Benzothiazole-Containing Analogues of Triclocarban with Potent Antibacterial Activity
Abstract
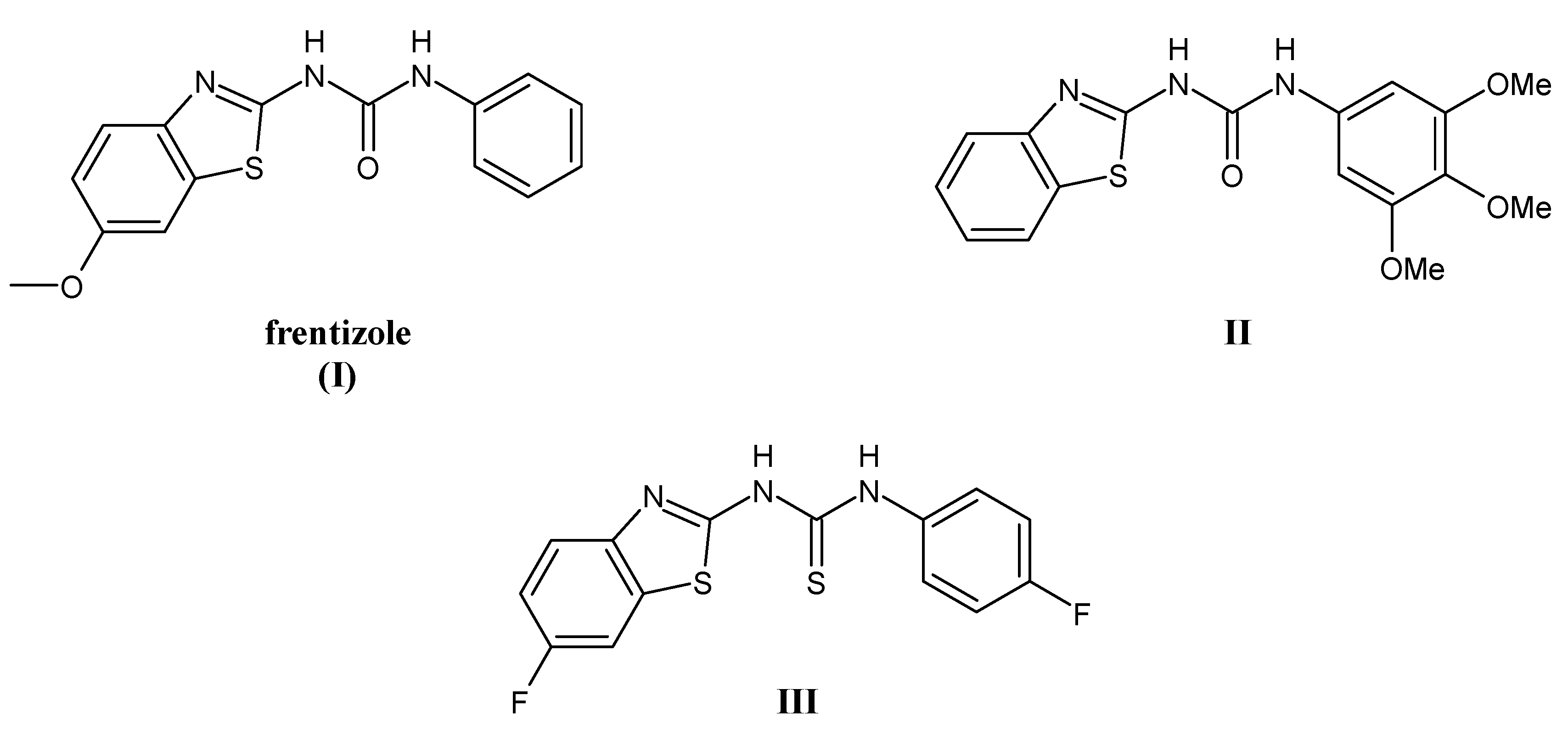
:1. Introduction
2. Results and Discussion
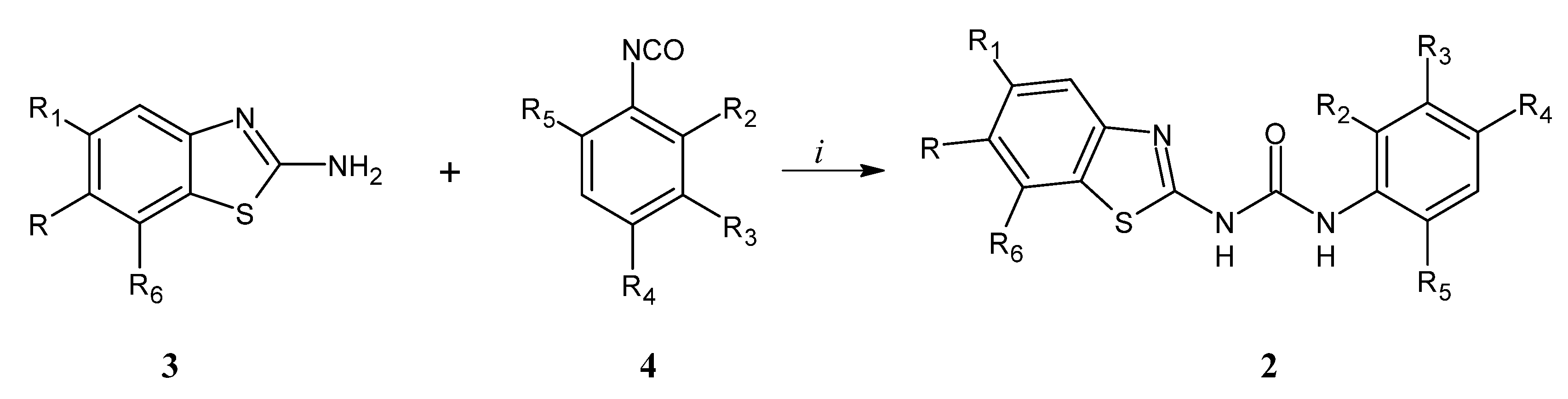
2.1. Chemistry
2.2. Antibacterial Studies
3. Materials and Methods
3.1. Chemistry
3.2. Antibacterial In Vitro Evaluation
3.3. Cell Cultures
3.4. Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liao, C.; Kannan, K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch. Environ. Contam. Toxicol. 2014, 67, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The Different Facets of Triclocarban: A Review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef]
- Yun, H.; Liang, B.; Kong, D.; Li, X.; Wang, A. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as antitumor agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef]
- Catalano, A. COVID-19: Could irisin become the handyman myokine of the 21st Century? Coronaviruses 2020, 1, 32–41. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Elangovan, M.; Kannan, K. Migration of parabens, bisphenols, benzophenone-type UV filters, triclosan, and triclocarban from teethers and its implications for infant exposure. Environ. Sci. Technol. 2016, 50, 13539–13547. [Google Scholar] [CrossRef]
- European Commission. Opinion on Triclocarban for Other Uses than as a Preservative. EC Scientific Committee on Consumer Products Report No. SCCP/0851/04. 2005, pp. 1–45. Available online: https://tinyurl.com/bd5bk5rj (accessed on 23 March 2021).
- De Bellis, M.; De Luca, A.; Rana, F.; Cavalluzzi, M.M.; Catalano, A.; Lentini, G.; Franchini, C.; Tortorella, V.; Conte Camerino, D. Evaluation of the pharmacological activity of the major mexiletine metabolites on skeletal muscle sodium currents. Br. J. Pharmacol. 2006, 149, 300–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Wei, L.; Shi, Y.; Zhang, J.; Wu, Q.; Shao, B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ. Geochem. Health 2016, 38, 1125–1135. [Google Scholar] [CrossRef]
- Wei, L.; Qiao, P.; Shi, Y.; Ruan, Y.; Yin, J.; Wu, Q.; Shao, B. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin. Chim. Acta 2017, 466, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Smarr, M.M.; Sun, L.; Chen, Z.; Honda, M.; Wang, W.; Karthikraj, R.; Weck, J.; Kannan, K. Endocrine disrupting chemicals in seminal plasma and couple fecundity. Environ. Res. 2018, 163, 64–70. [Google Scholar] [CrossRef]
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Fair, P.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The Florence statement on triclosan and triclocarban. Environ. Health Perspect. 2017, 125, 064501. [Google Scholar] [CrossRef]
- Musee, N. Environmental risk assessment of triclosan and triclocarban from personal care products in South Africa. Environ. Pollut. 2018, 242, 827–838. [Google Scholar] [CrossRef]
- Karthikraj, R.; Kannan, K.; Karthikraj, R. Mass loading and removal of benzotriazoles, benzothiazoles, benzophenones, and bisphenols in Indian sewage treatment plants. Chemosphere 2017, 181, 216–223. [Google Scholar] [CrossRef]
- Hu, Z.; He, L.; Wei, J.; Su, Y.; Wang, W.; Fan, Z.; Xu, J.; Zhang, Y.; Wang, Y.; Peng, M.; et al. tmbim4 protects against triclocarban-induced embryonic toxicity in zebrafish by regulating autophagy and apoptosis. Environ. Pollut. 2021, 277, 116873. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Safety and effectiveness of consumer antiseptics: Topical antimicrobial drug products for over-the-counter human use. Fed. Regist. 2016, 81, 61106–61130. [Google Scholar]
- Voelker, R. Say goodbye to some antibacterials. JAMA 2016, 316, 1538. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, V.S.; Lenz, K.A.; Svoboda, K.R.; Ma, H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol—Three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ. Pollut. 2018, 235, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlen, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Dhanyalayam, D.; Scrivano, L.; Parisi, O.I.; Sinicropi, M.S.; Fazio, A.; Saturnino, C.; Plutino, M.R.; Cristo, F.D.; Puoci, F.; Cappello, A.R.; et al. Biopolymeric self-assembled nanoparticles for enhanced antibacterial activity of Ag-based compounds. Int. J. Pharm. 2017, 517, 395–402. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Rosato, A.; Salvagno, L.; Ceramella, J.; Longo, F.; Sinicropi, M.S.; Franchini, C. Searching for small molecules as antibacterials: Non-cytotoxic diarylureas analogues of triclocarban. Antibiotics 2021, 10, 204. [Google Scholar] [CrossRef]
- Catalano, A.; Carocci, A.; Corbo, F.; Franchini, C.; Muraglia, M.; Scilimati, A.; De Bellis, M.; De Luca, A.; Camerino, D.C.; Sinicropi, M.S.; et al. Constrained analogues of tocainide as potent skeletal muscle sodium channel blockers towards the development of antimyotonic agents. Eur. J. Med. Chem. 2008, 43, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Muraglia, M.; De Bellis, M.; Catalano, A.; Carocci, A.; Franchini, C.; Carrieri, A.; Fortugno, C.; Bertucci, C.; Desaphy, J.F.; De Luca, A.; et al. N-aryl-2,6-dimethylbenzamides, a new generation of tocainide analogues as blockers of skeletal muscle voltage-gated sodium channels. J. Med. Chem. 2014, 57, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- de Luca, A.; Talon, S.; De Bellis, M.; Desaphy, J.-F.; Franchini, C.; Lentini, G.; Catalano, A.; Corbo, F.; Tortorella, V.; Conte-Camerino, D. Inhibition of skeletal muscle sodium currents by mexiletine analogues: Specific hydrophobic interactions rather than lipophilia per se account for drug therapeutic profile. Naunyn Schmiedeberg’s Arch. Pharmacol. 2003, 367, 318–327. [Google Scholar] [CrossRef]
- Pozzi, C.; Ferrari, S.; Cortesi, D.; Luciani, R.; Stroud, R.M.; Catalano, A.; Costi, M.P.; Mangani, S. The structure of Enterococcus faecalis thymidylate synthase provides clues about folate bacterial metabolism. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1232–1241. [Google Scholar] [CrossRef]
- Dahl, A.; Iversen, K.; Tonder, N.; Hoest, N.; Arpi, M.; Dalsgaard, M.; Chehri, M.; Soerensen, L.L.; Fanoe, S.; Junge, S. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J. Am. Coll. Cardiol. 2019, 74, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Trapani, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Mercurio, A.; Rosato, A.; Mandracchia, D.; Tripodo, G.; Schiavone, B.I.P.; Franchini, C.; et al. Effect of Methyl-beta-Cyclodextrin on the antimicrobial activity of a new series of poorly water-soluble benzothiazoles. Carbohydr. Polym. 2019, 207, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Defrenza, I.; Catalano, A.; Carocci, A.; Carrieri, A.; Muraglia, M.; Rosato, A.; Corbo, F.; Franchini, C. 1,3-Benzothiazoles as Antimicrobial Agents. J. Heterocycl. Chem. 2015, 52, 1705–1712. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Carradori, S.; Amoroso, R.; Fernández, I.F. 2-Substituted benzothiazoles as antiproliferative agents: Novel insights on structure-activity relationships. Eur. J. Med. Chem. 2020, 207, 112762. [Google Scholar] [CrossRef] [PubMed]
- Hroch, L.; Guest, P.; Benek, O.; Soukup, O.; Janockova, J.; Dolezal, R.; Kuca, K.; Aitken, L.; Smith, T.K.; Gunn-Moore, F.; et al. Synthesis and evaluation of frentizole-based indolyl thiourea analogues as MAO/ABAD inhibitors for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2016, 25, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Azam, F.; Prasad, M.V.V.; Thangavel, N. Structure-based design, synthesis, and molecular modeling studies of 1-(benzo[d]thiazol-2-yl)-3-(substituted aryl) urea derivatives as novel anti-Parkinsonian agents. Med. Chem. Res. 2012, 21, 2630–2643. [Google Scholar] [CrossRef]
- Kumbhare, R.M.; Dadmal, T.; Kosurkar, U.; Sridhar, V.; Rao, J.V. Synthesis and cytotoxic evaluation of thiourea and N-bis-benzothiazole derivatives: A novel class of cytotoxic agents. Bioorg. Med. Chem. Lett. 2012, 22, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Kaymakçıoğlu, B.K.; Rollas, S.; Körceğez, E.; Arıcıoğlu, F. Synthesis and bological evaluation of new N-substituted-N′-(3,5-di/1,3,5-trimethylpyrazole-4-yl)thiourea/urea derivatives. Eur. J. Pharm. Sci. 2005, 26, 97–103. [Google Scholar] [CrossRef]
- Catalano, A.; Carocci, A.; Defrenza, I.; Muraglia, M.; Carrieri, A.; Van Bambeke, F.; Rosato, A.; Corbo, F.; Franchini, C. 2-Aminobenzothiazole derivatives: Search for new antifungal agents. Eur. J. Med. Chem. 2013, 64, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armenise, D.; Carocci, A.; Catalano, A.; Muraglia, M.; Defrenza, I.; De Laurentis, N.; Rosato, A.; Corbo, F.; Franchini, C. Synthesis and antimicrobial evaluation of a new series of N-1, 3-benzothiazol-2-ylbenzamides. J. Chem. 2013, 2013, 181758. [Google Scholar] [CrossRef] [Green Version]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved [Document M7-A9]; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Rosato, A.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Franchini, C.; Corbo, F.; Carbonara, G.G.; Carrieri, A.; Fracchiolla, G. Elucidation of the synergistic action of Mentha piperita essential oil with common antimicrobials. PLoS ONE 2018, 13, e0200902. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Luciani, R.; Carocci, A.; Cortesi, D.; Pozzi, C.; Borsari, C.; Ferrari, S.; Mangani, S. X-ray crystal structures of Enterococcus faecalis thymidylate synthase with folate binding site inhibitors. Eur. J. Med. Chem. 2016, 123, 649–664. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Benek, O.; Vinklarova, L.; Hrabinova, M.; Zemanova, L.; Chribek, M.; Kralova, V.; Hroch, L.; Dolezal, R.; Lycka, A.; et al. Benzothiazolyl ureas are low micromolar and uncompetitive inhibitors of 17β-HSD10 with implications to Alzheimer’s disease treatment. Int. J. Mol. Sci. 2020, 21, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Xiao, S.; Tian, G.; Zhu, A.; Feng, X.; Liu, J. Microwave Promoted Environmentally Benign Synthesis of 2-Aminobenzothiazoles and Their Urea Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1124–1133. [Google Scholar] [CrossRef]
- CLSI M26A. Methods for Determining Bactericidal Activity of Antimicrobial Agents, 1st ed. 1999. Available online: https://clsi.org (accessed on 23 March 2021).
- Iacopetta, D.; Rosano, C.; Puoci, F.; Parisi, O.I.; Saturnino, C.; Caruso, A.; Longo, P.; Ceramella, J.; Malzert-Freon, A.; Dallemagne, P.; et al. Multifaceted properties of 1,4-dimethylcarbazoles: Focus on trimethoxybenzamide and trimethoxyphenylurea derivatives as novel human topoisomerase II inhibitors. Eur. J. Pharm. Sci. 2017, 96, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Caruso, A.; Conforti, F.; Marrelli, M.; El Kashef, H.; Lancelot, J.C.; Rault, S.; Statti, G.A.; Menichini, F. Synthesis, inhibition of NO production and antiproliferative activities of some indole derivatives. J. Enzyme Inhib. Med. Chem. 2009, 24, 1148–1153. [Google Scholar] [CrossRef] [PubMed]


| Microorganisms (MIC, µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | |||||||
| Compd | Structure | S.a. 25923 | S.a. 29213 | S.a. 6538 | S.a. 6538p | E.f. 29212 | P. a. 27853 | K.p. 13883 |
| 2eA | 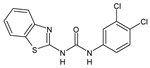 | nt | 64 | 32 | 16 | 32 | >256 | >256 |
| 2eB |  | nt | 64 | 128 | A.N. | 32 | >256 | A.N. |
| 2bB | 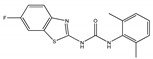 | nt | 16 | 128 | A.N. | 8 | >256 | A.N. |
| 2cC | 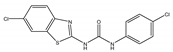 | A.N. | >512 | A.N. | >512 | A.N. | A.N. | A.N. |
| 2bC | 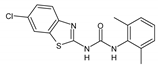 | 128 | 128 | 64 | 64 | 256 | >512 | >512 |
| 2bF |  | 32 | 32 | 8 | 8 | 32 | >512 | >512 |
| 2eE | 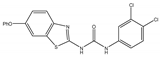 | 16 | 16 | 16 | 16 | 64 | >512 | >512 |
| 2eC | 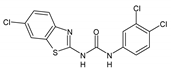 | 16 | 16 | 8 | 8 | 32 | >512 | >512 |
| 2dC | 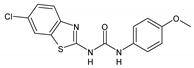 | 512 | 512 | 256 | 128 | 128 | >512 | >512 |
| 2bG a |  | 32 | 32 | 64 | 64 | 32 | >512 | >512 |
| NRF b | - | 0.5–2 | nt | nt | 2–8 | 1–4 | nt | |
| TCC | 16 | 16 | 128 | 16 | 64 | 256 | 256 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, A.; Rosato, A.; Salvagno, L.; Iacopetta, D.; Ceramella, J.; Fracchiolla, G.; Sinicropi, M.S.; Franchini, C. Benzothiazole-Containing Analogues of Triclocarban with Potent Antibacterial Activity. Antibiotics 2021, 10, 803. https://doi.org/10.3390/antibiotics10070803
Catalano A, Rosato A, Salvagno L, Iacopetta D, Ceramella J, Fracchiolla G, Sinicropi MS, Franchini C. Benzothiazole-Containing Analogues of Triclocarban with Potent Antibacterial Activity. Antibiotics. 2021; 10(7):803. https://doi.org/10.3390/antibiotics10070803
Chicago/Turabian StyleCatalano, Alessia, Antonio Rosato, Lara Salvagno, Domenico Iacopetta, Jessica Ceramella, Giuseppe Fracchiolla, Maria Stefania Sinicropi, and Carlo Franchini. 2021. "Benzothiazole-Containing Analogues of Triclocarban with Potent Antibacterial Activity" Antibiotics 10, no. 7: 803. https://doi.org/10.3390/antibiotics10070803
APA StyleCatalano, A., Rosato, A., Salvagno, L., Iacopetta, D., Ceramella, J., Fracchiolla, G., Sinicropi, M. S., & Franchini, C. (2021). Benzothiazole-Containing Analogues of Triclocarban with Potent Antibacterial Activity. Antibiotics, 10(7), 803. https://doi.org/10.3390/antibiotics10070803










