Antimicrobial and Antibiofilm Activity of the Probiotic Strain Streptococcus salivarius K12 against Oral Potential Pathogens
Abstract
1. Introduction
2. Results
2.1. Identification of S. salivarius K12
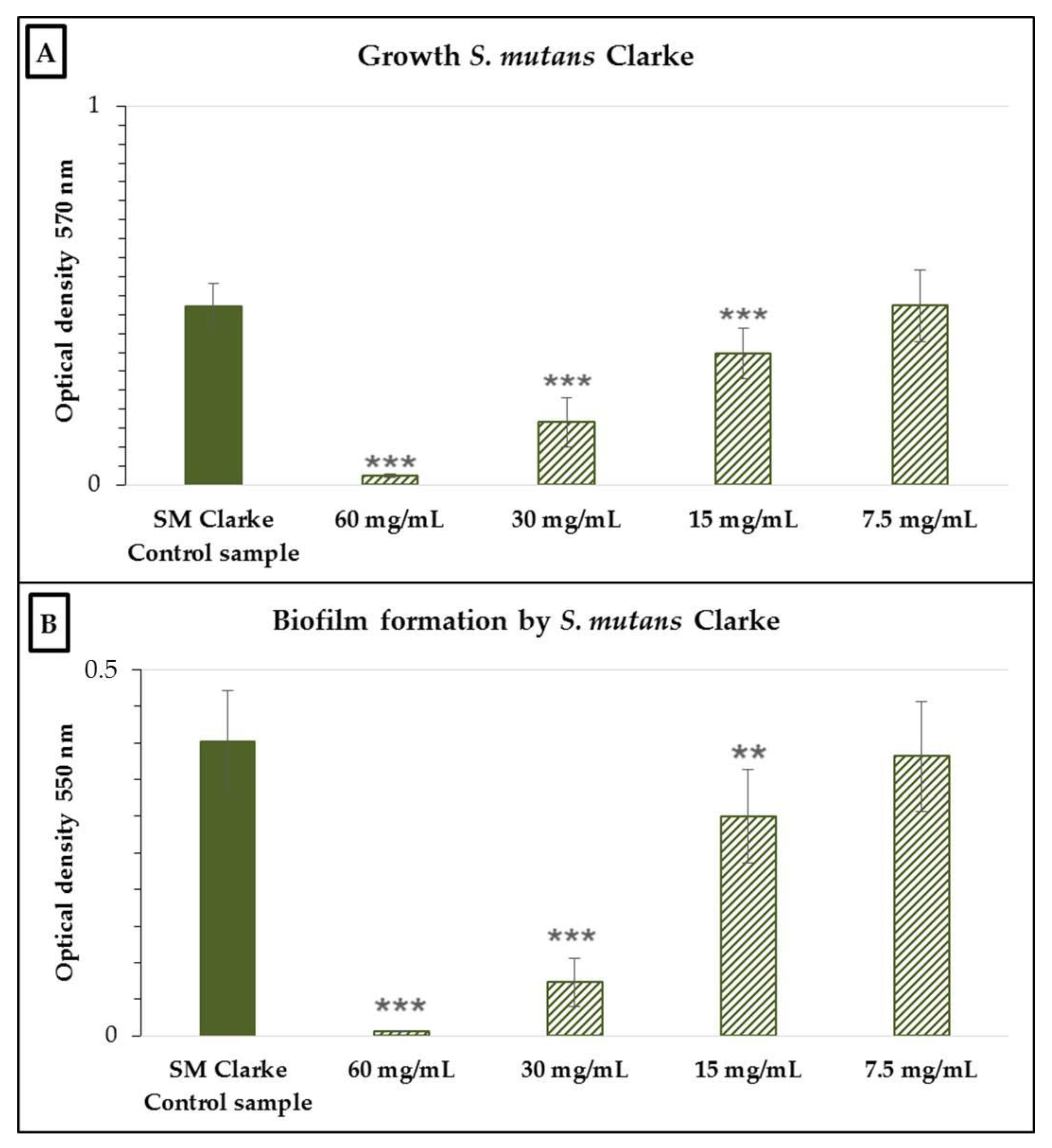
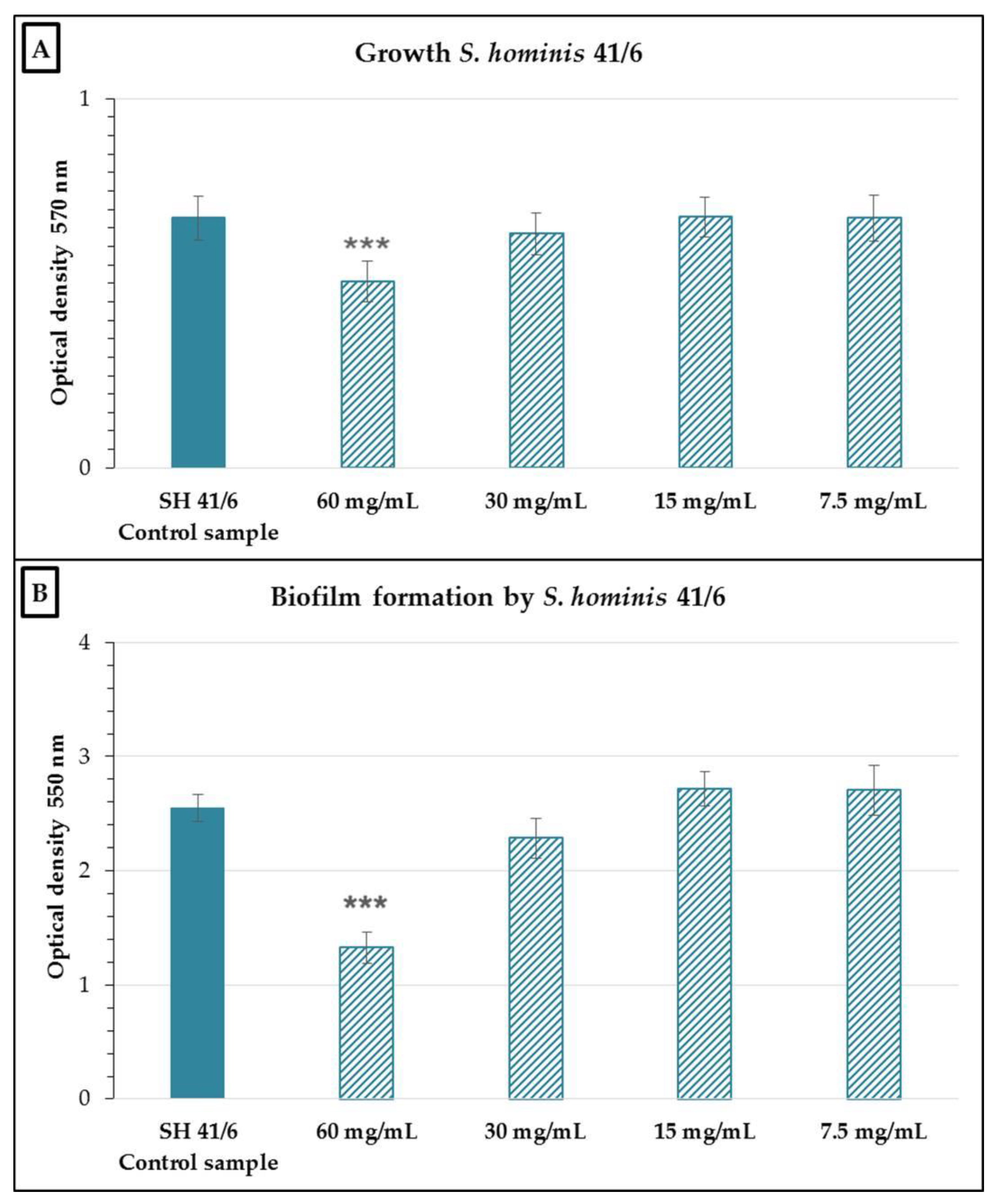
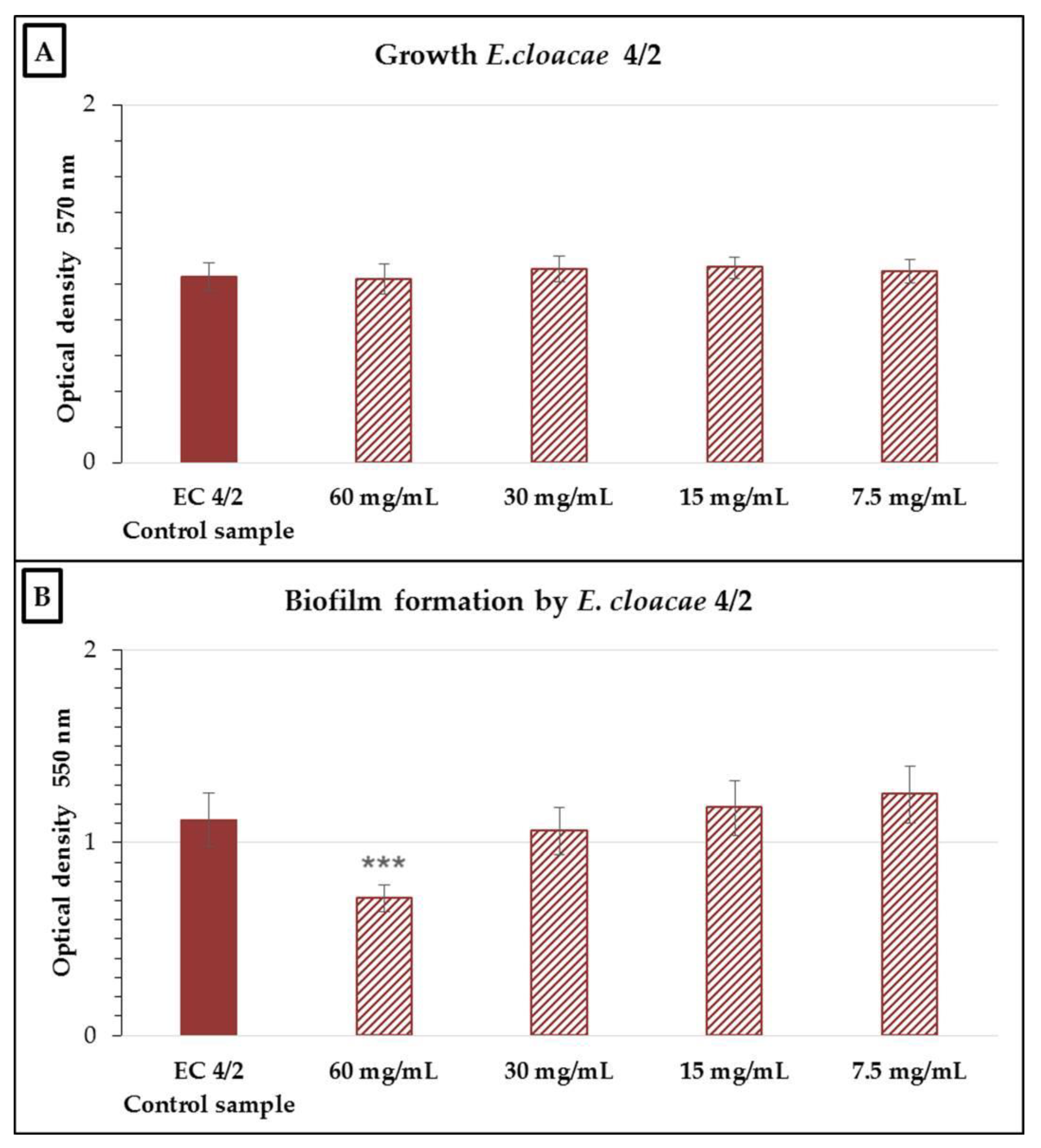
2.2. Antimicrobial and Antibiofilm Activity of the Neutralized Cell-Free Supernatant of S. salivarius K12 against Potential Oral Pathogens
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Preparation of the nCFS of S. salivarius K12
4.3. In Vitro Assay for Antibiofilm and Antimicrobial Activity of the nCFS
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Allaker, R.P.; Ian Douglas, C.W. Non-conventional therapeutics for oral infections. Virulence 2015, 6, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.; Bandara, N.; Pesee, S. Oral Biofilms: What Are They? In Oral Biofilms and Modern Dental Materials, 1st ed.; Ionescu, A.C., Hahnel, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–7. [Google Scholar]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B.; Takahashi, N. Integrated hypothesis of dental caries and periodontal diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Colombo, M.; Zanvit, A.; Rottoli, A.S. Positive clinical outcomes derived from using Streptococcus salivarius K12 to prevent streptococcal pharyngotonsillitis in children: A pilot investigation. Drug Healthc. Patient Saf. 2016, 8, 77–81. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, M.; Liu, R.; Tian, Y.; Vu, V.H.; Li, Y.; Liu, B.; Kushmaro, A.; Li, Y.; Sun, Q. Inhibition of Streptococcus mutans Biofilm Formation and Virulence by Lactobacillus plantarum K41 Isolated from Traditional Sichuan Pickles. Front. Microbiol. 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Wade, W.G. Actinomyces and related organisms in human infections. Clin. Microbiol. Rev. 2015, 28, 419–442. [Google Scholar] [CrossRef]
- Szczuka, E.; Telega, K.; Kaznowski, A. Biofilm formation by Staphylococcus hominis strains isolated from human clinical specimens. Folia Microbiol. 2015, 60, 1–5. [Google Scholar] [CrossRef]
- Wischer, D.; Schneider, D.; Poehlein, A.; Herrmann, F.; Oruc, H.; Meinhardt, J.; Wagner, O.; Ahmed, R.; Kharin, S.; Novikova, N.; et al. Novel Antimicrobial Cellulose Fleece Inhibits Growth of Human-Derived Biofilm-Forming Staphylococci During the SIRIUS19 Simulated Space Mission. Front. Microbiol. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Zurob, E.; Dennett, G.; Gentil, D.; Montero-Silva, F.; Gerber, U.; Naulín, P.; Gómez, A.; Fuentes, R.; Lascano, S.; Rodrigues da Cunha, T.H.; et al. Inhibition of Wild Enterobacter cloacae Biofilm Formation by Nanostructured Graphene- and Hexagonal Boron Nitride-Coated Surfaces. Nanomaterials 2019, 9, 49. [Google Scholar] [CrossRef]
- Sweileh, W.M.; Shraim, N.Y.; Al-Jabi, S.W.; Sawalha, A.F.; Rahhal, B.; Khayyat, R.A.; Zyoud, S.H. Assessing worldwide research activity on probiotics in pediatrics using Scopus database: 1994–2014. World Allergy Organ. J. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Mahasneh, S.A.; Mahasneh, A.M. Probiotics: A Promising Role in Dental Health. Dent. J. 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef]
- Dodoo, C.C.; Stapleton, P.; Basit, A.W.; Gaisford, S. The potential of Streptococcus salivarius oral films in the management of dental caries: An inkjet printing approach. Int. J. Pharm. 2020, 591, 119962. [Google Scholar] [CrossRef] [PubMed]
- Couvigny, B.; Lapaque, N.; Rigottier-Gois, L.; Guillot, A.; Chat, S.; Meylheuc, T.; Kulakauskas, S.; Rohde, M.; Mistou, M.Y.; Renault, P.; et al. Three glycosylated serine-rich repeat proteins play a pivotal role in adhesion and colonization of the pioneer commensal bacterium, Streptococcus salivarius. Environ. Microbiol. 2017, 19, 3579–3594. [Google Scholar] [CrossRef]
- Gong, S.G.; Chan, Y.; Lévesque, C.M. Complete Genome Sequence of Megaplasmid-Bearing Streptococcus salivarius Strain LAB813, Isolated from the Dental Plaque of a Caries-Free Child. Microbiol. Resour. Announc. 2019, 8, e01092-19. [Google Scholar] [CrossRef]
- Hols, P.; Ledesma-García, L.; Gabant, P.; Mignolet, J. Mobilization of Microbiota Commensals and Their Bacteriocins for Therapeutics. Trends Microbiol. 2019, 27, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Fantinato, V.; Camargo, H.R.; Sousa, A.L.O.P. Probiotics study with Streptococcus salivarius and its ability to produce bacteriocins and adherence to KB cells. Rev. Odontol. UNESP 2019, 48, e20190029. [Google Scholar] [CrossRef]
- Hu, L.; Mao, Q.; Zhou, P.; Lv, X.; Hua, H.; Yan, Z. Effects of Streptococcus salivarius K12 with nystatin on oral candidiasis-RCT. Oral Dis. 2019, 25, 1573–1580. [Google Scholar] [CrossRef]
- Zupancic, K.; Kriksic, V.; Kovacevic, I.; Kovacevic, D. Influence of Oral Probiotic Streptococcus salivarius K12 on Ear and Oral Cavity Health in Humans: Systematic Review. Probiotics Antimicrob. Proteins 2017, 9, 102–110. [Google Scholar] [CrossRef]
- Reid, P.; Heng, N.C.K.; Hale, J.D.; Krishnan, D.; Crane, J.; Tagg, J.R.; Milne, T.J. A TaqMan™-based quantitative PCR screening assay for the probiotic Streptococcus salivarius K12 based on the specific detection of its megaplasmid-associated salivaricin B locus. J. Microbiol. Methods 2020, 170, 105837. [Google Scholar] [CrossRef] [PubMed]
- Gregori, G.; Righi, O.; Risso, P.; Boiardi, G.; Demuru, G.; Ferzetti, A.; Galli, A.; Ghisoni, M.; Lenzini, S.; Marenghi, C.; et al. Reduction of group A beta-hemolytic streptococcus pharyngo-tonsillar infections associated with use of the oral probiotic Streptococcus salivarius K12: A retrospective observational study. Ther. Clin. Risk Manag. 2016, 12, 87–92. [Google Scholar] [CrossRef]
- Masdea, L.; Kulik, E.M.; Hauser-Gerspach, I.; Ramseier, A.M.; Filippi, A.; Waltimo, T. Antimicrobial activity of Streptococcus salivarius K12 on bacteria involved in oral malodour. Arch. Oral Biol. 2012, 57, 1041–1047. [Google Scholar] [CrossRef]
- Di Pierro, F.; Colombo, M.; Zanvit, A.; Risso, P.; Rottoli, A.S. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc. Patient Saf. 2014, 6, 15–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, T.Y.; Hale, J.D.F.; Tagg, J.R.; Jain, R.; Voss, A.L.; Mills, N.; Best, E.J.; Stevenson, D.S.; Bird, P.A.; Walls, T. In vitro Inhibition of Clinical Isolates of Otitis Media Pathogens by the Probiotic Streptococcus salivarius BLIS K12. Probiotics Antimicrob. Proteins 2021, 13, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.A.; Hayama, K.; Burton, J.P.; Reid, G.; Okada, M.; Matsushita, Y.; Abe, S. Effect of Streptococcus salivarius K12 on the in vitro growth of Candida albicans and its protective effect in an oral candidiasis model. Appl. Environ. Microbiol. 2012, 78, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, P.; Sjollema, J.; Cammue, B.P.; Lagrou, K.; Berman, J.; d’Enfert, C.; Andes, D.R.; Arendrup, M.C.; Brakhage, A.A.; Calderone, R.; et al. Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microb. Cell 2018, 5, 300–326. [Google Scholar] [CrossRef]
- Yoo, H.J.; Jwa, S.K.; Kim, D.H.; Ji, Y.J. Inhibitory effect of Streptococcus salivarius K12 and M18 on halitosis in vitro. Clin. Exp. Dent. Res. 2020, 6, 207–214. [Google Scholar] [CrossRef]
- Ogawa, A.; Furukawa, S.; Fujita, S.; Mitobe, J.; Kawarai, T.; Narisawa, N.; Sekizuka, T.; Kuroda, M.; Ochiai, K.; Ogihara, H.; et al. Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl. Environ. Microbiol. 2011, 77, 1572–1580. [Google Scholar] [CrossRef]
- Vacca, C.; Contu, M.P.; Rossi, C.; Ferrando, M.L.; Blus, C.; Szmukler-Moncler, S.; Scano, A.; Orrù, G. In vitro Interactions between Streptococcus intermedius and Streptococcus salivarius K12 on a Titanium Cylindrical Surface. Pathogens 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Dinu, L.D.; Luntraru, C.M.; Suciu, A. In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model. Appl. Sci. 2021, 11, 4315. [Google Scholar] [CrossRef]
- Chaffanel, F.; Charron-Bourgoin, F.; Libante, V.; Leblond-Bourget, N.; Payot, S. Resistance Genes and Genetic Elements Associated with Antibiotic Resistance in Clinical and Commensal Isolates of Streptococcus salivarius. Appl. Environ. Microbiol. 2015, 81, 4155–4163. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Höfling, J.F.; Klein, M.I.; Kamiya, R.U.; Gonçalves, R.B. Tansmission, diversity and virulence factors of Sreptococcus mutans genotypes. J. Oral Sci. 2005, 47, 59–64. [Google Scholar] [CrossRef]
- Hyink, O.; Wescombe, P.A.; Upton, M.; Ragland, N.; Burton, J.P.; Tagg, J.R. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl. Environ. Microbiol. 2007, 73, 1107–1113. [Google Scholar] [CrossRef]
- Frickmann, H.; Klenk, C.; Warnke, P.; Redanz, S.; Podbielski, A. Influence of Probiotic Culture Supernatants on In Vitro Biofilm Formation of Staphylococci. Eur. J. Microbiol. Immunol. 2018, 8, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bidossi, A.; De Grandi, R.; Toscano, M.; Bottagisio, M.; De Vecchi, E.; Gelardi, M.; Drago, L. Probiotics Streptococcus salivarius 24SMB and Streptococcus oralis 89a interfere with biofilm formation of pathogens of the upper respiratory tract. BMC Infect. Dis. 2018, 18, 653. [Google Scholar] [CrossRef]
- Barbour, A.; Wescombe, P.; Smith, L. Evolution of Lantibiotic Salivaricins: New Weapons to Fight Infectious Diseases. Trends Microbiol. 2020, 28, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Tagg, J.; Abou-Zied, O.K.; Philip, K. New insights into the mode of action of the lantibiotic salivaricin B. Sci. Rep. 2016, 6, 31749. [Google Scholar] [CrossRef] [PubMed]
- Wescombe, P.A.; Heng, N.C.; Burton, J.P.; Tagg, J.R. Something Old and Something New: An Update on the Amazing Repertoire of Bacteriocins Produced by Streptococcus salivarius. Probiotics Antimicrob. Proteins 2010, 2, 37–45. [Google Scholar] [CrossRef]
- Jalali, F.; Ellett, F.; Balani, P.; Duncan, M.J.; Dewhirst, F.E.; Borisy, G.G.; Irimia, D. No man’s land: Species-specific formation of exclusion zones bordering Actinomyces graevenitzii microcolonies in nanoliter cultures. Microbiologyopen 2021, 10, e1137. [Google Scholar] [CrossRef]
- Guo, H.; Rivailler, P.; Wang, J.; Wang, H.; Xu, W.; Xu, S.; Xu, H.; Hu, R. Metagenomic Analysis of a Throat Swab Sample Collected in China on A Patient Infected with Varicella Zoster Virus. Res. Sq. 2021, in press. [Google Scholar] [CrossRef]
- Davis, I.J.; Richards, H.; Mullany, P. Isolation of silver- and antibiotic-resistant Enterobacter cloacae from teeth. Oral Microbiol. Immunol. 2005, 20, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Davin-Regli, A.; Pagès, J.M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.E.; O’toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Llena, C.; Almarche, A.; Mira, A.; López, M.A. Antimicrobial efficacy of the supernatant of Streptococcus dentisani against microorganisms implicated in root canal infections. J. Oral Sci. 2019, 61, 184–194. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Dymock, D.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [CrossRef]
- Hoshino, T.; Kawaguchi, M.; Shimizu, N.; Hoshino, N.; Ooshima, T.; Fujiwara, T. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn. Microbiol. Infect. Dis. 2004, 48, 195–199. [Google Scholar] [CrossRef]
- O’Shea, E.F.; Gardiner, G.E.; O’Connor, P.M.; Mills, S.; Ross, R.P.; Hill, C. Characterization of enterocin- and salivaricin-producing lactic acid bacteria from the mammalian gastrointestinal tract. FEMS Microbiol. Lett. 2009, 291, 24–34. [Google Scholar] [CrossRef]
- Wescombe, P.A.; Burton, J.P.; Cadieux, P.A.; Klesse, N.A.; Hyink, O.; Heng, N.C.; Chilcott, C.N.; Reid, G.; Tagg, J.R. Megaplasmids encode differing combinations of lantibiotics in Streptococcus salivarius. Antonie Van Leeuwenhoek 2006, 90, 269–280. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Chen, Y.; Jiang, W.; Chen, H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015, 21, e128–e134. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Pratt, L.A.; Watnick, P.I.; Newman, D.K.; Weaver, V.B.; Kolter, R. Genetic approaches to study of biofilms. Methods Enzymol. 1999, 310, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stašková, A.; Sondorová, M.; Nemcová, R.; Kačírová, J.; Maďar, M. Antimicrobial and Antibiofilm Activity of the Probiotic Strain Streptococcus salivarius K12 against Oral Potential Pathogens. Antibiotics 2021, 10, 793. https://doi.org/10.3390/antibiotics10070793
Stašková A, Sondorová M, Nemcová R, Kačírová J, Maďar M. Antimicrobial and Antibiofilm Activity of the Probiotic Strain Streptococcus salivarius K12 against Oral Potential Pathogens. Antibiotics. 2021; 10(7):793. https://doi.org/10.3390/antibiotics10070793
Chicago/Turabian StyleStašková, Andrea, Miriam Sondorová, Radomíra Nemcová, Jana Kačírová, and Marián Maďar. 2021. "Antimicrobial and Antibiofilm Activity of the Probiotic Strain Streptococcus salivarius K12 against Oral Potential Pathogens" Antibiotics 10, no. 7: 793. https://doi.org/10.3390/antibiotics10070793
APA StyleStašková, A., Sondorová, M., Nemcová, R., Kačírová, J., & Maďar, M. (2021). Antimicrobial and Antibiofilm Activity of the Probiotic Strain Streptococcus salivarius K12 against Oral Potential Pathogens. Antibiotics, 10(7), 793. https://doi.org/10.3390/antibiotics10070793





