The Effect of Amoxicillin in Adult Patients Presenting to Primary Care with Acute Cough Predicted to Have Pneumonia or a Combined Viral-Bacterial Infection
Abstract
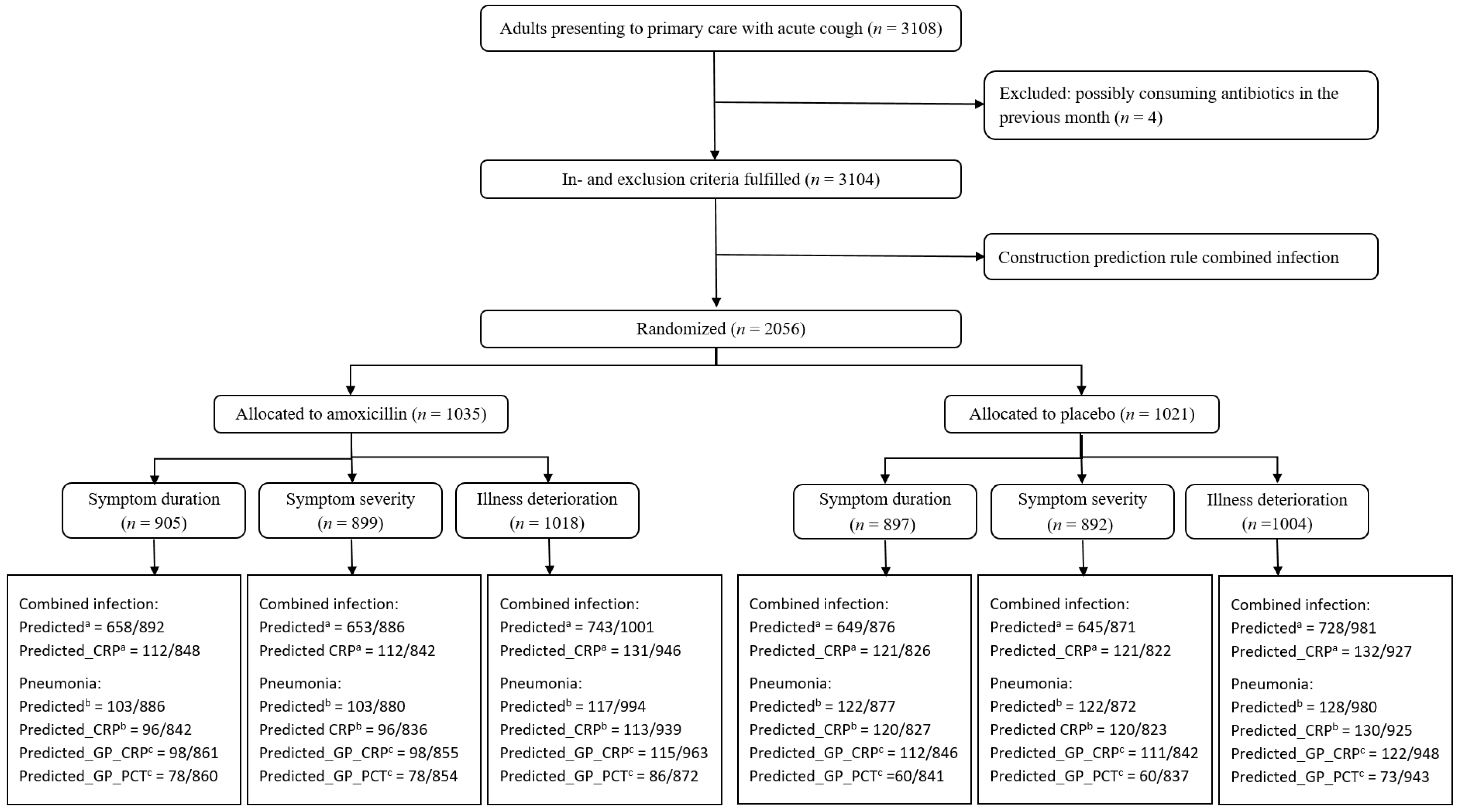
:1. Introduction
2. Results
2.1. Predicting Combined Infection
2.2. Predicting Pneumonia
2.3. Evaluation of Treatment Effect
2.3.1. Symptom Duration
2.3.2. Symptom Severity
2.3.3. Illness deterioration
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Data
4.2. Prediction Rules for Combined Infection
4.3. Prediction Rules for Pneumonia
4.4. Prediction Rule Evaluation
4.5. Evaluation of Treatment Effect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, G.J.; Loddenkemper, R.; Lundbäck, B.; Sibille, Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013, 42, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Hood, K.; Verheij, T.; Little, P.; Melbye, H.; Nuttall, J.; Kelly, M.J.; Mölstad, S.; Godycki-Cwirko, M.; Almirall, J.; et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: Prospective study in 13 countries. BMJ 2009, 338, b2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, P.; Stuart, B.; Moore, M.; Coenen, S.; Butler, C.C.; Godycki-Cwirko, M.; Mierzecki, A.; Chlabicz, S.; Torres, A.; Almirall, J.; et al. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: A 12-country, randomised, placebo-controlled trial. Lancet Infect. Dis. 2013, 13, 123–129. [Google Scholar] [CrossRef]
- Moore, M.; Stuart, B.; Coenen, S.; Butler, C.C.; Goossens, H.; Verheij, T.J.M.; Little, P. Amoxicillin for acute lower respiratory tract infection in primary care: Subgroup analysis of potential high-risk groups. Br. J. Gen. Pract. 2014, 64, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teepe, J.; Little, P.; Elshof, N.; Broekhuizen, B.D.L.; Moore, M.; Stuart, B.; Butler, C.C.; Hood, K.; Ieven, M.; Coenen, S.; et al. Amoxicillin for clinically unsuspected pneumonia in primary care: Subgroup analysis. Eur. Respir. J. 2015, 47, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Bruyndonckx, R.; Stuart, B.; Hens, N.; Ieven, G.; Butler, C.C.; Little, P.; Verheij, T.; Goossens, H.; Coenen, S.; GRACE Project Group. Amoxicillin for acute lower respiratory tract infection in primary care: Subgroup analysis by bacterial and viral etiology. Clin. Microbiol. Infect. 2018, 24, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vugt, S.F.; Broekhuizen, B.D.L.; Lammens, C.; Zuithoff, N.P.A.; de Jong, P.A.; Coenen, S.; Ieven, M.; Butler, C.C.; Goossens, H.; Little, P.; et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: Diagnostic study. BMJ 2013, 346, f2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vugt, S.F.; Verheij, T.J.M.; de Jong, P.A.; Butler, C.C.; Hood, K.; Coenen, S.; Goossens, H.; Little, P.; Broekhuizen, B.D.L.; GRACE Project Group. Diagnosing pneumonia in patients with acute cough: Clinical judgment compared to chest radiography. Eur. Respir. J. 2013, 42, 1076–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ieven, M.; Coenen, S.; Loens, K.; Lammens, C.; Coenjaerts, F.; Vanderstraeten, A.; Henriques-Normark, B.; Crook, D.; Huygen, K.; Butler, C.C.; et al. Aetiology of lower respiratory tract infection in adults in primary care: A prospective study in 11 European countries. Clin. Microbiol. Infect. 2018, 24, 1158–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, M.S.; Peterson, K.; Winthrop, K.; Cantor, A.; Lazur, B.H.; Buckley, D.I. Interventions to reduce inappropriate prescribing of antibiotics for acute respiratory tract infections: Summary and update of a systematic review. J. Int. Med. Res. 2018, 46, 3337–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, A.D.; Tilling, K. Can 88% of patients with acute lower respiratory infection all be special? Br. J. Gen. Pract. 2014, 64, 60–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruyndonckx, R.; Hens, N.; Verheij, T.J.M.; Aerts, M.; Ieven, M.; Butler, C.C.; Little, P.; Goossens, H.; Coenen, S. Development of a prediction tool for patients presenting with acute cough in primary care: A prognostic study spanning six European countries. Br. J. Gen. Pract. 2018, 68, e342–e350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teepe, J.; Broekhuizen, B.D.L.; Loens, K.; Lammens, C.; Ieven, M.; Goossens, H.; Little, P.; Butler, C.C.; Coenen, S.; Godycki-Cwirko, M.; et al. Predicting the presence of bacterial pathogens in the airways of primary care patients with acute cough. CMAJ 2016, 189, E50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Cox, D.R. Two further applications of a model for binary regression. Biometrika 1958, 45, 562–565. [Google Scholar] [CrossRef]
- Quandt, R.E. The Estimation of the Parameters of a Linear Regression System Obeying Two Separate Regimes. J. Am. Stat. Assoc. 1958, 53, 873. [Google Scholar] [CrossRef]
- Efron, B. The Efficiency of Logistic Regression Compared to Normal Discriminant Analysis. J. Am. Stat. Assoc. 1975, 70, 892. [Google Scholar] [CrossRef]

| Patient Characteristics | Number (%) of Patients a (n = 3104) | Number (%) with Missing Information | Patients with Combined Infection (n = 304) | |
|---|---|---|---|---|
| Number (%) a | OR (95% CI) | |||
| General characteristics | ||||
| Age (years): mean ± SD | 49.8 ± 16.8 | 0 (0) | 48.5 ± 16.6 | 0.99 [0.99–1.00] |
| Male | 1244 (40.1) | 0 (0) | 122 (40.1) | 1.00 [0.79–1.27] |
| Current smoker | 871 (28.1) | 3 (0.1) | 97 (31.9) | 1.22 [0.95–1.58] |
| No. of days coughing before consultation: mean ± SD | 8.7 ± 7.4 | 46 (1.5) | 7.5 ± 5.9 | 0.97 [0.95–0.99] |
| No. of days illness before consultation: mean ± SD | 9.7 ± 10.2 | 31 (1.0) | 8.1 ± 6.1 | 0.97 [0.95–0.99] |
| Clinical signs | ||||
| Abnormal consciousness | 44 (1.4) | 3 (0.1) | 5 (1.6) | 1.18 [0.41–2.76] |
| General toxicity | 800 (25.8) | 8 (0.3) | 92 (30.3) | 1.28 [0.98–1.65] |
| Lung auscultation: | ||||
| Diminished vesicular breathing | 393 (12.7) | 20 (0.6) | 48 (15.9) | 1.34 [0.96–1.85] |
| Wheeze | 539 (17.5) | 21 (0.7) | 65 (21.7) | 1.35 [1.00–1.79] |
| Crackles | 289 (9.4) | 18 (0.6) | 30 (10.0) | 1.08 [0.71–1.58] |
| Rhonchi | 521 (16.9) | 21 (0.7) | 65 (21.6) | 1.40 [1.04–1.87] |
| Tachycardia (>100 beats/min) | 85 (2.8) | 44 (1.4) | 7 (2.3) | 0.82 [0.34–1.68] |
| Tachypnoea (>24 breaths/min) | 61 (2.0) | 78 (2.5) | 8 (2.7) | 1.39 [0.61–2.79] |
| Prolonged expiration | 309 (10.1) | 45 (1.4) | 44 (14.8) | 1.64 [1.15–2.29] |
| Low blood pressure (<90/60 mmHg) | 10 (0.3) | 67 (2.2) | 0 (0) | 0.44 [0.00–3.44] d |
| Fever (oral temperature >37.8 °C) | 137 (4.5) | 31 (1.0) | 18 (6.1) | 1.45 [0.84–2.35] |
| Baseline symptoms (as reported by the patient during consultation) | ||||
| Phlegm | 2472 (79.7) | 4 (0.1) | 252 (83.2) | 1.28 [0.95–1.78] |
| Shortness of breath | 1754 (56.6) | 4 (0.1) | 180 (59.4) | 1.14 [0.89–1.45] |
| Wheeze | 1324 (42.7) | 5 (0.2) | 139 (45.9) | 1.15 [0.91–1.46] |
| Runny nose | 2212 (71.4) | 4 (0.1) | 235 (77.6) | 1.43 [1.09–1.91] |
| Fever | 1085 (35.0) | 5 (0.2) | 133 (43.9) | 1.52 [1.19–1.93] |
| Chest pain | 1433 (46.3) | 6 (0.2) | 151 (49.8) | 1.17 [0.92–1.49] |
| Muscle ache | 1573 (50.7) | 4 (0.1) | 170 (56.1) | 1.27 [1.00–1.61] |
| Headache | 1742 (56.2) | 3 (0.1) | 191 (62.8) | 1.36 [1.07–1.74] |
| Disturbed sleep | 1955 (63.1) | 5 (0.2) | 207 (68.1) | 1.28 [1.00–1.65] |
| Myalgia | 2349 (75.8) | 4 (0.1) | 244 (80.5) | 1.36 [1.02–1.84] |
| Interference with daily activities | 1955 (63.0) | 3 (0.1) | 212 (69.7) | 1.39 [1.08–1.81] |
| Confusion/disorientation | 137 (4.4) | 4 (0.1) | 17 (5.6) | 1.32 [0.76–2.17] |
| Diarrhoea | 222 (7.2) | 4 (0.1) | 24 (7.9) | 1.12 [0.71–1.71] |
| Comorbidities | ||||
| Pulmonary comorbidity b | 528 (17.0) | 2 (0.1) | 49 (16.1) | 0.93 [0.67–1.27] |
| Cardiac comorbidity c | 288 (9.3) | 3 (0.1) | 27 (8.9) | 0.95 [0.61–1.41] |
| Diabetes | 200 (6.5) | 4 (0.1) | 14 (4.6) | 0.68 [0.37–1.14] |
| Previous hospitalisation for respiratory illness | 129 (4.2) | 2 (0.1) | 14 (4.6) | 1.13 [0.61–1.92] |
| Antibiotic treatment in previous six months | 460 (14.8) | 1 (0.0) | 34 (11.2) | 0.70 [0.48–1.00] |
| Allergic disease | 562 (18.1) | 5 (0.2) | 59 (19.4) | 1.10 [0.81–1.47] |
| Other regular medication | ||||
| Inhaled bronchodilators | 348 (11.2) | 2 (0.1) | 33 (10.9) | 0.96 [0.65–1.38] |
| Inhaled steroids | 270 (8.7) | 2 (0.1) | 26 (8.6) | 0.98 [0.63–1.47] |
| Oral steroids | 43 (1.4) | 1 (0.0) | 2 (0.7) | 0.45 [0.07–1.46] |
| Oral agents for diabetes | 153 (4.9) | 1 (0.0) | 9 (3.0) | 0.56 [0.26–1.05] |
| Insulin | 45 (1.5) | 1 (0.0) | 4 (1.3) | 0.90 [0.27–2.24] |
| Antihypertensives/diuretics | 734 (23.7) | 2 (0.1) | 66 (21.7) | 0.88 [0.66–1.17] |
| Nonsteroidal anti-inflammatories | 251 (8.1) | 1 (0.0) | 26 (8.6) | 1.07 [0.68–1.61] |
| Benzodiazepines/antidepressants | 307 (9.9) | 1 (0.0) | 25 (8.2) | 0.80 [0.51–1.20] |
| Influenza vaccination received this autumn/winter | 732 (23.6) | 2 (0.1) | 62 (20.4) | 0.81 [0.60–1.08] |
| Blood test results | ||||
| C-reactive protein: mean ± SD | 1.8 ± 3.5 | 174 (5.6) | 3.1 ± 4.8 | 1.08 [1.06–1.11] |
| Procalcitonin: mean ± SD | 0.06 ± 0.22 | 164 (5.3) | 0.10 ± 0.66 | 1.99 [1.10–5.17] |
| Amoxicillin | Placebo | Interaction Term b (95% CI) | p-Value | Hazard Ratio for Subgroup b (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| WHOLE COHORT (N = 1802) | 6 (3–11) | 7 (3–13) | 1.06 [0.96, 1.17] | 0.278 | ||
| COMBINED INFECTION: | ||||||
| PREDICTED c (N = 1307) | 6 (3–11) | 7 (4–12) | 1.01 [0.80, 1.27] | 0.945 | 1.07 [0.95, 1.20] | 0.287 |
| PREDICTED_CRP c (N = 233) | 8 (4–16) | 8 (5–14) | 0.87 [0.64, 1.17] | 0.351 | 0.90 [0.68, 1.20] | 0.474 |
| PNEUMONIA: | ||||||
| PREDICTED d (N = 225) | 7 (4–15) | 7 (5–11) | 0.83 [0.61, 1.11] | 0.207 | 0.88 [0.67, 1.16] | 0.370 |
| PREDICTED_CRP d (N = 216) | 7 (4–15) | 7 (5–11) | 0.78 [0.57, 1.07] | 0.119 | 0.80 [0.59, 1.07] | 0.126 |
| PREDICTED_GP_CRP e (N = 210) | 8 (4–14) | 8 (5–13) | 1.03 [0.75, 1.41] | 0.851 | 1.04 [0.77, 1.39] | 0.818 |
| PREDICTED_GP_PCT e (N = 138) | 6 (3–14) | 7 (3–13) | 0.80 [0.55, 1.17] | 0.250 | 0.81 [0.56, 1.17] | 0.260 |
| Amoxicillin | Placebo | Interaction Term b (95% CI) | p-Value | Difference for Subgroup b (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| WHOLE COHORT (N = 1791) | 1.59 (0.96) | 1.70 (1.01) | −0.07 [−0.15, 0.00] | 0.065 | ||
| COMBINED INFECTION: | ||||||
| PREDICTED c (N = 1298) | 1.73 (0.97) | 1.83 (1.01) | 0.00 [−0.17, 0.18] | 0.968 | −0.06 [−0.16, 0.03] | 0.187 |
| PREDICTED_CRP c (N = 233) | 2.15 (1.04) | 2.21 (1.03) | 0.10 [−0.13, 0.33] | 0.384 | 0.02 [−0.24, 0.27] | 0.907 |
| PNEUMONIA: | ||||||
| PREDICTED d (N = 225) | 1.93 (0.99) | 1.95 (1.02) | 0.12 [−0.12, 0.35] | 0.321 | 0.03 [−0.19, 0.25] | 0.783 |
| PREDICTED_CRP d (N = 216) | 2.06 (1.02) | 2.09 (1.12) | 0.09 [−0.15, 0.33] | 0.451 | 0.02 [−0.23, 0.28] | 0.852 |
| PREDICTED_GP_CRP e (N = 209) | 1.97 (0.97) | 2.17 (1.07) | -0.07 [−0.32, 0.17] | 0.554 | −0.14 [−0.40, 0.12] | 0.298 |
| PREDICTED_GP_PCT e (N = 138) | 1.72 (0.88) | 1.85 (1.12) | -0.06 [−0.36, 0.23] | 0.684 | −0.12 [−0.41, 0.17] | 0.417 |
| Amoxicillin | Placebo | Interaction Term b (95% CI) | p-Value | Odds Ratio for Subgroup b (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| WHOLE COHORT (N = 2022) | 162/1018 | 192/1004 | 0.81 [0.64, 1.02] | 0.073 | ||
| COMBINED INFECTION: | ||||||
| PREDICTED c (N = 1471) | 117/743 | 134/728 | 1.08 [0.64, 1.82] | 0.785 | 0.84 [0.64, 1.10] | 0.207 |
| PREDICTED_CRP c (N = 263) | 27/131 | 28/132 | 1.26 [0.65, 2.41] | 0.492 | 0.98 [0.54, 1.79] | 0.952 |
| PNEUMONIA: | ||||||
| PREDICTED d (N = 245) | 20/117 | 26/128 | 1.02 [0.50, 2.03] | 0.963 | 0.82 [0.42, 1.56] | 0.542 |
| PREDICTED_CRP d (N = 243) | 21/113 | 27/130 | 1.11 [0.55, 2.21] | 0.762 | 0.87 [0.46, 1.64] | 0.665 |
| PREDICTED_GP_CRP e (N = 237) | 20/115 | 27/122 | 0.93 [0.46, 1.86] | 0.839 | 0.75 [0.39, 1.41] | 0.381 |
| PREDICTED_GP_PCT e (N = 159) | 16/86 | 19/73 | 0.81 [0.36, 1.79] | 0.602 | 0.65 [0.30, 1.38] | 0.262 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruyndonckx, R.; Stuart, B.; Little, P.; Hens, N.; Ieven, M.; Butler, C.C.; Verheij, T.J.M.; Goossens, H.; Coenen, S.; The GRACE Project Group. The Effect of Amoxicillin in Adult Patients Presenting to Primary Care with Acute Cough Predicted to Have Pneumonia or a Combined Viral-Bacterial Infection. Antibiotics 2021, 10, 817. https://doi.org/10.3390/antibiotics10070817
Bruyndonckx R, Stuart B, Little P, Hens N, Ieven M, Butler CC, Verheij TJM, Goossens H, Coenen S, The GRACE Project Group. The Effect of Amoxicillin in Adult Patients Presenting to Primary Care with Acute Cough Predicted to Have Pneumonia or a Combined Viral-Bacterial Infection. Antibiotics. 2021; 10(7):817. https://doi.org/10.3390/antibiotics10070817
Chicago/Turabian StyleBruyndonckx, Robin, Beth Stuart, Paul Little, Niel Hens, Margareta Ieven, Christopher C. Butler, Theo J. M. Verheij, Herman Goossens, Samuel Coenen, and The GRACE Project Group. 2021. "The Effect of Amoxicillin in Adult Patients Presenting to Primary Care with Acute Cough Predicted to Have Pneumonia or a Combined Viral-Bacterial Infection" Antibiotics 10, no. 7: 817. https://doi.org/10.3390/antibiotics10070817
APA StyleBruyndonckx, R., Stuart, B., Little, P., Hens, N., Ieven, M., Butler, C. C., Verheij, T. J. M., Goossens, H., Coenen, S., & The GRACE Project Group. (2021). The Effect of Amoxicillin in Adult Patients Presenting to Primary Care with Acute Cough Predicted to Have Pneumonia or a Combined Viral-Bacterial Infection. Antibiotics, 10(7), 817. https://doi.org/10.3390/antibiotics10070817








