Antibacterial, Antibiofilm and Anti-Virulence Activity of Biactive Fractions from Mucus Secretion of Giant African Snail Achatina fulica against Staphylococcus aureus Strains
Abstract
:1. Introduction
2. Results
2.1. Purification and Characterization of Fractions from Mucus of Achatina fulica
2.2. In Vitro Determination of MIC
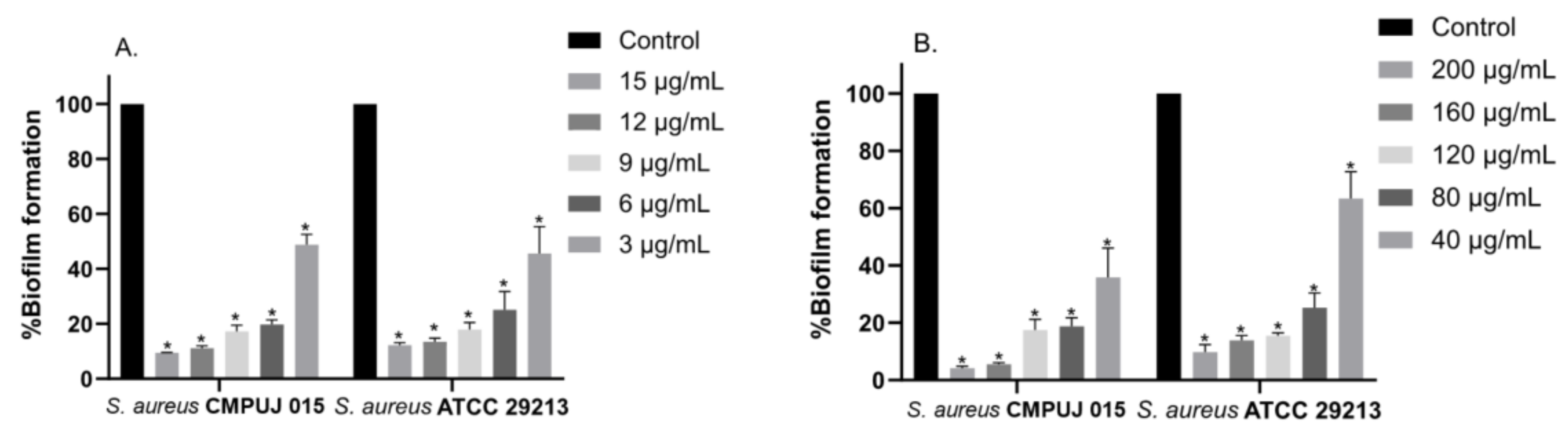
2.3. In Vitro Inhibition of Biofilm Formation
2.4. Inhibition of Virulence Factors
3. Discussion
4. Materials and Methods
4.1. Mucus Collection and Sample Fractionation
4.2. SDS-PAGE Electrophoresis
4.3. Molecular Mass Analysis
4.4. Bacterial Strains and Growth Conditions
4.5. Determination of Minimum Inhibitory Concentration (MIC50)
4.6. Antibiofilm Activity Assay
4.7. Anti-Virulence Activity Assay
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.; M’ikanatha, N.M.; Read, A.F. Antibiotic Resistance: A Primer and Call to Action. Health Commun. 2015, 30, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO|Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Galdiero, E.; Lombardi, L.; Falanga, A.; Libralato, G.; Guida, M.; Carotenuto, R. Biofilms: Novel strategies based on antimicrobial peptides. Pharmaceutics 2019, 11, 322. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Parisi, M.G.; Parrinello, N.; Cammarata, M.; Roch, P. Molluscan antimicrobial peptides, a review from activity-based evidences to computer-assisted sequences. Invertebr. Surviv. J. 2011, 8, 85–97. [Google Scholar]
- Balandin, S.V.; Ovchinnikova, T.V. Antimicrobial peptides of invertebrates. Part 1. structure, biosynthesis, and evolution. Russ. J. Bioorganic Chem. 2016, 42, 229–248. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Liu, L.; Benkendorff, K. Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease. Mar. Drugs 2020, 18, 570. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.; Rey, A.; López, J.P.; Castro, J.P.; Uribe, N. Physicochemical characterization and antimicrobial activity of mucus of Achatina fulica. Rev. Univ. Ind. Santander. Salud 2016, 48, 188–195. [Google Scholar]
- Courchamp, F. Alien species: Monster fern makes IUCN invader list. Nature 2013, 498, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iguchi, S.M.; Aikawa, T.; Matsumoto, J.J. Antibacterial Activity of Snail Mucus Mucin. Comp. Biochem. Physiol. 1982, 72, 571–574. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, W.; Yang, X.; Yan, X.; Liu, R. A novel cysteine-rich antimicrobial peptide from the mucus of the snail of Achatina fulica. Peptides 2013, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hirasawa, N.; Lee, Y.S.; Kim, Y.S.; Shin, K.H.; Ryu, N.; Ohuchi, K. Inhibition by acharan sulphate of angiogenesis in experimental inflammation models. Br. J. Pharmacol. 2010, 137, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmester, T. Molecular Evolution of the Arthropod Hemocyanin Superfamily. Mol. Biol. Evol. 2001, 18, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiumiento, I.R.; Ituarte, S.; Sun, J.; Qiu, J.W.; Heras, H.; Sebastiá, N.; Dreonid, M. Hemocyanin of the caenogastropod Pomacea canaliculata exhibits evolutionary differences among gastropod clades. PLoS ONE 2020, 15, e0228325. [Google Scholar] [CrossRef] [Green Version]
- Riciluca, K.C.T.; Sayegh, R.S.R.; Melo, R.L.; Silva, P.I. Rondonin an antifungal peptide from spider (Acanthoscurria rondoniae) haemolymph. Results Immunol. 2012, 2, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, B.L.; Söderhäll, K. Processing of an antibacterial peptide from hemocyanin of the freshwater crayfish Pacifastacus leniusculus. J. Biol. Chem. 2003, 278, 7927–7933. [Google Scholar] [CrossRef] [Green Version]
- Ishwarya, R.; Vaseeharan, B.; Jayakumar, R.; Ramasubramanian, V.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-anbr, M.N.; Benelli, G. Bio-mining drugs from the sea: High antibiofilm properties of haemocyanin purified from the haemolymph of flower crab Portunus pelagicus (L.) (Decapoda: Portunidae). Aquaculture 2018, 489, 130–140. [Google Scholar] [CrossRef]
- Obara, K.; Otsuka-Fuchino, H.; Sattayasai, N.; Nonomura, Y.; Tsuchiya, T.; Tamiya, T. Molecular cloning of the antibacterial protein of the giant African snail, Achatina fulica Ferussac. Eur. J. Biochem. 1992, 209, 1–6. [Google Scholar] [CrossRef]
- Ogawa, M.; Nakamura, S.; Atsuchi, T.; Tamiya, T.; Tsuchiya, T.; Nakai, S. Macromolecular antimicrobial glycoprotein, achacin, expressed in a methylotrophic yeast Pichia pastoris. FEBS Lett. 1999, 448, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Kriswandini, I.L. Antimicrobial proteins of Snail mucus (Achatina fulica) against Streptococcus mutans and Aggregatibacter actinomycetemcomitans. Dent. J. 2014, 47, 31–36. [Google Scholar]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.-H.; Kim, S.-I.; Cho, M.H.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, A.; Srinivasan, R.; Nivetha, A.; Annapoorani, A.; Pandian, S.K.; Ravi, A.V. Anti-virulence potential of 2-hydroxy-4-methoxybenzaldehyde against methicillin-resistant Staphylococcus aureus and its clinical isolates. Appl. Microbiol. Biotechnol. 2019, 103, 6747–6758. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-Ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and antibiofilm peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2017, 122, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sequeda, N.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Antimicrobial and antibiofilm activities of essential oils against Escherichia coli O157:H7 and methicillin-resistant staphylococcus aureus (MRSA). Antibiotics 2020, 9, 730. [Google Scholar] [CrossRef]
- Patel, M.; Ashraf, M.S.; Siddiqui, A.J.; Ashraf, S.A.; Sachidanandan, M.; Snoussi, M.; Adnan, M.; Hadi, S. Profiling and role of bioactive molecules from puntius sophore (Freshwater/brackish fish) skin mucus with its potent antibacterial, antiadhesion, and antibiofilm activities. Biomolecules 2020, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Ranjan, A.; Asokan, G.V.; Kasimanickam, V.R.; Kastelic, J.P. Prevention and treatment of biofilms by hybrid- and nanotechnologies. Int. J. Nanomed. 2013, 8, 2809–2819. [Google Scholar] [CrossRef]
- Dostert, M.; Belanger, C.R.; Hancock, R.E.W. Design and Assessment of Anti-Biofilm Peptides: Steps Toward Clinical Application. J. Innate Immun. 2019, 11, 193–204. [Google Scholar] [CrossRef]
- Ullal, A.J.; Wayne Litaker, R.; Noga, E.J. Antimicrobial peptides derived from hemoglobin are expressed in epithelium of channel catfish (Ictalurus punctatus, Rafinesque). Dev. Comp. Immunol. 2008, 32, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Aweya, J.J.; Wang, F.; Yao, D.; Zhong, M.; Chen, J.; Li, S.; Zhang, Y. Litopenaeus Vannamei Attenuates White Spot Syndrome Virus Replication by Specific Antiviral Peptides Generated from Hemocyanin; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 91, ISBN 8675486502. [Google Scholar]
- Petit, V.W.; Rolland, J.L.; Blond, A.; Cazevieille, C.; Djediat, C.; Peduzzi, J.; Goulard, C.; Bachère, E.; Dupont, J.; Destoumieux-Garzón, D.; et al. A hemocyanin-derived antimicrobial peptide from the penaeid shrimp adopts an alpha-helical structure that specifically permeabilizes fungal membranes. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, M.L.; Lima, D.B.; de Menezes, R.R.P.P.B.; Sampaio, T.L.; Silva, B.P.; Nunes, J.V.S.; Cavalcanti, M.M.; Morlighem, J.-E.; Martins, A.M.C. Antichagasic effect of hemocyanin derived from antimicrobial peptides of penaeus monodon shrimp. Exp. Parasitol. 2020, 215, 107930. [Google Scholar] [CrossRef]
- Zhuang, J.; Coates, C.J.; Zhu, H.; Zhu, P.; Wu, Z.; Xie, L. Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev. Comp. Immunol. 2015, 49, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Dolashki, A.; Velkova, L.; Daskalova, E.; Zheleva, N.; Topalova, Y.; Atanasov, V.; Voelter, W.; Dolashka, P. Antimicrobial Activities of Different Fractions from Mucus of the Garden Snail Cornu aspersum. Biomedicines 2020, 8, 315. [Google Scholar] [CrossRef]
- Ortiz López, C. Design, synthesis, characterization and in vitro evaluation of antimicrobial peptides against pathogenic bacteria resistant to antibiotics. Rev. Acad. Colomb. Cienc. Exact. Fis. Nat. 2019, 43, 614–627. [Google Scholar] [CrossRef]
- Cruz, J.; Ortiz, C.; Guzman, F.; Cardenas, C.; Fernandez-Lafuente, R.; Torres, R. Design and activity of novel lactoferrampin analogues against O157:H7 enterohemorrhagic escherichia coli. Biopolymers 2014, 101, 319–328. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 1-56238-988-2. [Google Scholar]
- Cruz, J.; Flórez, J.; Torres, R.; Urquiza, M.; Gutiérrez, J.A.; Guzmán, F.; Ortiz, C.C. Antimicrobial activity of a new synthetic peptide loaded in polylactic acid or poly(lactic-co-glycolic) acid nanoparticles against Pseudomonas aeruginosa, Escherichia coli O157:H7 and methicillin resistant Staphylococcus aureus (MRSA). Nanotechnology 2017, 28, 135102. [Google Scholar] [CrossRef] [PubMed]
- Molhoek, E.M.; van Dijk, A.; Veldhuizen, E.J.A.; Haagsman, H.P.; Bikker, F.J. A cathelicidin-2-derived peptide effectively impairs Staphylococcus epidermidis biofilms. Int. J. Antimicrob. Agents 2011, 37, 476–479. [Google Scholar] [CrossRef] [Green Version]

| # | Sequence | Origin | ID |
|---|---|---|---|
| 1 | IYSRPADTFDYRN | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VFQ5 |
| 2 | RLLTVQAENALRKH | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VFQ5 |
| 3 | RKPLQPFQDKT | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VFQ5 |
| 4 | RLHGIGVSADVRV | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VFQ5 |
| 5 | REMPWAYERL | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VFQ5 |
| 6 | RKPLQPFQDKK | Hemocyanin alphaD OS = Helix pomatia OX = 6536 PE = 2 SV = 1 | A0A3G2VHR9 |
| 7 | RYSRPADTFDYRN | Hemocyanin 1 OS = Lymnaea stagnalis OX = 6523 PE = 2 SV = 1 | A0A3G2VM51 |
| 8 | RAIDAFDYDRL | Hemocyanin 1 OS = Lymnaea stagnalis OX = 6523 PE = 2 SV = 1 | A0A3G2VM51 |
| 9 | KYDVTNVFNKL | Hemocyanin 1 OS = Lymnaea stagnalis OX = 6523 PE = 2 SV = 1 | A0A3G2VM51 |
| 10 | KEMPWAYERI | Hemocyanin 1 OS = Lymnaea stagnalis OX = 6523 PE = 2 SV = 1 | A0A3G2VM51 |
| 11 | SGRVEFEHVDTERD | Hemocyanin alphaN-subunit (Fragment) OS = Helix lucorum OX = 31,229 PE = 2 SV = 1 | G3FPE7 |
| 12 | RYDVTNVFNKL | Hemocyanin alphaN-subunit (Fragment) OS = Helix lucorum OX = 31,229 PE = 2 SV = 1 | G3FPE7 |
| 13 | RLYVVQLEQALKEKG | Hemocyanin 1 OS = Lymnaea stagnalis OX = 6523 PE = 2 SV = 1 | A0A3G2VM51 |
| 14 | DPLFLLHHSNVDRQ | Hemocyanin 1 OS = Lymnaea stagnalis OX = 6523 PE = 2 SV = 1 | A0A3G2VM51 |
| 15 | KYSRPIDTFDYRN | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VHN3 |
| 16 | RLLTVQAENALRN | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VHN3 |
| 17 | RIYIVVEDH | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VHN3 |
| 18 | RAIDAFDYDRF | Hemocyanin 1 OS = Aplysia californica OX = 6500 PE = 2 SV = 1 | A0A3G9M8B7 |
| 19 | RLLTVQAENALRR | Hemocyanin 1 OS = Aplysia californica OX = 6500 PE = 2 SV = 1 | A0A3G9M8B7 |
| 20 | KVAGEDAVTTRD | Hemocyanin alphaD OS = Cornu aspersum OX = 6535 PE = 2 SV = 1 | A0A3G2VFQ5 |
| Mucus Fraction | Antimicrobial Activity | |||
|---|---|---|---|---|
| S. aureus CMPUJ 015 | S. aureus ATCC 29213 | |||
| MIC20 | MIC50 | MIC20 | MIC50 | |
| FMA30 | 10 | 25 | 12 | 125 |
| FME30 | 120 | 500 | 250 | 750 |
| Mucus Fraction | S. aureus CMPUJ 015 | S. aureus ATCC 29213 | ||
|---|---|---|---|---|
| Hemolytic Activity Inhibition (%) | Protease Activity Inhibition (%) | Hemolytic Activity Inhibition (%) | Protease Activity Inhibition (%) | |
| FMA30 | 98.60 ± 1.44 | 73.55 ± 2.08 | 97.43 ± 2.97 | <20 |
| FME30 | 96.69 ± 1.89 | 80.34 ± 3.72 | 75.74 ± 3.39 | 31.46 ± 6.15 |
| Mucus Fraction | S. aureus CMPUJ 015 | S. aureus ATCC 29213 |
|---|---|---|
| FMA30 | +++ | ++ |
| FME30 | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, L.; Pereira, A.; Hidalgo, W.; Uribe, N. Antibacterial, Antibiofilm and Anti-Virulence Activity of Biactive Fractions from Mucus Secretion of Giant African Snail Achatina fulica against Staphylococcus aureus Strains. Antibiotics 2021, 10, 1548. https://doi.org/10.3390/antibiotics10121548
Suárez L, Pereira A, Hidalgo W, Uribe N. Antibacterial, Antibiofilm and Anti-Virulence Activity of Biactive Fractions from Mucus Secretion of Giant African Snail Achatina fulica against Staphylococcus aureus Strains. Antibiotics. 2021; 10(12):1548. https://doi.org/10.3390/antibiotics10121548
Chicago/Turabian StyleSuárez, Libardo, Andrés Pereira, William Hidalgo, and Nelson Uribe. 2021. "Antibacterial, Antibiofilm and Anti-Virulence Activity of Biactive Fractions from Mucus Secretion of Giant African Snail Achatina fulica against Staphylococcus aureus Strains" Antibiotics 10, no. 12: 1548. https://doi.org/10.3390/antibiotics10121548
APA StyleSuárez, L., Pereira, A., Hidalgo, W., & Uribe, N. (2021). Antibacterial, Antibiofilm and Anti-Virulence Activity of Biactive Fractions from Mucus Secretion of Giant African Snail Achatina fulica against Staphylococcus aureus Strains. Antibiotics, 10(12), 1548. https://doi.org/10.3390/antibiotics10121548






