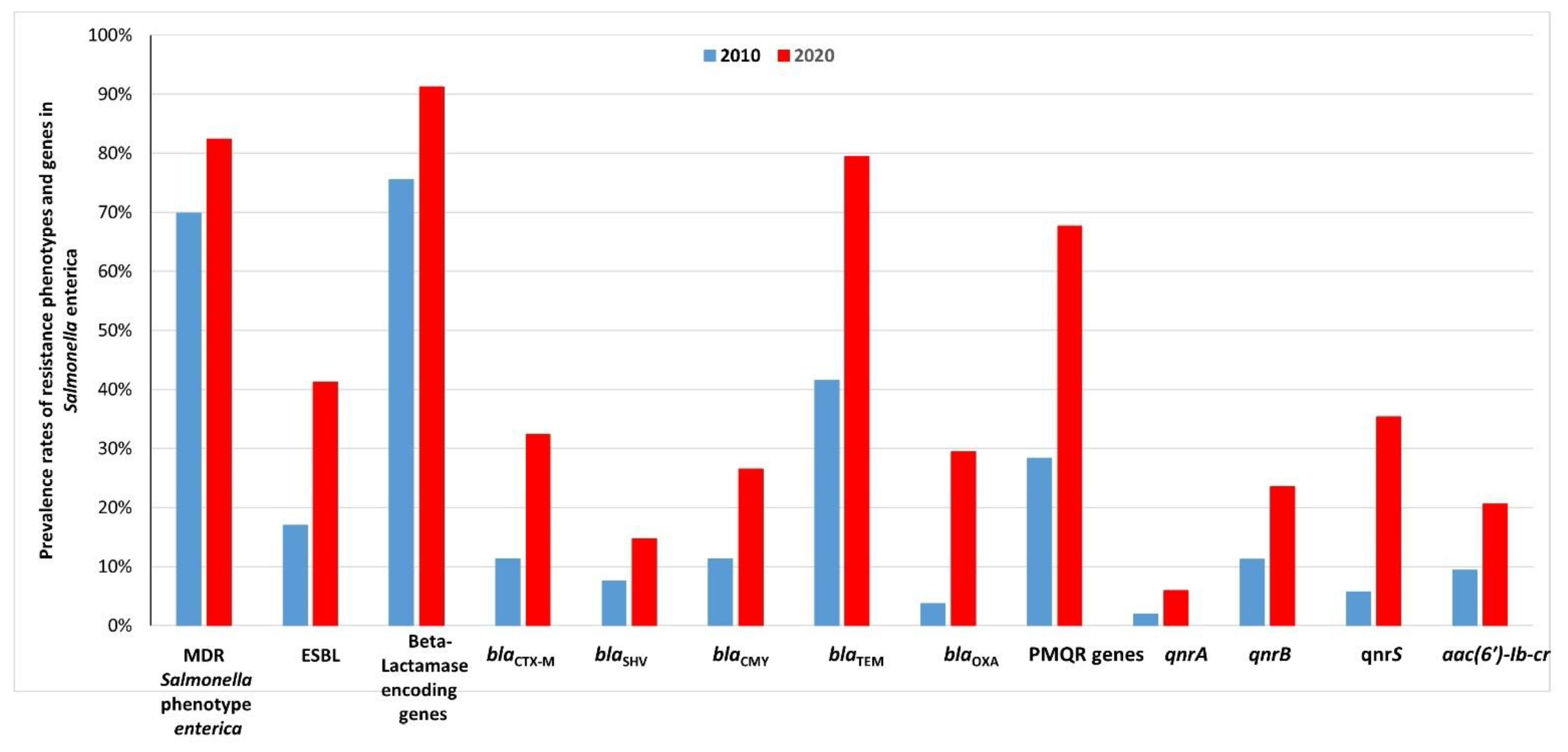
2.4. Comparison between the Prevalence Rates of Resistance Phenotypes and Genes in Salmonella Enterica Isolated from Retail Meats and Beef Carcasses in 2010 and 2020 in Egypt
2.5. Transferability and Replicon Typing of Plasmids
3. Discussion
3.1. High Prevalence of MDR and ESBL-Producing S. enterica Isolated from Retail Meat and Beef Carcasses in Egypt
3.2. High Prevalence of β-Lactamase-Encoding Genes in S. enterica Isolated from Retail Meat and Beef Carcasses in Egypt
3.3. High Prevalence of Plasmid-Mediated Quinolone Resistance Genes in S. enterica Isolated from Retail Meats and Beef Carcasses in Egypt
3.4. Common Plasmid Replicon Types in S. enterica Isolated from Retail Meat and Beef Carcasses in Egypt
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation and Identification of S. enterica
4.3. Antimicrobial Sensitivity Testing and ESBL-Resistance Phenotyping
4.4. Preparation of Salmonella DNA
4.5. PCR and DNA Sequencing for β-Lactamase-Encoding Genes and Plasmid-Mediated Quinolone Resistance Genes
4.6. Plasmid Incompatibility Grouping and Transconjugation Experiments
4.7. BLAST Analysis of the Sequence Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO (World Health Organization). Food Safety Fact Sheet. 30 April 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 4 June 2021).
- Chen, H.M.; Wang, Y.; Su, L.H.; Chiu, C.H. Nontyphoid Salmonella infection: Microbiology, clinical features, and antimicrobial therapy. Pediatr. Neonatol. 2013, 54, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunguya, O.; Lejon, V.; Phoba, M.F.; Bertrand, S.; Vanhoof, R.; Glupczynski, Y.; Verhaegen, J.; Muyembe-Tamfum, J.J.; Jacobs, J. Antimicrobial resistance in invasive non-typhoid Salmonella from the Democratic Republic of the Congo: Emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl. Trop. Dis. 2013, 7, e2103. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Critically Important Antimicrobials for Human Medicine, 3rd ed.; WHO: Geneva, Switzerland, 2012; Available online: http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf?ua=1&ua=1 (accessed on 4 June 2021).
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic resistance in the food chain: A developing country-perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). Final Report FAO/WHO Regional Conference On Food Safety for Africa. 3–6 October 2005, Harare, Zimbabwe. Available online: http://www.fao.org/documents/card/en/c/4d192b73-849b-516d-acdc-c27664f72949/ (accessed on 4 June 2021).
- WHO (World Health Organization). Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017–2018; WHO: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/279656/9789241515061-eng.pdf (accessed on 13 July 2021).
- DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2017. Available online: https://backend.orbit.dtu.dk/ws/files/161713656/Rapport_DANMAP_2017.pdf (accessed on 13 July 2021).
- NARMS. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/narms/index.html (accessed on 13 July 2021).
- Schnall, J.; Rajkhowa, A.; Ikuta, K.; Rao, P.; Moore, C.E. Surveillance and Monitoring of Antimicrobial Resistance: Limitations and Lessons from the GRAM Project. BMC Med. 2019, 17, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernet, G.; Mary, C.; Altmann, D.M.; Doumbo, O.; Morpeth, S.; Bhutta, Z.A.; Klugman, K.P. Surveillance for antimicrobial drug resistance in under-resourced countries. Emerg. Infect. Dis. 2014, 20, 434–441. [Google Scholar] [CrossRef] [Green Version]
- McMillan, E.A.; Wasilenko, J.L.; Tagg, K.A.; Chen, J.C.; Simmons, M.; Gupta, S.K.; Tillman, G.E.; Folster, J.; Jackson, C.R.; Frye, J.G. Carriage and Gene Content Variability of the pESI-Like Plasmid Associated with Salmonella Infantis Recently Established in United States Poultry Production. Genes 2020, 11, 1516. [Google Scholar] [CrossRef]
- Proietti, P.C.; Stefanetti, V.; Musa, L.; Zicavo, A.; Dionisi, A.M.; Bellucci, S.; Mensa, A.L.; Menchetti, L.; Branciari, R.; Ortenzi, R.; et al. Genetic profiles and antimicrobial resistance patterns of Salmonella Infantis strains isolated in Italy in the food chain of broiler meat production. Antibiotics 2020, 9, 814. [Google Scholar] [CrossRef]
- Lapierre, L.; Cornejo, J.; Zavala, S.; Galarce, N.; Sánchez, F.; Benavides, M.B.; Guzmán, M.; Sáenz, L. Phenotypic and genotypic characterization of virulence factors and susceptibility to antibiotics in Salmonella infantis strains isolated from chicken meat: First findings in Chile. Animals 2020, 10, 1049. [Google Scholar] [CrossRef]
- Parvin, M.S.; Hasan, M.M.; Ali, M.Y.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Prevalence and multidrug resistance pattern of Salmonella carrying extended-spectrum β-lactamase in frozen chicken meat in Bangladesh. J. Food Prot. 2020, 83, 2107–2121. [Google Scholar] [CrossRef]
- Souza, A.I.; Saraiva, M.M.; Casas, M.R.; Oliveira, G.M.; Cardozo, M.V.; Benevides, V.P.; Barbosa, F.O.; Freitas Neto, O.C.; Almeida, A.M.; Berchieri, A. High occurrence of β-lactamase-producing Salmonella Heidelberg from poultry origin. PLoS ONE 2020, 15, e0230676. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Characterization of integrons and resistance genes in multidrug-resistant Salmonella enterica isolated from meat and dairy products in Egypt. Int. J. Food Microbiol. 2014, 189, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO (World Health Organization). Antimicrobial Resistance. 13 October 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 4 June 2021).
- CDC (Centers for Disease Control and Prevention). Antibiotic Resistance Threats to the United States. 2013. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 4 June 2021).
- Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, X.; Zhang, J.; Yang, S.; Zhang, S.; Chen, M.; Xue, L.; Ding, Y.; Zeng, H.; Gu, Q.; et al. Molecular characterisation of antimicrobial resistance determinants and class 1 integrons of Salmonella enterica subsp. enterica serotype Enteritidis strains from retail food in China. Food Control 2021, 128, 108191. [Google Scholar] [CrossRef]
- Sin, M.; Yoon, S.; Kim, Y.B.; Noh, E.B.; Seo, K.W.; Lee, Y.J. Molecular characteristics of antimicrobial resistance determinants and integrons in Salmonella isolated from chicken meat in Korea. J. Appl. Poult. Res. 2020, 29, 502–514. [Google Scholar] [CrossRef]
- Awad, A.; Gwida, M.; Khalifa, E.; Sadat, A. Phenotypes, antibacterial-resistant profile, and virulence-associated genes of Salmonella serovars isolated from retail chicken meat in Egypt. Vet. World 2020, 13, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Vitas, A.I.; Naik, D.; Pérez-Etayo, L.; González, D. Increased exposure to extended-spectrum β-lactamase-producing multidrug-resistant Enterobacteriaceae through the consumption of chicken and sushi products. Int. J. Food Microbiol. 2018, 269, 80–86. [Google Scholar] [CrossRef]
- Gawish, M.F.; Ahmed, A.M.; Torky, H.A.; Shimamoto, T. Prevalence of extended-spectrum β-lactamase (ESBL)-producing Salmonella enterica from retail fishes in Egypt: A major threat to public health. Int. J. Food Microbiol. 2021, 351, 109268. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M. Beta-lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 1995, 8, 557–584. [Google Scholar] [CrossRef]
- Britto, C.D.; Wong, V.K.; Dougan, G.; Pollard, A.J. A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Negl. Trop. Dis. 2018, 12, e0006779. [Google Scholar] [CrossRef] [Green Version]
- Bogomazova, A.N.; Gordeeva, V.D.; Krylova, E.V.; Soltynskaya, I.V.; Davydova, E.E.; Ivanova, O.E.; Komarov, A.A. Mega-plasmid found worldwide confers multiple antimicrobial resistance in Salmonella Infantis of broiler origin in Russia. Int. J. Food Microbiol. 2020, 319, 108497. [Google Scholar] [CrossRef]
- Na, S.H.; Moon, D.C.; Kang, H.Y.; Song, H.J.; Kim, S.J.; Choi, J.H.; Yoon, J.W.; Yoon, S.S.; Lim, S.K. Molecular characteristics of extended-spectrum β-lactamase/AmpC-producing Salmonella enterica serovar Virchow isolated from food-producing animals during 2010–2017 in South Korea. Int. J. Food Microbiol. 2020, 322, 108572. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.W.; Zhang, Y.; Wang, X.C.; Gao, Y.F.; Wang, H.N. Draft genome sequence of a multidrug-resistant Salmonella enterica serotype Kentucky ST198 with chromosomal integration of blaCTX-M-14b isolated from a poultry slaughterhouse in China. J. Glob. Antimicrob. Resist. 2020, 20, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Lay, K.K.; Jeamsripong, S.; Sunn, K.P.; Angkititrakul, S.; Prathan, R.; Srisanga, S.; Chuanchuen, R. Colistin Resistance and ESBL Production in Salmonella and Escherichia coli from Pigs and Pork in the Thailand, Cambodia, Lao PDR, and Myanmar Border Area. Antibiotics 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, H.; Sakatsume, E.; Sekizuka, T.; Yokoyama, H.; Hamada, K.; Etoh, Y.; Carle, Y.; Mizumoto, S.; Hirai, S.; Matsui, M.; et al. Food workers as a reservoir of extended-spectrum-cephalosporin-resistant Salmonella strains in Japan. Appl. Environ. Microbiol. 2020, 86, e00072-20. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puchol, S.; Riveros, M.; Ruidias, K.; Granda, A.; Ruiz-Roldán, L.; Zapata-Cachay, C.; Ochoa, T.J.; Pons, M.J.; Ruiz, J. Dissemination of a multidrug resistant CTX-M-65 producer Salmonella enterica serovar Infantis clone between marketed chicken meat and children. Int. J. Food Microbiol. 2021, 344, 109109. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [Green Version]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [Green Version]
- Strahilevitz, J.; Jacoby, G.A.; Hooper, D.C.; Robicsek, A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin. Microbiol. Rev. 2009, 22, 664–689. [Google Scholar] [CrossRef] [Green Version]
- Tyson, G.H.; Tate, H.P.; Zhao, S.; Li, C.; Dessai, U.; Simmons, M.; McDermott, P.F. Identification of plasmid-mediated quinolone resistance in Salmonella isolated from swine ceca and retail pork chops in the United States. Antimicrob. Agents Chemother. 2017, 22, 61. [Google Scholar] [CrossRef] [Green Version]
- Bae, D.; Cheng, C.M.; Khan, A.A. Characterization of extended-spectrum β-lactamase (ESBL) producing non-typhoidal Salmonella (NTS) from imported food products. Int. J. Food Microbiol. 2015, 214, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Fang, T.; Zhou, X.; Zhang, D.; Shi, X.; Shi, C. IncHI2 plasmids are predominant in antibiotic-resistant Salmonella isolates. Front. Microbiol. 2016, 7, 1566. [Google Scholar] [CrossRef]
- Dolejska, M.; Villa, L.; Hasman, H.; Hansen, L.; Carattoli, A. Characterization of IncN plasmids carrying blaCTX-M-1 and qnr genes in Escherichia coli and Salmonella from animals, the environment and humans. J. Antimicrob. Chemother. 2013, 68, 333–339. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, J.K.; Kim, K.S. Prevalence and characterization of plasmid-mediated quinolone resistance genes in Salmonella isolated from poultry in Korea. Avian Pathol. 2013, 42, 221–229. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids in Gram negatives: Molecular typing of resistance plasmids. Int. J. Med. Microbiol. 2011, 301, 654–658. [Google Scholar] [CrossRef]
- Folster, J.P.; Pecic, G.; Bolcen, S.; Theobald, L.; Hise, K.; Carattoli, A.; Zhao, S.; McDermott, P.F.; Whichard, J.M. Characterization of extended-spectrum cephalosporin–resistant Salmonella enterica serovar Heidelberg isolated from humans in the United States. Foodborne Pathog. Dis. 2010, 7, 181–187. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 6579-1: Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella spp.; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- Grimont, P.A.D.; Weill, F.X. Antigenic Formulae of the Salmonella Serovars, 9th ed.; Institut Pasteur: Paris, France, 2007. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; CLSI: Annapolis Junction, MD, USA, 2018; Available online: https://clsi.org/standards/products/microbiology/documents/m02/ (accessed on 19 July 2021).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twenty Fifth Informational Supplement. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 19 July 2021).
- Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. 2013, 303, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kado, C.I.; Liu, S. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 1981, 145, 1365–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef] [PubMed]


| No. | Isolate | Serovar | Source | Resistance Phenotype | ESBL Phenotype | Resistance Gene(s) |
|---|---|---|---|---|---|---|
| 1 | SI-CM1 | S. Infantis | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CIP, CPD, CRO, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-1, blaCMY-2, blaOXA-1, qnrB, aac(6′)-Ib-cr |
| 2 | SI-CM2 | S. Infantis | Chicken meat | AMC, AMP, ATM, CHL, CPD, CTT, CTX, FOX, GEN, OXA, STR, SXT, TET | + | blaTEM-1, blaSHV-12 |
| 3 | SI-CM3 | S. Infantis | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CPD, CTT, CTX, FOX, GEN, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-14, blaCMY-2 |
| 4 | SI-CM4 | S. Infantis | Chicken meat | AMP, CHL, CTT, FOX, OXA, STR, TET | - | blaOXA-1 |
| 5 | SI-CM5 | S. Infantis | Chicken meat | AMP, CHL, CIP, NAL, STR, TET | - | blaTEM-1, qnrS |
| 6 | SI-CM6 | S. Infantis | Chicken meat | AMC, AMP, ATM, CTT, FOX, GEN, OXA, STR, SXT, TET | - | blaCMY-2 |
| 7 | SI-BM1 | S. Infantis | Beef meat | AMP, ATM, CTT, FOX, OXA, STR, SXT, TET | - | blaTEM-1 |
| 8 | SI-BC1 | S. Infantis | Beef carcass | AMP, CHL, NAL, STR | - | blaTEM-1, qnrB |
| 9 | SI-BC2 | S. Infantis | Beef carcass | AMP, CTT, FOX, OXA, STR | - | blaOXA-1 |
| 10 | SI-BC3 | S. Infantis | Beef carcass | AMP, NAL | - | blaTEM-1, qnrS |
| 11 | ST-CM1 | S. Typhimurium | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CIP, CPD, CRO, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-15, blaCMY-2, blaOXA-1, qnrB, aac(6′)-Ib-cr |
| 12 | ST-CM2 | S. Typhimurium | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CIP, CPD, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-3, blaSHV-12, qnrB |
| 13 | ST-BM1 | S. Typhimurium | Beef meat | AMC, AMP, ATM, CHL, CTT, FOX, OXA, STR, SXT, TET | - | blaTEM-1 |
| 14 | ST-BM2 | S. Typhimurium | Beef meat | AMC, AMP, ATM, CAZ, CHL, CIP, CPD, CRO, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-14, blaOXA-1, qnrS |
| 15 | ST-BC1 | S. Typhimurium | Beef carcass | AMC, AMP, ATM, CAZ, CHL, CPD, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-13, qnrS |
| 16 | ST-BC2 | S. Typhimurium | Beef carcass | AMP, OXA, CHL, NAL, STR | - | blaTEM-1, qnrA |
| 17 | ST-BC3 | S. Typhimurium | Beef carcass | AMP, ATM, CHL, CTT, FOX, GEN, NAL, OXA, STR, SXT, TET | - | blaTEM-1, qnrS |
| 18 | SE-CM1 | S. Enteritidis | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CIP, CPD, CRO, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-3, blaCMY-2, blaOXA-1, qnrB, aac(6′)-Ib-cr |
| 19 | SE-CM2 | S. Enteritidis | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CPD, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-15, qnrS |
| 20 | SE-CM3 | S. Enteritidis | Chicken meat | AMC, AMP, ATM, CHL, CTT, CTX, FOX, NAL, OXA, STR, SXT TET | + | blaTEM-1, blaSHV-12, qnrS |
| 21 | SE-BC1 | S. Enteritidis | Beef carcass | AMP, ATM, CTT, FOX, OXA, STR | - | blaOXA-1, blaCMY-2 |
| 22 | SV-CM1 | S. Virchow | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CPD, CRO, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-15, aac(6′)-Ib-cr |
| 23 | SV-BM1 | S. Virchow | Beef meat | AMP, CHL, CTT, GEN, NAL, OXA, STR, SXT, TET | - | blaTEM-1, qnrS |
| 24 | SV-BC1 | S. Virchow | Beef carcass | AMC, AMP, ATM, CHL, CTT, FOX, GEN, OXA, STR, SXT, TET | - | blaTEM-1, blaOXA-1, blaCMY-2 |
| 25 | SV-BC2 | S. Virchow | Beef carcass | AMP, NAL, CHL | - | qnrB |
| 26 | SH-CM1 | S. Heidelberg | Chicken meat | AMC, AMP, ATM, CAZ, CHL, CIP, CPD, CRO, CTT, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-2, blaCMY-2, blaSHV-12, qnrB |
| 27 | SH-CM2 | S. Heidelberg | Chicken meat | AMP, ATM, CHL, CTT, FOX, GEN, OXA, STR, SXT, TET | - | blaTEM-1, blaCMY-2 |
| 28 | SH-CM3 | S. Heidelberg | Chicken meat | AMP, OXA, NAL, TET | - | blaTEM-1, qnrB |
| 29 | SK-CM1 | S. Kentucky | Chicken meat | AMC, AMP, CAZ, CHL, CIP, CPD, CRO, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaCTX-M-15, blaCMY-2, blaOXA-1, qnrS, aac(6′)-Ib-cr |
| 30 | SK-CM2 | S. Kentucky | Chicken meat | AMC, AMP, ATM, CHL, CIP, CTT, CTX, FOX, GEN, NAL, OXA, STR, SXT, TET | + | blaTEM-1, blaSHV-12, aac(6′)-Ib-cr |
| 31 | SAN-CM1 | S. Anatum | Chicken meat | AMP, CHL, CTT, NAL, OXA, STR, SXT, TET | - | blaOXA-1, qnrS |
| 32 | SAG-CM1 | S. Agona | Chicken meat | NAL | - | qnrA |
| 33 | SM-BC1 | S. Montevideo | Beef carcass | AMP, ATM, CHL, CTT, GEN, NAL, OXA, STR, SXT, TET | - | blaTEM-1, qnrS |
| 34 | SS-BC1 | S. Stanley | Beef carcass | NAL, TET | - | qnrS |
| S. enterica Serovar | B-Lactamases Resistance Genes | Plasmid-Mediated Quinolone Resistance Genes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ESBL-Type (No.) | Narrow-Spectrum Types (No.) | AmpC (blaCMY) (No.) | qnrA | qnrB | qnrS | aac(6′)-Ib-cr | |||
| blaCTX-M | blaSHV | blaTEM | blaOXA | ||||||
| Infantis | blaCTX-M-1 (1) blaCTX-M-14 (1) | blaSHV-12 (1) | blaTEM-1 (7) | blaOXA-1 (3) | blaCMY-2 (3) | - | 2 | 2 | 1 |
| Typhimurium | blaCTX-M-3 (1) blaCTX-M-13 (1) blaCTX-M-14(1) blaCTX-M-15 (1) | blaSHV-12 (1) | blaTEM-1 (7) | blaOXA-1 (2) | blaCMY-2 (1) | 1 | 2 | 3 | 2 |
| Enteritidis | blaCTX-M-3 (1) blaCTX-M-15 (1) | blaSHV-12 (1) | blaTEM-1 (3) | blaOXA-1 (2) | blaCMY-2 (1) | - | 1 | 2 | 1 |
| Virchow | blaCTX-M-15 (1) | - | blaTEM-1 (3) | blaOXA-1 (1) | blaCMY-2 (1) | - | 1 | 1 | 1 |
| Heidelberg | blaCTX-M-2 (1) | blaSHV-12 (1) | blaTEM-1 (3) | - | blaCMY-2 (2) | - | 2 | - | - |
| Kentucky | blaCTX-M-15 (1) | blaSHV-12 (1) | blaTEM-1 (2) | blaOXA-1 (1) | blaCMY-2 (1) | - | - | 1 | 2 |
| Anatum | - | - | - | blaOXA-1 (1) | - | - | - | 1 | - |
| Agona | - | - | - | - | - | 1 | - | - | - |
| Montevideo | - | - | blaTEM-1 (2) | - | - | - | - | 1 | - |
| Stanley | - | - | - | - | - | - | - | 1 | - |
| Total | 11 (32.4%) | 5 (14.7%) | 27 (79.4%) | 10 (29.4%) | 9 (26.5%) | 2 (5.9%) | 8 (23.5%) | 12 (35.3%) | 7 (20.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adel, W.A.; Ahmed, A.M.; Hegazy, Y.; Torky, H.A.; Shimamoto, T. High Prevalence of ESBL and Plasmid-Mediated Quinolone Resistance Genes in Salmonella enterica Isolated from Retail Meats and Slaughterhouses in Egypt. Antibiotics 2021, 10, 881. https://doi.org/10.3390/antibiotics10070881
Adel WA, Ahmed AM, Hegazy Y, Torky HA, Shimamoto T. High Prevalence of ESBL and Plasmid-Mediated Quinolone Resistance Genes in Salmonella enterica Isolated from Retail Meats and Slaughterhouses in Egypt. Antibiotics. 2021; 10(7):881. https://doi.org/10.3390/antibiotics10070881
Chicago/Turabian StyleAdel, Wesam A., Ashraf M. Ahmed, Yamen Hegazy, Helmy A. Torky, and Tadashi Shimamoto. 2021. "High Prevalence of ESBL and Plasmid-Mediated Quinolone Resistance Genes in Salmonella enterica Isolated from Retail Meats and Slaughterhouses in Egypt" Antibiotics 10, no. 7: 881. https://doi.org/10.3390/antibiotics10070881
APA StyleAdel, W. A., Ahmed, A. M., Hegazy, Y., Torky, H. A., & Shimamoto, T. (2021). High Prevalence of ESBL and Plasmid-Mediated Quinolone Resistance Genes in Salmonella enterica Isolated from Retail Meats and Slaughterhouses in Egypt. Antibiotics, 10(7), 881. https://doi.org/10.3390/antibiotics10070881






