Quality Appraisal of Ambulatory Oral Cephalosporin and Fluoroquinolone Use in the 16 German Federal States from 2014–2019
Abstract
:1. Introduction
2. Results
2.1. Quality Assessment of Oral Antibiotic Use in 2019
2.2. Use of Cephalosporins in 2019
2.3. Use of Fluoroquinolones in 2019
2.4. Assessment of Federal States Based on Quality Indicators
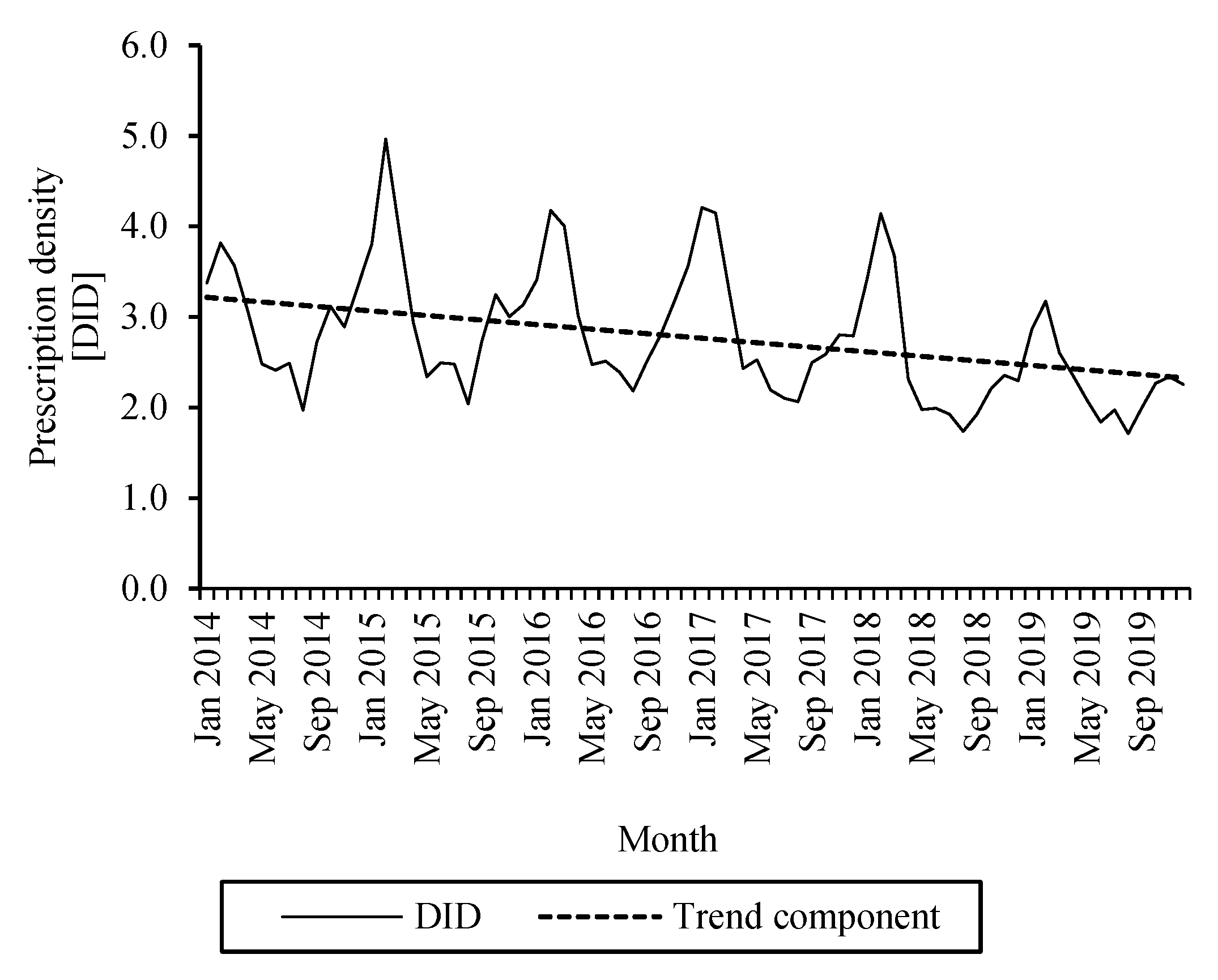
2.5. Changes in the Use of Cephalosporins from 2014–2019
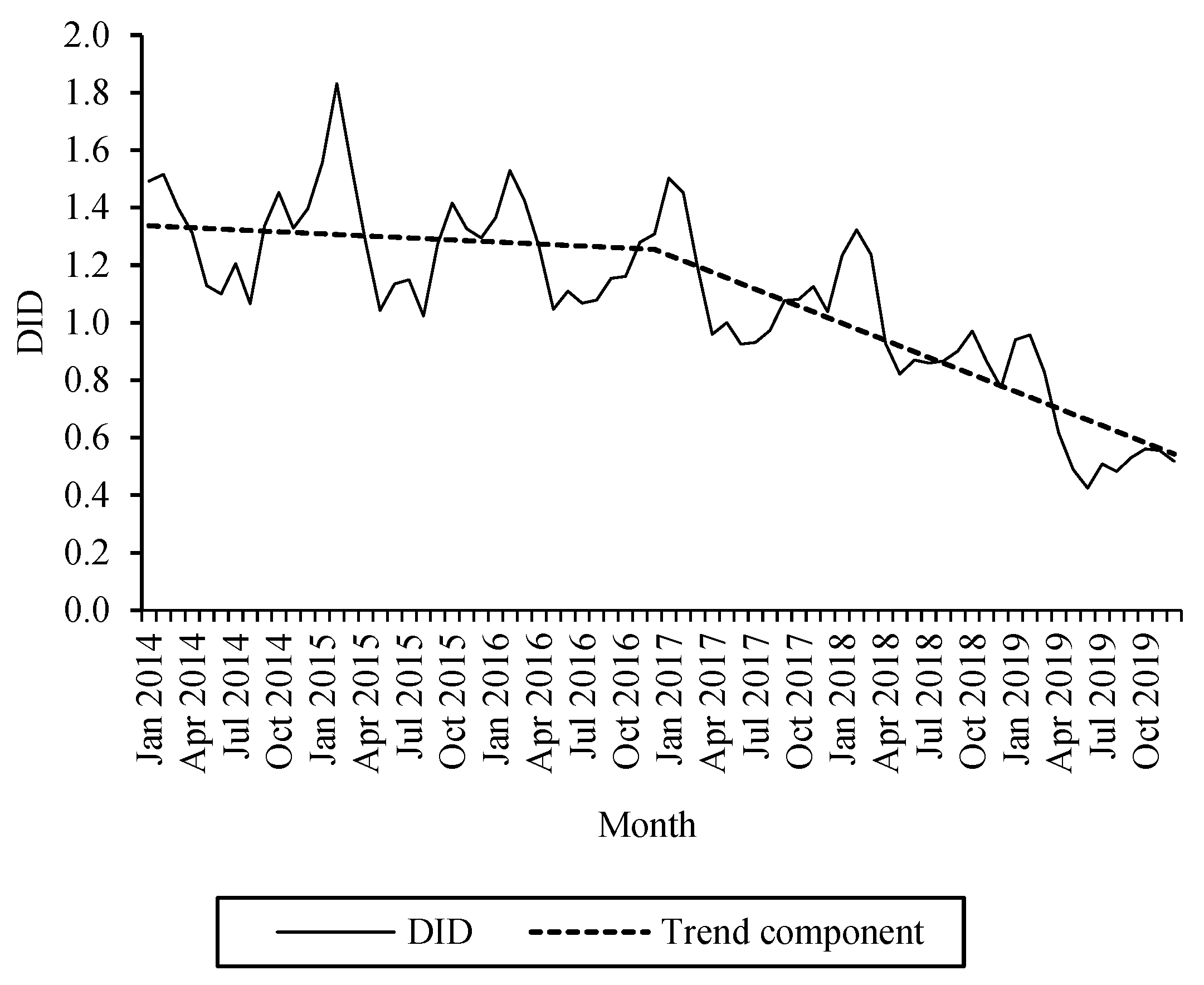
2.6. Changes in the Use of Fluoroquinolones from 2014–2019
3. Discussion
3.1. Quality of Oral Cephalosporin Use
3.2. Quality of Oral Fluoroquinolone Use
3.3. Conclusions and Recommendations towards Measures for Rational Use of Antibiotics
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Study Design
4.2. Measurement of Antibiotic Use
4.3. Assessment of the Quality of Antibiotic Drug Use
4.4. Trend Analysis of Oral Cephalosporin and Fluoroquinolone Use
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Pharmaceutical Strategy for Europe COM/2020/761 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0761&from=EN (accessed on 7 July 2021).
- Federal Office of Consumer Protection and Food Safety, Paul-Ehrlich-Gesellschaft für Chemotherapie e.V. GERMAP 2015—Bericht über den Antibiotikaverbrauch und Die Verbreitung von Antibiotikaresistenzen in der Human- und Veterinärmedizin in Deutschland, Rheinbach: Antiinfectives Intelligence. 2016. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/05_Tierarzneimittel/germap2015.html (accessed on 6 July 2021).
- Federal Ministry of Health. DART Deutsche Antibiotika-Resistenzstrategie. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Berichte/Bericht_DART_Deutsche_Antibiotika-Resistenzstrategie.pdf (accessed on 7 July 2021).
- Helmholtz Centre for Infection Research. Wirksamkeit von Antibiotika-Schulungen in der Niedergelassenen Ärzteschaft (WASA). Available online: http://www.wasa.helmholtz-hzi.de/de/home (accessed on 4 February 2021).
- aQua-Institute. CHANGE-3—Converting Habits of Antibiotic Use for Respiratory Tract Infections in German Primary Care. Available online: https://www.klinikum.uni-heidelberg.de/kliniken-institute/institute/abteilung-allgemeinmedizin-und-versorgungsforschung/forschung/schwerpunkte/projekte/arzneimitteleinsatz/change-3-converting-habits-of-antibiotic-use-for-respiratory-tract-infections-in-german-primary-care (accessed on 4 February 2021).
- German College of General Practitioners and Family Physicians. Halsschmerzen—S3-Leitlinie, AWMF-Reg.-Nr. 053-010. Available online: https://www.awmf.org/uploads/tx_szleitlinien/053-010l-S3_Halsschmerzen_2021-01.pdf (accessed on 7 July 2021).
- German Society of Oto-Rhino-Laryngology, Head and Neck Surgery, German College of General Practitioners and Family Physicians. S2k Leitlinie: Rhinosinusitis, AWMF-Reg.-Nr. 017/049 (HNO) und 053/012 (DEGAM). 2017. Available online: https://www.awmf.org/uploads/tx_szleitlinien/017-049_und_053-012l_S2k_Rhinosinusitis_2019-04_01.pdf (accessed on 7 July 2021).
- Borgmann, S.; Jakobiak, T.; Gruber, H.; Reil, M.; Schröder, H.; Kist, M.; Sagel, U. Association of ciprofloxacin prescriptions to outpatients to Clostridium difficile infections. Eurosurveillance 2010, 15, 19479. [Google Scholar] [CrossRef]
- Nakai, H.; Hagihara, M.; Kato, H.; Hirai, J.; Nishiyama, N.; Koizumi, Y.; Sakanashi, D.; Suematsu, H.; Yamagishi, Y.; Mikamo, H. Prevalence and risk factors of infections caused by extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. J. Infect. Chemother. 2016, 22, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Gatermann, S.; Kresken, M.; Kern, W.V. Grenzwerte sind hilfreich [Limit values are helpful]. Dtsch. Arztebl. 2017, 114, A-1314. [Google Scholar]
- World Health Organization. WHO Releases the 2019 AWaRe Classification Antibiotics. Available online: https://www.who.int/publications/i/item/WHOEMPIAU2019.11 (accessed on 24 April 2021).
- Federal Institute for Drugs and Medical Devices. Rote-Hand-Brief zu Fluorchinolon-Antibiotika: Schwerwiegende und Anhaltende, die Lebensqualität Beeinträchtigende und Möglicherweise Irreversible Nebenwirkungen. Available online: https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/DE/RHB/2019/rhb-fluorchinolone.html (accessed on 18 September 2020).
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Kern, W.V.; Steffen, A.; Bätzing, J. The decline in outpatient antibiotic use. Dtsch. Aerzteblatt Online 2020, 117, 679–686. [Google Scholar] [CrossRef]
- Kern, W.V.; De With, K.; Nink, K.; Steib-Bauert, M.; Schröder, H. Regional variation in outpatient antibiotic prescribing in Germany. Infection 2006, 34, 269–273. [Google Scholar] [CrossRef]
- Bätzing-Feigenbaum, J.; Schulz, M.; Schulz, M.; Hering, R.; Kern, W.V. Outpatient antibiotic prescription. Dtsch. Arztebl. 2016, 113, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Gradl, G.; Teichert, M.; Kieble, M.; Werning, J.; Schulz, M. Comparing outpatient oral antibiotic use in Germany and the Netherlands from 2012 to 2016. Pharmacoepidemiol. Drug Saf. 2018, 27, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Trend of Antimicrobial Consumption by Country. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/trend-country (accessed on 29 June 2021).
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Steffen, A.; Bätzing, J. Update: Die Ambulante Anwendung Systemischer Antibiotika in Deutschland im Zeitraum 2010 bis 2018—Eine Populationsbasierte Studie. Available online: https://www.versorgungsatlas.de/fileadmin/ziva_docs/104/VA_19-07_Bericht_UpdateAntibiotika_2019-08-21V1.pdf (accessed on 7 July 2021).
- Schulz, M.; Kern, W.V.; Hering, R.; Bätzig-Feigenbaum, J. Antibiotikaverordnungen in der Ambulanten Versorgung in Deutschland bei Bestimmten Infektionserkrankungen; Teil 2—Krankheitsspezifische Analyse von Qualitätsindikatoren auf Regionaler Ebene. Available online: https://www.versorgungsatlas.de/fileadmin/ziva_docs/46/Antibiotika_best_Infektionskrankheiten_Nebenbericht.pdf (accessed on 7 July 2021).
- Coenen, S.; Ferech, M.; Haaijer-Ruskamp, F.M.; Butler, C.; Stichele, R.H.V.; Verheij, T.J.M.; Monnet, D.L.; Little, P.; Goossens, P.L.H. The ESAC project group European Surveillance of Antimicrobial Consumption (ESAC): Quality indicators for outpatient antibiotic use in Europe. Qual. Saf. Health Care 2007, 16, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Vankerckhoven, V.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Quality appraisal of antibiotic use in Europe. J. Antimicrob. Chemother. 2011, 66, vi71–vi77. [Google Scholar] [CrossRef] [Green Version]
- Federal Ministry of Health. KM6-Statistics. Available online: https://www.bundesgesundheitsministerium.de/themen/krankenversicherung/zahlen-und-fakten-zur-krankenversicherung/mitglieder-und-versicherte.html (accessed on 23 April 2021).
- National Association of Statutory Health Insurance Accredited Physicians. Regionale Verteilung der Ärzte in der vertragsärztlichen Versorgung. Available online: https://gesundheitsdaten.kbv.de/cms/html/16402.php (accessed on 17 February 2021).
- German Society for Pediatric Infectious Diseases, The Society for Pediatric Pneumology. S2k Leitlinie: Management der ambulant erworbenen Pneumonie bei Kindern und Jugendlichen (pädiatrische ambulant erworbene Pneumonie, pCAP), AWMF-Reg.-Nr. 048/013. 2017. Available online: http://www.awmf.org/uploads/tx_szleitlinien/048-013l_S2k_pCAP_ambulant_erworbene_Pneumonie__Kinder_Jugendliche_2017-06.pdf (accessed on 7 July 2021).
- German Society of Oto-Rhino-Laryngology, Head and Neck Surgery, German College of General Practitioners and Family Physicians. S2K-Leitlinie: Antibiotikatherapie bei HNO-Infektionen, AMWF-Register-Nr. 017/166. Available online: https://www.awmf.org/leitlinien/detail/ll/017-066.html (accessed on 7 July 2021).
- Kern, W.V. Rationale Antibiotikaverordnung in der Humanmedizin. Available online: https://www.rki.de/DE/Content/Infekt/Antibiotikaresistenz/Grundwissen/BGBL_61_11_Kern.pdf?__blob=publicationFile (accessed on 21 April 2021).
- Goossens, H.; Ferech, M.; Stichele, R.V.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Gerlach, F.M.; Greiner, W.; Haubitz, M.; Schaeffer, D.; Thürmann, P.; Thüsing, G.; Wille, E. Bedarfsgerechte Versorgung-Perspektiven für ländliche Regionen und ausgewählte Leistungsbereiche [Demand-Oriented Care—Perspectives for Rural Regions and Selected Service Areas]. Available online: https://www.svr-gesundheit.de/fileadmin/Gutachten/Gutachten_2014/Langfassung2014.pdf (accessed on 10 February 2021).
- Faber, M.S.; Heckenbach, K.; Velasco, E.; Eckmanns, T. Antibiotics for the common cold: Expectations of Germany’s general population. Eurosurveillance 2010, 15, 1–7. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Foley, R.N. Method of physician remuneration and rates of antibiotic prescription. Can. Med Assoc. J. 1999, 160, 1013–1017. [Google Scholar]
- Velasco, E.; Eckmanns, T.; Espelage, W.; Barger, A.; Krause, G. Einflüsse auf die ärztliche Verschreibung von Antibiotika in Deutschland (EVA-Studie): Abschlussbericht an das Bundesministerium für Gesundheit. Available online: http://www.mre-netzwerk-mittelhessen.de/images/DOWNLOADS_NEU/EVA_BMG_Schlussbericht.pdf (accessed on 28 June 2021).
- Hoffmann, F.; Glaeske, G.; Pfannkuche, M.S. The effect of introducing rebate contracts to promotegeneric drug substitution, on doctors’ prescribing practices. Dtsch. Arztebl. 2009, 106, 783–788. [Google Scholar] [CrossRef]
- WIdO–AOK Research Institute. PharMaAnalyst. Available online: https://www.wido.de/publikationen-produkte/pharmaanalyst/ (accessed on 29 June 2021).
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Steffen, A.; Bätzing, J. Marked reductions in outpatient antibiotic prescriptions for children and adolescents—A population-based study covering 83% of the paediatric population, Germany, 2010 to 2018. Eurosurveillance 2020, 25, 1900599. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.T.; Antonelli, P.J.; Hincapie-Castillo, J.M.; Winterstein, A.G. Association of US Food and Drug Administration removal of indications for use of oral quinolones with prescribing trends. JAMA Intern. Med. 2021, 181, 808. [Google Scholar] [CrossRef]
- Witte, M.D.-P.C.; Ludwig, R.N.S.; Zahn, I.T.P. Entwicklung der Antibiotikaverordnungen bei Kindern und Jugendlichen. Monit. Versorg. 2018, 11, 51–57. [Google Scholar] [CrossRef]
- Altiner, A.; Brockmann, S.; Sielk, M.; Wilm, S.; Wegscheider, K.; Abholz, H.-H. Reducing antibiotic prescriptions for acute cough by motivating GPs to change their attitudes to communication and empowering patients: A cluster-randomized intervention study. J. Antimicrob. Chemother. 2007, 60, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Information Service of the City of Hamburg. Antibiotika Gezielt Einsetzen. Available online: https://www.hamburg.de/antibiotika-gezielt/ (accessed on 4 February 2021).
- Ministerium für Arbeit, Gesundheit und Soziales des Landes Nordrhein-Westfalen. Antibiotika Sinnvoll Einsetzen. Available online: https://www.mags.nrw/antibiotika (accessed on 4 February 2021).
- Verband der Privaten Krankenversicherung e.V. PKV-Zahlenportal. Available online: https://www.pkv.de/wissen/pkv-zahlenportal/ (accessed on 28 April 2021).
- Van Bodegraven, B.; Palin, V.; Mistry, C.; Sperrin, M.; White, A.; Welfare, W.; Ashcroft, D.M.; Van Staa, T.P. Infection-related complications after common infection in association with new antibiotic prescribing in primary care: Retrospective cohort study using linked electronic health records. BMJ Open 2021, 11, e041218. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.M.; Enners, S.; Kieble, M.; Mahfoud, F.; Böhm, M.; Laufs, U.; Schulz, M. Impact of angiotensin receptor blocker product recalls on antihypertensive prescribing in Germany. J. Hum. Hypertens. 2020. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.; Ritchie, S.; Metcalfe, S.; Van Bakel, B.; Thomas, M.G. Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J. Clin. Pharm. Ther. 2017, 43, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Federal Institute for Drugs and Medical Devices. ATC-Classification. Available online: https://www.dimdi.de/dynamic/en/drugs/atc-classification/index.html (accessed on 20 January 2021).
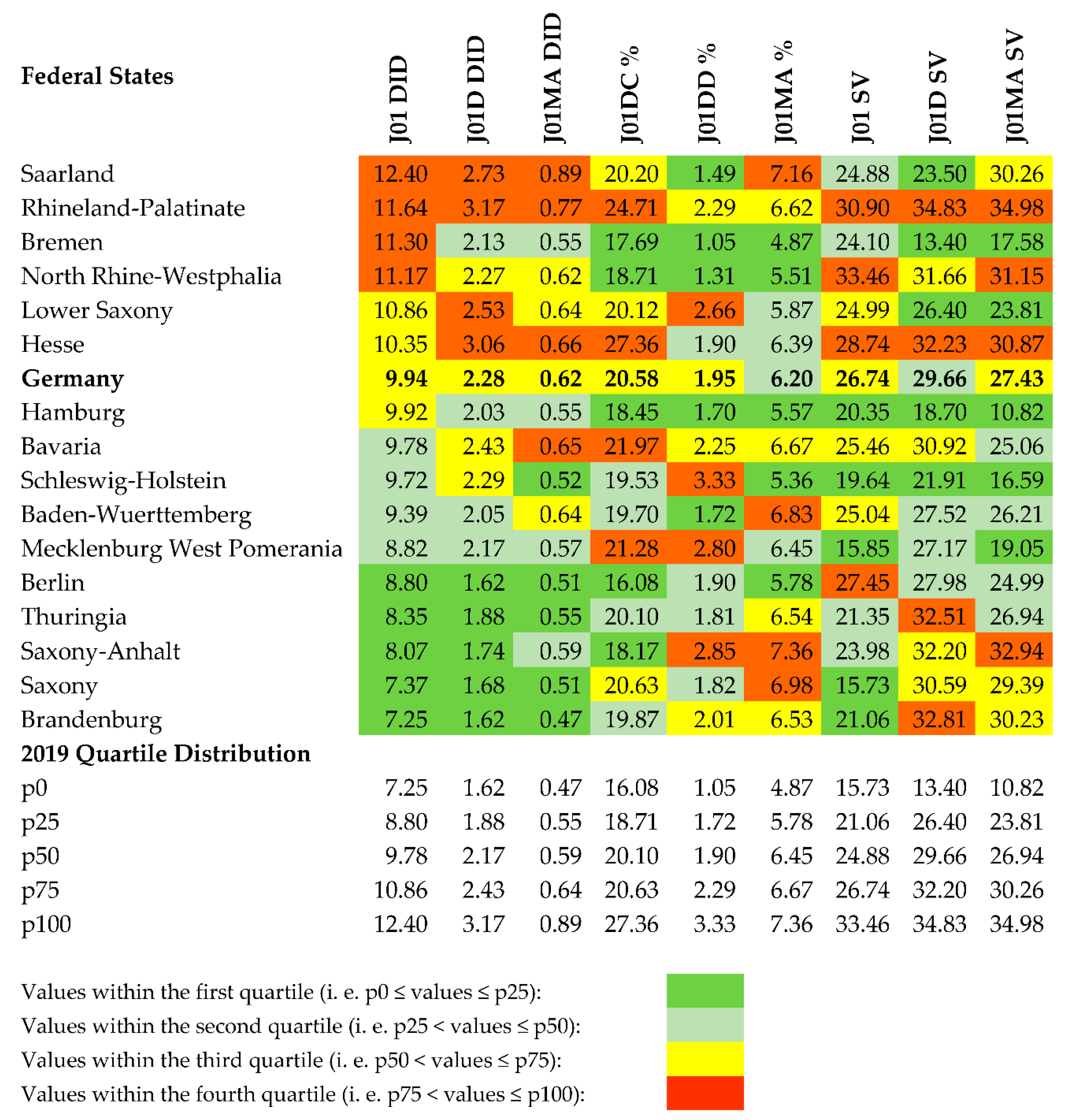
| Label | Description |
|---|---|
| J01 DID | use of oral antibacterials for systemic use (J01), expressed in DID |
| J01D DID | consumption of oral cephalosporins (J01D), expressed in DID |
| J01MA DID | consumption of oral fluoroquinolones (J01MA), expressed in DID |
| J01DC % | consumption of 2nd generation oral cephalosporins (J01DC), expressed as percentage 1 |
| J01DD % | consumption of 3rd generation oral cephalosporins (J01DD), expressed as percentage 1 |
| J01MA % | consumption of oral fluoroquinolones (J01MA), expressed as percentage 1 |
| J01 SV | seasonal variation of total oral antibiotic consumption (J01) 2 |
| J01D SV | seasonal variation of oral cephalosporin consumption (J01D) 2 |
| J01MA SV | seasonal variation of oral fluoroquinolone consumption (J01MA) 2 |
| Federal State | State Type TS = Territorial State CS = City-State | Area [km2] | Number of SHI-Insured Persons in 2019 | Number of Physicians per 100,000 Inhabitants in 2019 | Number of Pharmacists 2 per 100,000 Inhabitants in 2019 | ||||
|---|---|---|---|---|---|---|---|---|---|
| All Physicians | General Physicians 1 | Pediatricians | Ear-Nose- and Throat Specialists | Urologists | |||||
| Baden-Wuerttemberg | TS | 35,748 | 9,422,699 | 206.8 | 64.9 | 9.9 | 4.8 | 3.7 | 63.8 |
| Bavaria | TS | 70,542 | 11,157,806 | 217.3 | 70.6 | 8.9 | 5.1 | 3.8 | 67.7 |
| Berlin | CS | 891 | 3,159,945 | 283.7 | 71.5 | 10.5 | 7.7 | 5.1 | 76.3 |
| Brandenburg | TS | 29,654 | 2,264,526 | 188.3 | 65.4 | 8.5 | 5.1 | 3.8 | 49.4 |
| Bremen | CS | 419 | 599,781 | 301.1 | 68.2 | 14.3 | 8.2 | 5.7 | 55.2 |
| Hamburg | CS | 755 | 1,597,846 | 292.9 | 72.9 | 11.7 | 7.6 | 4.6 | 79.4 |
| Hesse | TS | 21,116 | 5,431,318 | 215.2 | 64.6 | 8.4 | 5.1 | 3.7 | 71.7 |
| Lower Saxony | TS | 47,710 | 7,050,786 | 202.2 | 65.2 | 9.0 | 5.4 | 3.9 | 61.2 |
| Mecklenburg West Pomerania | TS | 23,295 | 1,467,844 | 207.1 | 72.8 | 10.5 | 6.5 | 4.3 | 55.6 |
| North Rhine-Westphalia | TS | 34,112 | 15,762,659 | 211.8 | 62.9 | 9.3 | 5.6 | 4.4 | 59.0 |
| Rhineland-Palatinate | TS | 19,858 | 3,442,438 | 195.8 | 66.0 | 9.3 | 4.8 | 4.1 | 70.0 |
| Saarland | TS | 2,571 | 855,115 | 221.1 | 66.4 | 9.4 | 6.2 | 4.9 | 84.9 |
| Saxony | TS | 18,450 | 3,760,346 | 211.6 | 66.4 | 11.2 | 6.3 | 4.6 | 49.8 |
| Saxony-Anhalt | TS | 20,457 | 2,062,627 | 196.4 | 65.1 | 8.9 | 5.8 | 4.6 | 59.4 |
| Schleswig-Holstein | TS | 15,810 | 2,497,277 | 203.6 | 69.9 | 8.9 | 5.1 | 3.8 | 67.9 |
| Thuringia | TS | 16,202 | 1,965,431 | 202.8 | 69.1 | 10.4 | 5.6 | 4.8 | 52.6 |
| Federal State | DID2014 | DID2019 | Δ | Avg | p-Value | R2 |
|---|---|---|---|---|---|---|
| Thuringia | 2.65 | 1.88 | −29.2% | −0.016 | <0.001 * | 0.49 |
| Saxony | 2.31 | 1.68 | −27.3% | −0.012 | <0.001 * | 0.56 |
| Baden-Wuerttemberg | 2.82 | 2.05 | −27.2% | −0.014 | <0.001 * | 0.74 |
| Hesse | 4.06 | 3.06 | −24.6% | −0.018 | <0.001 * | 0.56 |
| North Rhine-Westphalia | 2.97 | 2.27 | −23.4% | −0.014 | <0.001 * | 0.55 |
| Berlin | 2.10 | 1.62 | −22.8% | −0.009 | <0.001 * | 0.58 |
| Germany | 2.93 | 2.28 | −22.1% | −0.013 | <0.001 * | 0.57 |
| Hamburg | 2.60 | 2.03 | −21.9% | −0.011 | <0.001 * | 0.56 |
| Bremen | 2.69 | 2.13 | −20.8% | −0.010 | <0.001 * | 0.42 |
| Brandenburg | 2.04 | 1.62 | −20.8% | −0.008 | <0.001 * | 0.49 |
| Saxony-Anhalt | 2.19 | 1.74 | −20.5% | −0.009 | <0.001 * | 0.32 |
| Bavaria | 3.05 | 2.43 | −20.3% | −0.012 | <0.001 * | 0.51 |
| Mecklenburg West Pomerania | 2.71 | 2.17 | −19.8% | −0.012 | <0.001 * | 0.39 |
| Rhineland Palatinate | 3.83 | 3.17 | −17.2% | −0.013 | <0.001 * | 0.40 |
| Lower Saxony | 3.05 | 2.53 | −17.1% | −0.012 | <0.001 * | 0.35 |
| Schleswig-Holstein | 2.66 | 2.29 | −13.9% | −0.007 | <0.001 * | 0.38 |
| Saarland | 3.17 | 2.73 | −13.7% | −0.008 | <0.001 * | 0.22 |
| Federal State | DID2014 | DID2019 | Δ | Avg2014–2016 | p-Value 2014–2016 | Avg 2017–2019 | p-Value 2017–2019 | R2 |
|---|---|---|---|---|---|---|---|---|
| Mecklenburg West Pomerania | 1.286 | 0.569 | −55.8% | −0.004 | 0.002 * | −0.016 | <0.001 * | 0.899 |
| Hesse | 1.488 | 0.661 | −55.6% | −0.002 | 0.081 | −0.021 | <0.001 * | 0.895 |
| Rhineland Palatinate | 1.735 | 0.771 | −55.6% | −0.005 | 0.001 * | −0.021 | <0.001 * | 0.903 |
| Schleswig-Holstein | 1.165 | 0.521 | −55.3% | −0.003 | <0.001 * | −0.015 | <0.001 * | 0.940 |
| Baden-Wuerttemberg | 1.433 | 0.641 | −55.2% | −0.007 | <0.001 * | −0.012 | <0.001 * | 0.925 |
| Bremen | 1.201 | 0.550 | −54.2% | 0.000 | 0.846 | −0.019 | <0.001 * | 0.866 |
| Hamburg | 1.203 | 0.553 | −54.1% | −0.004 | <0.001 * | −0.014 | <0.001 * | 0.958 |
| North Rhine-Westphalia | 1.331 | 0.615 | −53.7% | −0.001 | 0.297 | −0.020 | <0.001 * | 0.903 |
| Thuringia | 1.170 | 0.546 | −53.4% | −0.003 | 0.002 * | −0.014 | <0.001 * | 0.901 |
| Saarland | 1.892 | 0.888 | −53.0% | −0.002 | 0.237 | −0.029 | <0.001 * | 0.885 |
| Germany | 1.310 | 0.616 | −53.0% | −0.002 | 0.019 | −0.017 | <0.001 * | 0.918 |
| Berlin | 1.058 | 0.509 | −51.9% | −0.001 | 0.345 | −0.015 | <0.001 * | 0.884 |
| Saxony-Anhalt | 1.213 | 0.594 | −51.0% | 0.001 | 0.609 | −0.020 | <0.001 * | 0.820 |
| Bavaria | 1.329 | 0.652 | −50.9% | −0.003 | 0.001 * | −0.016 | <0.001 * | 0.926 |
| Saxony | 1.032 | 0.514 | −50.2% | −0.001 | 0.448 | −0.015 | <0.001 * | 0.891 |
| Brandenburg | 0.929 | 0.474 | −49.0% | −0.001 | 0.140 | −0.012 | <0.001 * | 0.882 |
| Lower Saxony | 1.242 | 0.637 | −48.7% | 0.000 | 0.669 | −0.021 | <0.001 * | 0.903 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradl, G.; Werning, J.; Enners, S.; Kieble, M.; Schulz, M. Quality Appraisal of Ambulatory Oral Cephalosporin and Fluoroquinolone Use in the 16 German Federal States from 2014–2019. Antibiotics 2021, 10, 831. https://doi.org/10.3390/antibiotics10070831
Gradl G, Werning J, Enners S, Kieble M, Schulz M. Quality Appraisal of Ambulatory Oral Cephalosporin and Fluoroquinolone Use in the 16 German Federal States from 2014–2019. Antibiotics. 2021; 10(7):831. https://doi.org/10.3390/antibiotics10070831
Chicago/Turabian StyleGradl, Gabriele, Johanna Werning, Salka Enners, Marita Kieble, and Martin Schulz. 2021. "Quality Appraisal of Ambulatory Oral Cephalosporin and Fluoroquinolone Use in the 16 German Federal States from 2014–2019" Antibiotics 10, no. 7: 831. https://doi.org/10.3390/antibiotics10070831
APA StyleGradl, G., Werning, J., Enners, S., Kieble, M., & Schulz, M. (2021). Quality Appraisal of Ambulatory Oral Cephalosporin and Fluoroquinolone Use in the 16 German Federal States from 2014–2019. Antibiotics, 10(7), 831. https://doi.org/10.3390/antibiotics10070831







