Antibacterial Peptides Produced by Alcalase from Cowpea Seed Proteins
Abstract
1. Introduction
2. Results
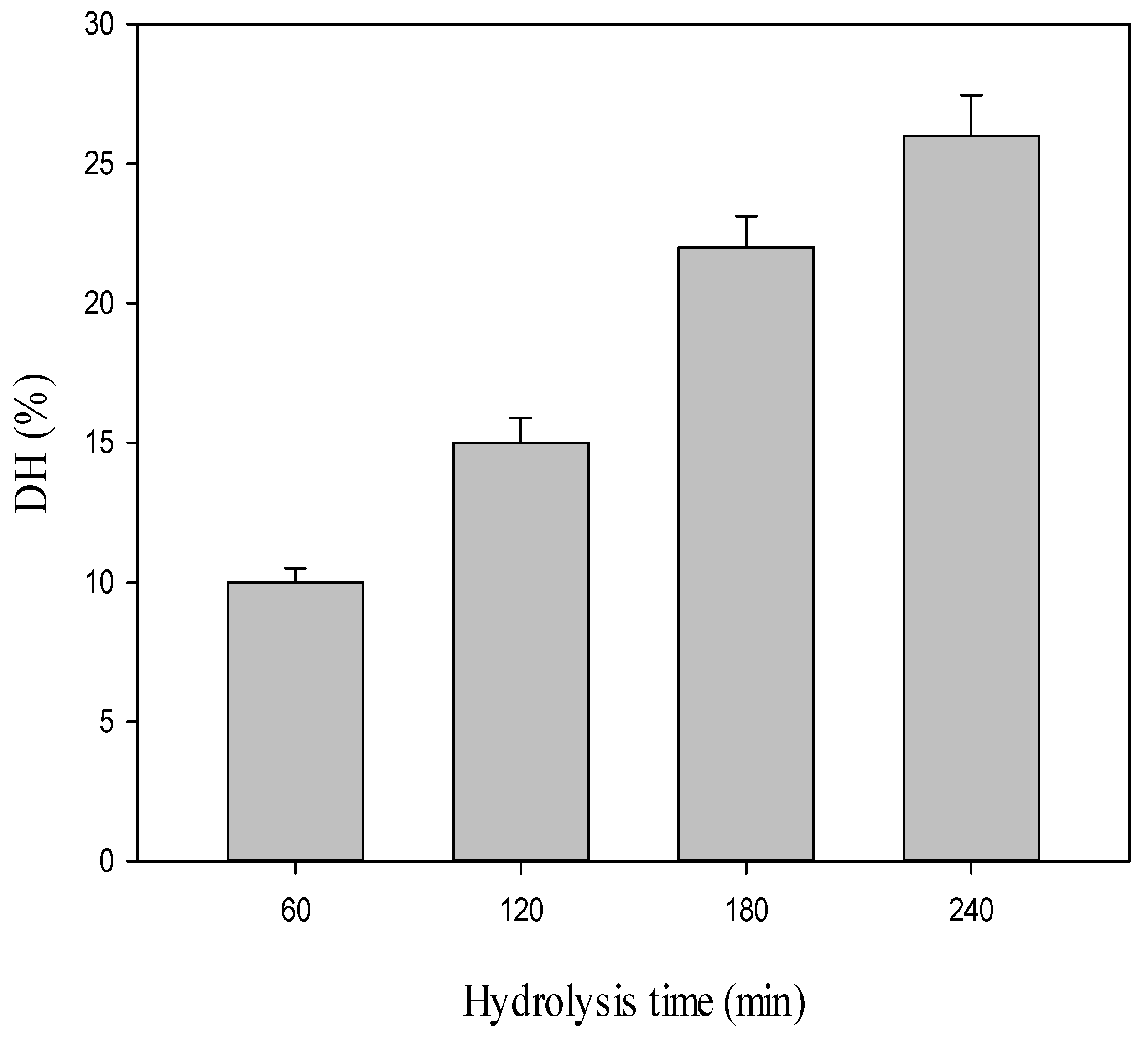
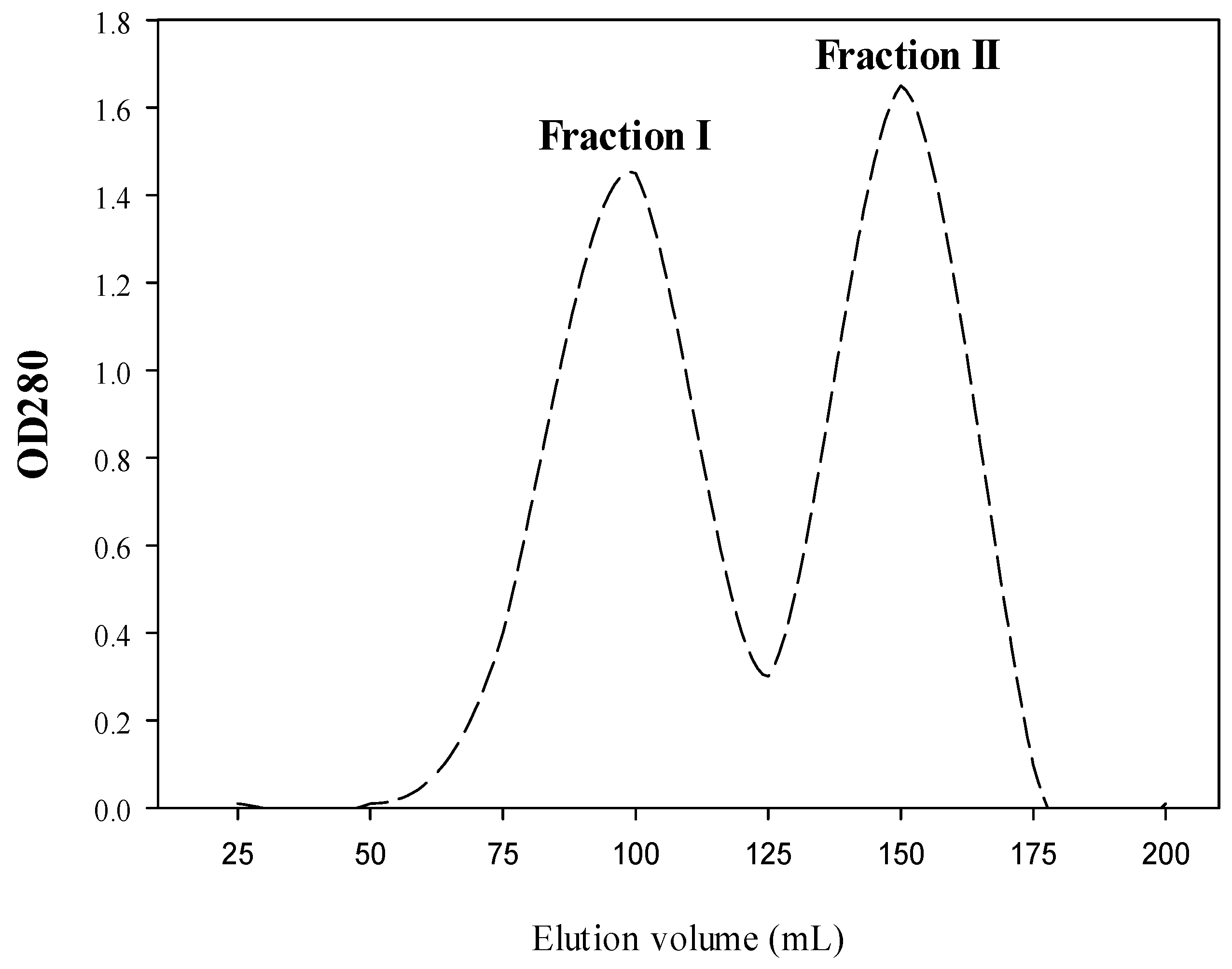
2.1. Production of Both CPH and SEC-Fractions
2.2. Electro-Spray-Ionization-Mass-Spectrometry (ESI-MS)of SEC-F1
2.3. Antibacterial Activity
2.4. Minimum Inhibitory Concentration (MIC) of Both CPHs and SEC-Fractions
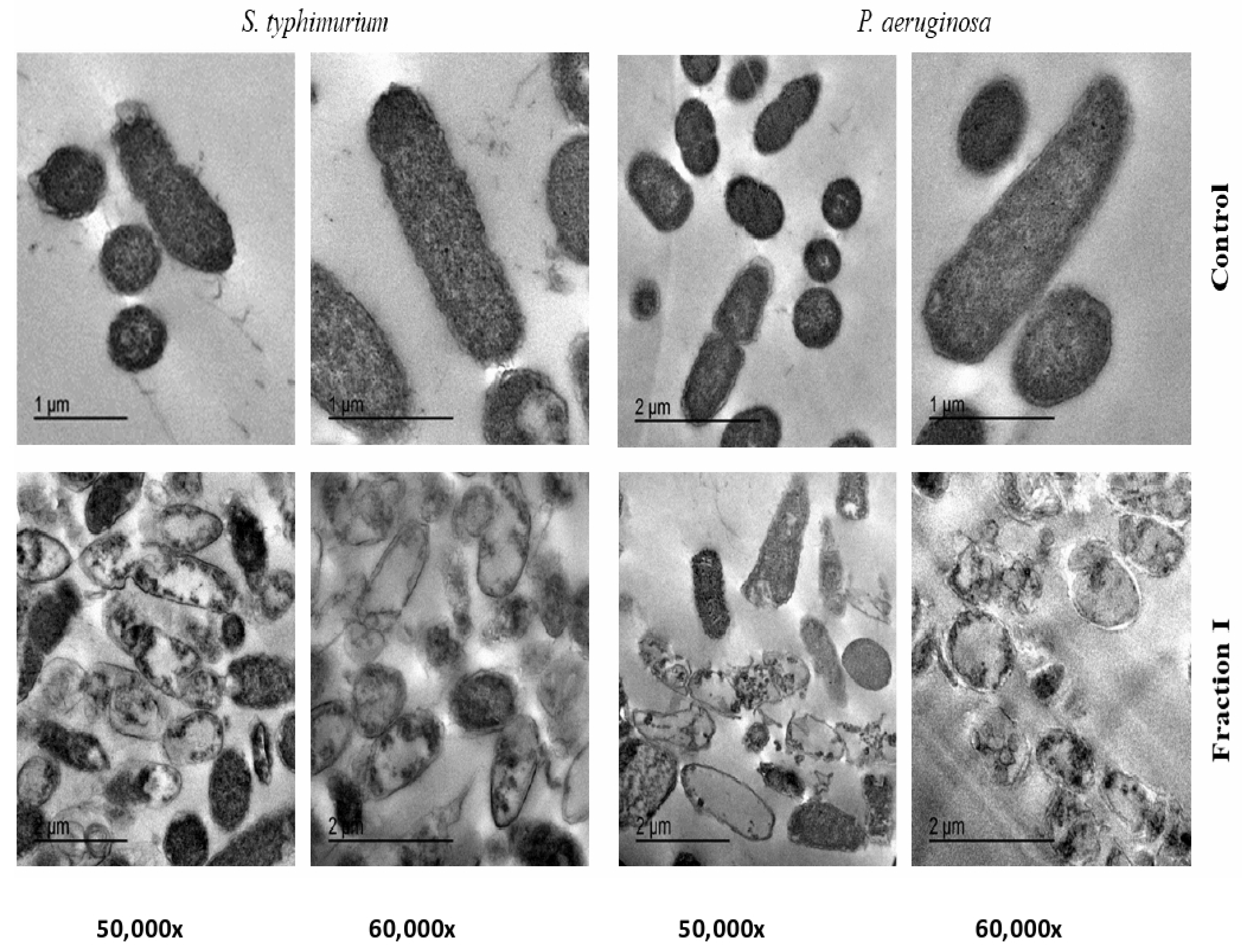
2.5. Transmission Electron Microscope (TEM) of SEC-F1
3. Discussion
4. Materials and Methods
4.1. Collection of Pathogenic Bacteria
4.2. Plant Materials and Chemicals
4.3. Cowpea Protein Isolation
4.4. Cowpea Seed Protein Hydrolysates Preparation
4.5. Fractionation of CPH by Size Exclusion Chromatography (SEC)
4.6. Electro-Spray-Ionization-Mass-Spectrometry (ESI-MS) of SEC-F1
4.7. Antibacterial Activityof CPH and Fractions Obtained by SEC
4.8. Minimum Inhibitory Concentration (MIC) Determination of Both CPH and SEC-F1
4.9. Transmission Electron Microscope (TEM) of SEC-F1
4.10. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Enan, G.; Abdel-Shafi, S.; Ouda, S.; Negm, S. Novel antibacterial activity of Lactococcus lactis subspecies Lactis Z11 isolated from Zabady. Int. J. Biomed. Sci. 2013, 9, 174–180. [Google Scholar]
- Abdel-shafi, S.; Ouda, S.M.; Elbalat, I.; Enan, G. Characterization and identification of multidrug resistant bacteria from some Egyptian patients. Biotechnology 2013, 12, 65–73. [Google Scholar] [CrossRef]
- El-Sayed, T.I.; Atef, D.; Amer, M.; Mahdy, A.; Enan, G. Molecular characterization and inhibition by natural agents of multidrug resistant Candida strains causing vaginal candidiasis. Res. J. Med. Sci. 2015, 9, 1–7. [Google Scholar]
- Enan, G.; Osman, M.E.; Abdel-Haliem, M.E.F.; Abdel-Ghany, S.E. Advances in microbial and nuclei acids biotechnology. BioMed Res. Int. 2018, 2018, 3102374. [Google Scholar] [CrossRef]
- Mune, M.A.M. Influence of Degree of Hydrolysis on the Functional Properties of Cowpea Protein Hydrolysates. J. Food Process. Preserv. 2015, 39, 2386–2392. [Google Scholar] [CrossRef]
- Enan, G.; Seham, A.S.; Abdel-Halem, M.F.; Negm, S. Characterization of probiotic lactic acid bacteria to be used as starter and protective cultures for dairy fermentations. Int. J. Probiotics Prebiotics 2013, 8, 157–163. [Google Scholar]
- Abdel-Shafi, S.; Osman, A.; Enan, G.; El-Nemer, M.; Sitohy, M. Antibacterial activity of methylated egg white proteins against pathogenic G+ and G− bacteria matching antibiotics. SpringerPlus 2016, 5, 983. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafi, S.; Al-Mohammadi, A.R.; Almanaa, T.N.; Moustafa, A.H.; Saad, T.M.M.; Ghonemy, A.; Anacarso, I.; Enan, G.; El-Gazzar, N. Identification and testing antidermatophyticoxaborole-6-derivative (OXBS) from Streptomyces atrovirens KM192347 isolated from soil. Antibiotics 2020, 9, 176. [Google Scholar] [CrossRef]
- Power, O.; Nongonierma, A.B.; Jakeman, P.; FitzGerald, R.J. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc. Nutr. Soc. 2013, 73, 34–46. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, N.S.; Enan, G. Advances in Phage Inspired Nanosciencebased Therapy. In Nanobioscience; Saxena, S.K., Khurana, S.P., Eds.; Springer Nature Singapore PteLtd.: Singapore, 2020. [Google Scholar]
- El-Gazzar, N.; Almanaa, T.N.; Reda, R.M.; El Gaafary, M.; Rashwan, A.; Mahsoub, F. Assessment the using of silica nanoparticles (SiO2NPs) biosynthesized from rice husks by Trichoderma harzianum MF780864 as water lead adsorbent for immune status of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2021. [Google Scholar] [CrossRef]
- Askoura, M.; Saed, N.; Enan, G.; Askora, A. Characterization of polyvalent bacteriophages targeting multidrug resistant Klebsiella pneumonia with enhanced anti-biofilm activity. Appl. Biochem. Microbiol. 2021, 57, 117–126. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Osman, A.; Enan, G.; Abdel-Hameid, S.; Sitohy, M. Characterization and Antibacterial Activity of 7S and 11S Globulins Isolated from Cowpea Seed Protein. Molecules 2019, 24, 1082. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Mahgoub, S.; Osman, A. In vitro and in situ antimicrobial action and mechanism of glycinin and its basic subunit. Int. J. Food Microbiol. 2012, 154, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.; Osman, A. Bioactive Compounds in Soybean Proteins and Its Applications in Food Systems. In Sustainability of Agricultural Environment in Egypt: Part I; Springer: Cham, Switzerland, 2018; pp. 147–160. [Google Scholar] [CrossRef]
- Osman, A.O.; Mahgoub, S.A.; Sitohy, M.Z. Preservative action of 11S (glycinin) and 7S (β-conglycinin) soy globulin on bovine raw milk stored either at 4 or 25 °C. J. Dairy Res. 2013, 80, 174–183. [Google Scholar] [CrossRef]
- Abbas, E.; Osman, A.; Sitohy, M. Biochemical control of Alternaria tenuissima infecting post-harvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020, 100, 2889–2897. [Google Scholar] [CrossRef]
- Enan, G. Behaviour of Listeria monocytogenes LMG 10470 in Poultry Meat and its Control by the Bacteriocin Plantaricin UG 1. Int. J. Poult. Sci. 2006, 5, 355–359. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Separovic, F.; O’Brien-Simpson, M.N.; Wade, D.J. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef]
- Sitohy, M.; Osman, A. Antimicrobial activity of native and esterified legume proteins against Gram-negative and Gram-positive bacteria. Food Chem. 2010, 120, 66–73. [Google Scholar] [CrossRef]
- Osman, A.; Mahgoub, S.; Sitohy, M. Hindering milk quality storage deterioration by mild thermization combined with methylated chickpea protein. Int. Food Res. J. 2014, 21, 693–701. [Google Scholar]
- Osman, A.; Mahgoub, S.; El-Masry, R.; Al-Gaby, A.; Sitohy, M. Extending the technological validity of Raw Buffalo Milk at room temperature by esterified legume proteins. J. Food Process. Preserv. 2014, 38, 223–231. [Google Scholar] [CrossRef]
- Mahgoub, S.A.; Sitohy, M.Z.; Osman, A.O. Counteracting Recontamination of Pasteurized Milk by Methylated Soybean Protein. Food Bioprocess Technol. 2011, 6, 101–109. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Osman, A.O. Enhancing Milk Preservation with Esterified Legume Proteins. Probiotics Antimicrob. Proteins 2011, 3, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Goda, H.A.; Abdel-Hamid, M.; Badran, S.M.; Otte, J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. LWT 2016, 65, 480–486. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Hanan, A.; Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Saporito, P.; Osman, A.; Mateiu, R.V.; Mojsoska, B.; Jenssen, H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control 2020, 111, 107056. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.-S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Segura Campos, M.R.; Chel Guerrero, L.A.; Betancur Ancona, D.A. Angiotensin-I converting enzyme inhibitory and antioxidant activities of peptide fractions extracted by ultrafiltration of cowpea Vigna unguiculata hydrolysates. J. Sci. Food Agric. 2010, 90, 2512–2518. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Chel-Guerrero, L.; Betancur-Ancona, D.A. Purification of angiotensin I-converting enzyme inhibitory peptides from a cowpea (Vigna unguiculata) enzymatic hydrolysate. Process. Biochem. 2011, 46, 864–872. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Jiménez-Morales, K.; Quintal-Novelo, C.; Moo-Puc, R.; Chel-Guerrero, L.; Betancur-Ancona, D. Enzymatic protein hydrolysates and ultrafiltered peptide fractions from Cowpea Vigna unguiculata L. bean with in vitro antidiabetic potential. J. Iran. Chem. Soc. 2019, 16, 1773–1781. [Google Scholar] [CrossRef]
- Enan, G.; Al-Mohammadi, A.-R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.; Taha, M.; El-Gazzar, N. Inhibition of Staphylococcus aureus LC554891 by Moringa oleifera Seed Extract either Singly or in Combination with Antibiotics. Mol. 2020, 25, 4583. [Google Scholar] [CrossRef]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Marques, M.; Fontanari, G.; Pimenta, D.; Soares-Freitas, R.M.; Arêas, J.A.G. Proteolytic hydrolysis of cowpea proteins is able to release peptides with hypocholesterolemic activity. Food Res. Int. 2015, 77, 43–48. [Google Scholar] [CrossRef]
- Marques, M.; Freitas, R.A.M.S.; Carlos, A.C.C.; Siguemoto, É.S.; Fontanari, G.; Arêas, J.A.G. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2015, 168, 288–293. [Google Scholar] [CrossRef]
- Enan, G.; Abdelshafi, S.; Ouda, S.M.; El-Balat, I. Genetic linkage of the antibiotic resistance ability in the Echerichia coli uR4 strain isolated from urine. J. Med. Sci. 2014, 3, 261–268. [Google Scholar]
- Sukan, G.; Andrews, A.T. Application of the plastein reaction to caseins and to skim-milk powder: I. Protein hydrolysis and plastein formation. J. Dairy Res. 1982, 49, 265–278. [Google Scholar] [CrossRef]
- Naima, N.; Karima, H.; Gabrielle, C.; Rafik, B.; Moncef, N.; Pascal, D.; Ali, B. Antibacterial peptides from barbel muscle protein hydrolysates: Activity against some pathogenic bacteria. LWTFood Sci. Technol. 2014, 55, 183–188. [Google Scholar]
- Al-Mohammadi, A.-R.; Osman, A.; Enan, G.; Abdel-Shafi, S.; El-Nemer, M.; Sitohy, M.; Taha, M. Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics 2020, 9, 901. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Lin, H.-L.; Shiu, Y.-L.; Tyan, Y.-C.; Liu, C.-H. Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing Vibrio infection in shrimp aquaculture. Fish Shellfish Immunol. 2017, 67, 270–279. [Google Scholar] [CrossRef]
- Xu, S.; Shen, Y.; Li, Y. Antioxidant Activities of Sorghum KafirinAlcalase Hydrolysates and Membrane/Gel Filtrated Fractions. Antioxidants 2019, 8, 131. [Google Scholar] [CrossRef]
- Yust, M.D.M.; Pedroche, J.; Millán-Linares, M.D.C.; Hidalgo, J.M.A.; Millán, F. Improvement of functional properties of chickpea proteins by hydrolysis with immobilisedAlcalase. Food Chem. 2010, 122, 1212–1217. [Google Scholar] [CrossRef]
- Cumby, N.; Zhong, Y.; Naczk, M.; Shahidi, F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008, 109, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Rao, G.N.; Rao, D.G.; Jyothirmay, T. Protein hydrolysates from meriga (Cirrhinusmrigala) egg and evaluation of their functional properties. Food Chem. 2010, 120, 652–657. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Rinaldi, A.C. Antimicrobial Peptides: The LPS Connection. In Antimicrobial Peptides; Humana Press: Totowa, NJ, USA, 2010; pp. 137–154. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Osman, A.; Al-Mohammadi, A.-R.; Enan, G.; Kamal, N.; Sitohy, M. Biochemical, biological characteristics and antibacterial activity of glycoprotein extracted from the epidermal mucus of African catfish (Clariasgariepinus). Int. J. Biol. Macromol. 2019, 138, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Surówka, K.; Żmudziński, D.; Surówka, J. Enzymic modification of extruded soy protein concentrates as a method of obtaining new functional food components. Trends Food Sci. Technol. 2004, 15, 153–160. [Google Scholar] [CrossRef]
- MuneMunea, A.M.; Minkab, R.S. Production and characterization of cowpea protein hydrolysate with optimum nitrogen solubility by enzymatic hydrolysis using pepsin. J. Sci. Food Agric. 2017, 97, 2561–2568. [Google Scholar] [CrossRef]
- Sitohy, M.; Mahgoub, S.; Osman, A.; El-Masry, R.; Al-Gaby, A. Extent and Mode of Action of Cationic Legume Proteins against Listeria monocytogenes and Salmonella Enteritidis. Probiotics Antimicrob. Proteins 2013, 5, 195–205. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.-R.; Ibrahim, R.; Moustafa, A.; Ismaiel, A.; Zeid, A.A.; Enan, G. Chemical Constitution and Antimicrobial Activity of Kefir Fermented Beverage. Molecules 2021, 26, 2635. [Google Scholar] [CrossRef]
- Enan, G.; El-Essawy, A.A.; Uyttendael, M.; Debevere, J. Antibacterial activity of Lactobacillus planetarium UG1 isolated from dry sausage: Characterization, production and bactericidal action of plantaricin UG1. Int. J. Food Microbiol. 1996, 30, 189–215. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Otte, J.; Abdel-Hamid, M.; Osman, A. Comparative Assessment of Peptide Concentration in Milk Protein Hydrolysates and Fractions. Int. J. Dairy Sci. 2015, 10, 228–235. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers LTD: Essex, UK, 1986. [Google Scholar]
- Bauer, A.T. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, R.; Negm, S.; Enan, G. Antibacterial activity of Lactobacillus delbreukii subspecies bulgaricus isolated from Zabady. Life Sci. J. 2014, 11, 264–270. [Google Scholar]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Sitohy, M.; Mosa, B.; Ismaiel, A.; Enan, G.; Osman, A. Antimicrobial Activity and Chemical Constitution of the Crude, Phenolic-Rich Extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- El-Gazzar, N.; Ismail, A.M. The potential use of Titanium, Silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 2020, 27, 101708. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almaary, K.H.; Ismail, A.; Polizzi, G. Influence of Funneliformismosseae enhanced with titanium dioxide nanoparticles (TiO2NPs) on Phaseolus vulgaris L. Under salinity stress. PLoS ONE 2020, 15, e0235355. [Google Scholar]
- El-Bahr, S.; Elbakery, A.; El-Gazzar, N.; Amin, A.; Al-Sultan, S.; Alfattah, M.; Shousha, S.; Alhojaily, S.; Shathele, M.; Sabeq, I.; et al. Biosynthesized Iron Oxide Nanoparticles from Petroselinum crispum Leaf Extract Mitigate Lead-Acetate-Induced Anemia in Male Albino Rats: Hematological, Biochemical and Histopathological Features. Toxics 2021, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Enan, G.; Al-Mohammadi, A.-R.; Moustafa, H.A.; El-Gazzar, N. Detection, Purification and Elucidation of Chemical Structure and Antiproliferative Activity of Taxol Produced by Penicillium chrysogenum. Molecules 2020, 25, 4822. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; El-Gazzar, N.; Almanaa, T.N.; El-Hadary, A.; Sitohy, M. Lipolytic Postbiotic from Lactobacillus paracasei Manages Metabolic Syndrome in Albino Wistar Rats. Molecules 2021, 26, 472. [Google Scholar] [CrossRef] [PubMed]
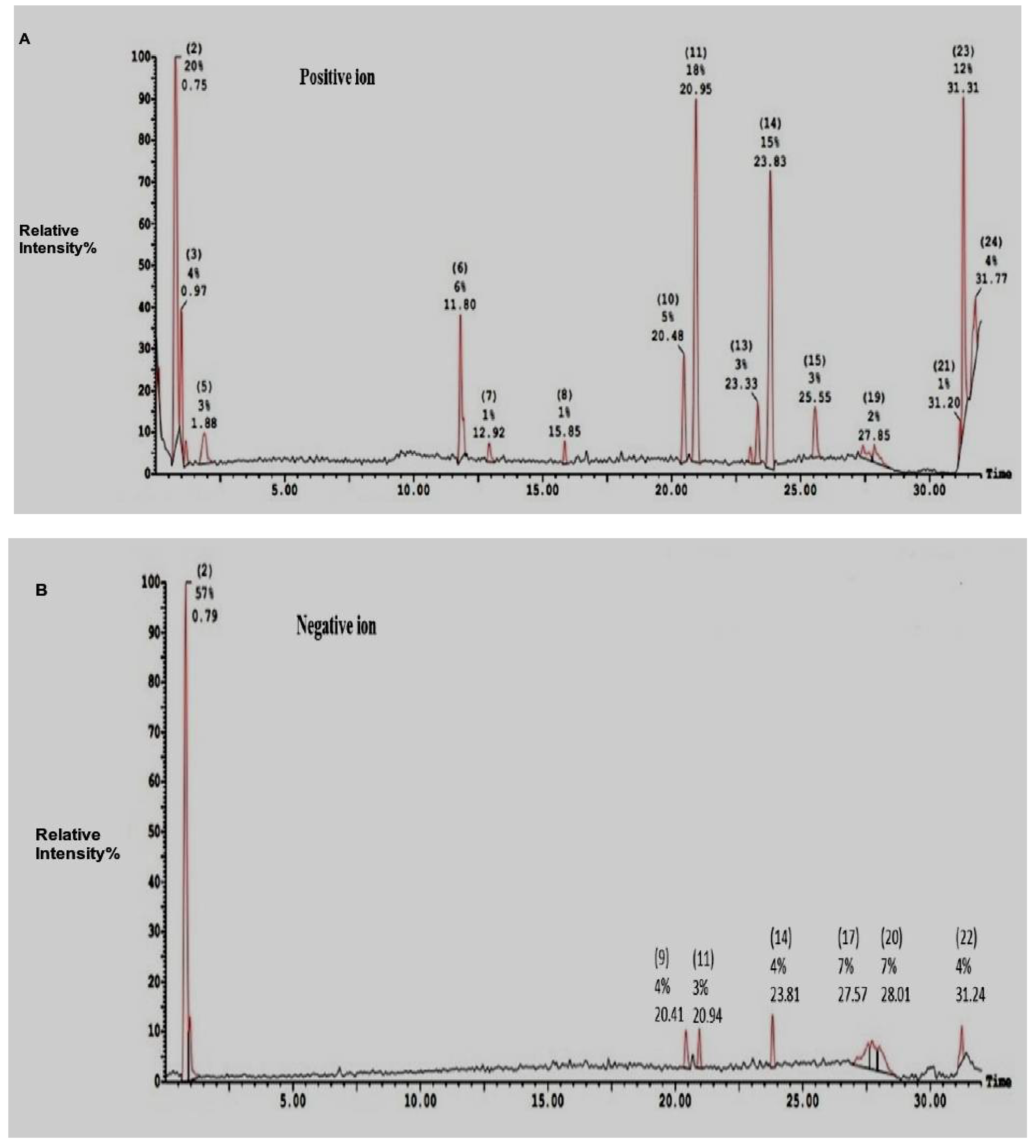
| Ions Mode/Peaks Number | Area (%) | Molecular Weight | Composition | |
|---|---|---|---|---|
| Positive ions | 2 | 20.20 | 707.32 (364) | Cys am-Trp |
| 3 | 4.10 | 707.28 (364) | Cys am-Trp | |
| 5 | 2.57 | 192.05 | Ser-Cys | |
| 6 | 6.46 | 274.20 | Trp-Ser | |
| 10 | 4.57 | 331.28 | Cys-Arg | |
| 11 | 18.28 | 659.43 (312 & 318) | His-Cysam & Trp-Met & Arg-Cys am | |
| 13 | 2.54 | 359.31 | Trp-Cys am | |
| 14 | 15.29 | 359.31 | Trp-Cys am | |
| 23 | 12.09 | 184.94 | Leu-Ala | |
| 226.89 | Gln-Pro | |||
| 24 | 3.86 | 214.06 | Asn-Val; Arg-Gly | |
| Negative ions | 2 | 57.28 | 719.35 fragmented into (365) | Trp-Cys am |
| 9 | 3.94 | 373 | Trp-Trp | |
| 11 | 3.27 | 656.97 fragmented into (318) | Trp-Met & Arg-Cys am | |
| 17 | 7.07 | 1133.01 fragmented into (330) | Arg-Cys am | |
| 20 | 7.31 | 532.97 fragmented into (332) | Phe-Trp | |
| Bacteria | Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|---|
| Ciprofloxacin (100µg/mL) | CPH | SEC-Fraction 1 | SEC-Fraction 2 | ||
| G +VeBacteria | St. pyogenes | 20.0 ± 0.19 | 24.70 a ± 0.51 | 26.50 a ± 0.67 | 8.50 b ± 0.31 |
| L. monocytogens | 23.0 ± 0.45 | 22.30 a ± 0.47 | 21.50 b ± 0.34 | 00.00 | |
| L. innocua | 22.0 ± 0.47 | 21.50 b ± 0.67 | 22.60 a ± 0.67 | 7.60 c ± 0.27 | |
| S. aureus | 18.0 ± 0.22 | 26.30 a ± 0.34 | 23.50 b ± 0.66 | 00.00 | |
| G-VeBacteria | S. typhimurium | 21.0 ± 0.12 | 24.30 a ± 0.31 | 21.63 b ± 0.12 | 9.00 c ± 0.11 |
| k. pneumonia | 18.0 ± 0.21 | 20.80 b ± 0.19 | 21.50 a ± 0.81 | 8.300 c ± 0.17 | |
| P. auriginosa | 21.0 ± 0.12 | 25.30 a ± 0.12 | 18.3.50 b ± 0.22 | 9.30 c ± 0.61 | |
| E. coli | 18.0 ± 0.22 | 17.3.50 b ± 0.57 | 23.50 a ± 0.45 | 8.60 c ± 0.86 | |
| Microorganisms | Inhibition Zone Diameter (mm/µg mL−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 250 | 500 | 1000 | |||||||
| CPH | F1 | CPH | F1 | CPH | F1 | CPH | F1 | CPH | F1 | CPH | F1 | |
| S. typhimurum | 11.0d± 0.3 | 15.0c ± 0.1 | 17.0b ± 0.3 | 18.0b ± 0.5 | 18.0b ± 0.1 | 18.6b ± 0.4 | 21.0b ± 0.5 | 22.0b ± 0.1 | 21.0c ± 0.7 | 29.0a ± 0.6 | 22.0d ± 0.5 | 29.6a ± 0.5 |
| K. pneumoniae | 15.6b± 0.3 | 12.0d ± 0.1 | 15.7c ± 0.8 | 12.7c ± 0.2 | 16.0 c ± 0.3 | 14.0 c ± 0.7 | 20.0b ± 0.6 | 15.0d ± 0.9 | 21.0c ± 0.4 | 18.0d ± 0.2 | 24.0c ± 0.4 | 2.02c ± 0.3 |
| St. pyogenes | 13.0c± 0.7 | 24.0a ± 0.4 | 17.9b ± 0.8 | 26.0a ± 0.7 | 20.0a ± 0.7 | 28.0 a ± 0.1 | 24.0a ± 0.2 | 18.0c ± 0.3 | 26.0b ± 0.1 | 20.0c ± 0.2 | 28.3b ± 0.3 | 27.0b ± 0.4 |
| L.monocytogen | 9.0e± 0.1 | 9.0e ± 0.3 | 9.5d ± 0.2 | 10.3d ± 0.3 | 10.0d ± 0.8 | 13.0 c ± 0.8 | 23.0ab ± 0.1 | 15.3d ± 0.4 | 18.3d ± 02 | 25.0b ± 0.1 | 19.6e ± 0.8 | 20.3d ± 0.1 |
| L. innocua | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 11.3d ± 0.2 | 14.0c ± 0.5 | 12.7 ± 0.3 | 20.3c ± 0.4 | 13.3f ± 0.5 | 22.0d ± 0.2 | 22.0c ± 0.1 |
| P. aeruginosa | 18.0a± 0.5 | 18.0b ± 0.3 | 19.0a ± 0.4 | 19.0b ± 0.2 | 19.3a ± 0.5 | 19.3b ± 0.4 | 23.0ab ± 0.6 | 23.0b ± 0.9 | 20.00c ± 3 | 20.0c ± 0.2 | 20.0e ± 0.5 | 16.7e ± 0.2 |
| S. aureus | 0.0 | 0.0 | 12.3 ± 0.4 | 0.0 | 9.0d ± 0.93 | 11.0d ± 0.7 | 13.0c ± 0.3 | 13.0e ± 0.2 | 22.0c ± 0.3 | 16.0e ± 0.5 | 31.0a ± 0.8 | 17.7e ± 0.4 |
| E. coli | 15.0b± 0.15 | 24.0a ± 0.3 | 20.6a ± 0.3 | 25.7a ± 0.1 | 19.3a ± 0.1 | 27.0a ± 0.5 | 26.0a ± 0.4 | 29.0a ± 0.1 | 30.0a ± 0.4 | 31.0a ± 0.4 | 32.0a ± 0.7 | 31.3a ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, A.; Enan, G.; Al-Mohammadi, A.-R.; Abdel-Shafi, S.; Abdel-Hameid, S.; Sitohy, M.Z.; El-Gazzar, N. Antibacterial Peptides Produced by Alcalase from Cowpea Seed Proteins. Antibiotics 2021, 10, 870. https://doi.org/10.3390/antibiotics10070870
Osman A, Enan G, Al-Mohammadi A-R, Abdel-Shafi S, Abdel-Hameid S, Sitohy MZ, El-Gazzar N. Antibacterial Peptides Produced by Alcalase from Cowpea Seed Proteins. Antibiotics. 2021; 10(7):870. https://doi.org/10.3390/antibiotics10070870
Chicago/Turabian StyleOsman, Ali, Gamal Enan, Abdul-Raouf Al-Mohammadi, Seham Abdel-Shafi, Samar Abdel-Hameid, Mahmoud Z. Sitohy, and Nashwa El-Gazzar. 2021. "Antibacterial Peptides Produced by Alcalase from Cowpea Seed Proteins" Antibiotics 10, no. 7: 870. https://doi.org/10.3390/antibiotics10070870
APA StyleOsman, A., Enan, G., Al-Mohammadi, A.-R., Abdel-Shafi, S., Abdel-Hameid, S., Sitohy, M. Z., & El-Gazzar, N. (2021). Antibacterial Peptides Produced by Alcalase from Cowpea Seed Proteins. Antibiotics, 10(7), 870. https://doi.org/10.3390/antibiotics10070870








