Beehive Products as Antibacterial Agents: A Review
Abstract
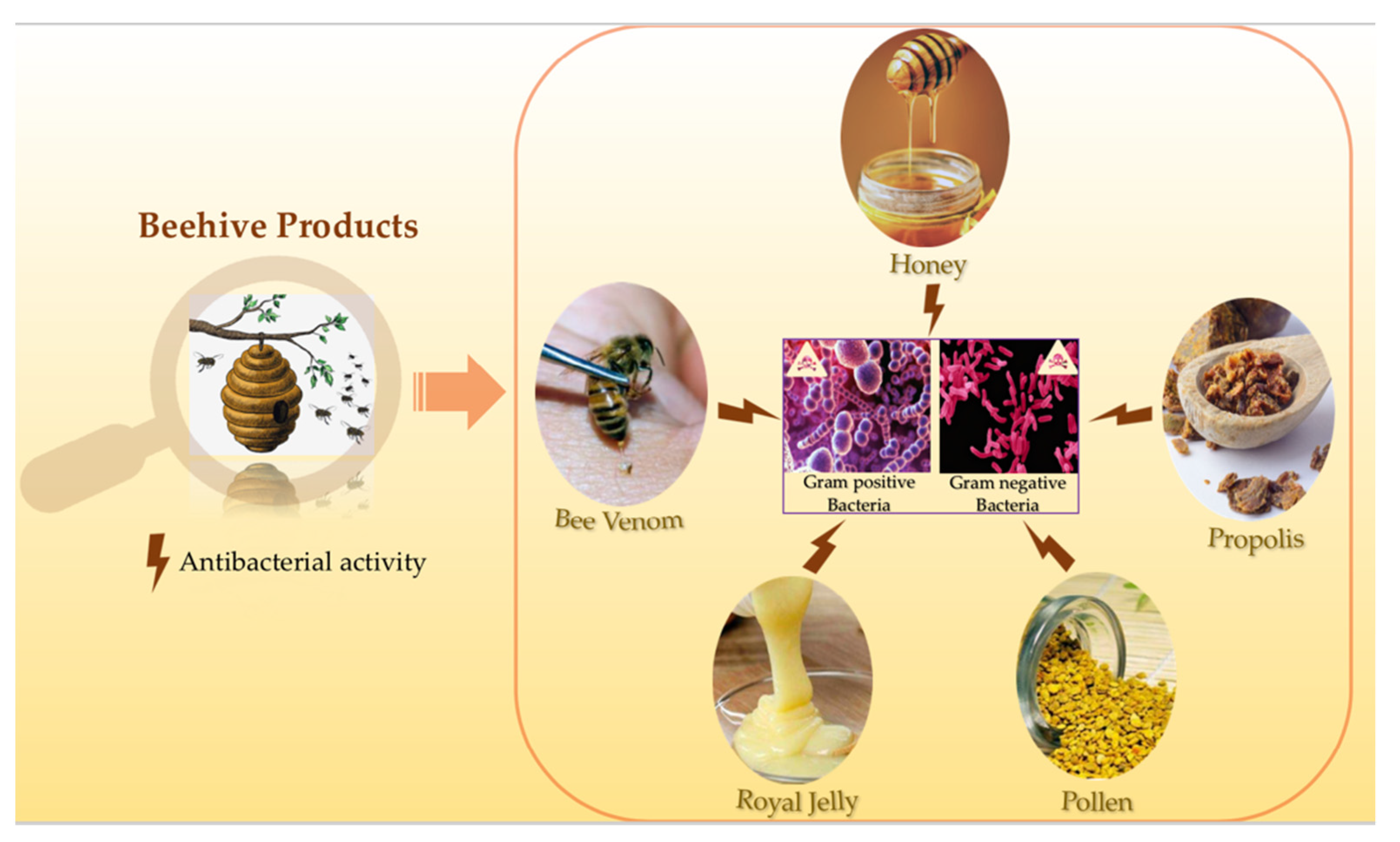
1. Introduction
2. Antibacterial Activity of Bee Honey
3. Antibacterial Activity of Bee Venom
4. Antibacterial Activity of Propolis
5. Antibacterial Activity of Bee Pollen
6. Antibacterial Activity of Royal Jelly
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Apolito Pessoa, S.M.; Balestieri, F.C.; de LM Balestieri, J.B.P. Pollen harvest by Apis mellifera L. (Hymenoptera: Apidae) in the Dourados region, Mato Grosso do Sul state (Brazil). Acta Bot. Bras. 2010, 24, 898–904. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Kremen, C. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. USA 2006, 103, 13890–13895. [Google Scholar] [CrossRef]
- Bogdanov, S. Pollen: Nutrition, Functional Properties, Health. Bee Prod. Sci. 2016, 20, 350. [Google Scholar]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Kassim, M.; Achoui, M.; Mansor, M.; Yusoff, K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia 2010, 81, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.K.B.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, V.; Lamas, A.; Regal, P.; Vazquez, B.; Miranda, J.; Cepeda, A.; Franco, C.M. Antimicrobial activity of apitoxin from Apis mellifera in Salmonella enterica strains isolated from poultry and its effects on motility, biofilm formation and gene expression. Microb. Pathog. 2019, 137, 103771. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A. Chapter 13 Bee Venom Composition: From Chemistry to Biological Activity. In Studies in Natural Products Chemistry; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.-M.; Coutard, B.; Fajloun, Z. The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components-Melittin and PLA2-On Colon Cancer HCT116 Cell Lines. Molecules 2021, 26, 2264. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front Pharmacol. 2017. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2017.00412/full (accessed on 2 June 2021).
- Paulino, N.; Abreu, S.R.L.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 1, S20–S28. [Google Scholar] [CrossRef]
- Buchwald, R.; Breed, M.D.; Greenberg, A.R.; Otis, G. Interspecific variation in beeswax as a biological construction material. J. Exp. Biol. 2006, 209, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- Bridi, R.; Atala, E.; Pizarro, P.N.; Montenegro, G. Honeybee Pollen Load: Phenolic Composition and Antimicrobial Activity and Antioxidant Capacity. J. Nat. Prod. 2019, 82, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of Bee-Pollen? J. ApiProduct ApiMedical Sci. 2010, I, 131–144. [Google Scholar] [CrossRef]
- Ahmed, R.; Tanvir, E.M.; Hossen, M.S.; Afroz, R.; Ahmmed, I.; Rumpa, N.-E.-N.; Paul, S.; Gan, S.H.; Sulaiman, S.A.; Khalil, M.I. Antioxidant Properties and Cardioprotective Mechanism of Malaysian Propolis in Rats. Evid. Based Complementary Altern. Med. 2017, 2017, e5370545. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef]
- Simúth, J.; Bíliková, K.; Kovácová, E.; Kuzmová, Z.; Schroder, W. Immunochemical approach to detection of adulteration in honey: Physiologically active royal jelly protein stimulating TNF-alpha release is a regular component of honey. J. Agric. Food Chem. 2004, 52, 2154–2158. [Google Scholar] [CrossRef]
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of Royal Jelly on Bisphenol A-Induced Proliferation of Human Breast Cancer Cells. Biosci. Biotechnol. Biochem. 2007, 71, 253–255. [Google Scholar] [CrossRef]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Ohta, S.; Sakamoto, T.; Mishima, S.; Furukawa, S. Royal jelly facilitates restoration of the cognitive ability in trimethyltin-intoxicated mice. Evid Based Complement Altern. Med. 2011, 2011, 165968. [Google Scholar] [CrossRef] [PubMed]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.; Albertini, M.; Piatti, E. Raw Milefiori honey is packed full of antioxidant. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Ahmed, A.K.J.; Hoekstra, M.J.; Hage, J.J.; Kariml, R.B. Honey-medicated dressing: Transformation of an ancient remedy into modern therapy. Ann. Plast. Surg. 2003, 50, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complementary Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, M.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef]
- Brown, E.; O’Brien, M.; Georges, K.; Suepaul, S. Physical Characteristics and Antimicrobial Properties of Apis Mellifera, Frieseomelitta Nigra and Melipona Favosa Bee Honeys from Apiaries in Trinidad and Tobago. BMC Complement Med. Ther. 2020, 20. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7076972/ (accessed on 27 April 2021). [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Da CAzeredo, L.; Azeredo, M.A.; De Souza, S.R.; Dutra, V.M. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
- Kumar, P.; Sindhu, R.K.; Narayan, S.; Singh, I. Honey collected from different floras of Chandigarh Tricity: A comparative study involving physicochemical parameters and biochemical activities. J. Diet Suppl. 2010, 7, 303–313. [Google Scholar] [CrossRef]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef]
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement Med. 2003, 9, 267–273. [Google Scholar] [CrossRef]
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89. [Google Scholar] [CrossRef]
- Taormina, P.J.; Niemira, B.A.; Beuchat, L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001, 69, 217–225. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; Miotto, D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H₂O₂-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012, 133, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M.A.; Mekawy, M.A.; Awadalla, M.M.; Mohamed, A.H. Bee honey added to the oral rehydration solution in treatment of gastroenteritis in infants and children. J. Med. Food 2010, 13, 605–609. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Bukowska, M.; Ligor, M.; Buszewski, B. Correlation Study of Honey Regarding their Physicochemical Properties and Sugars and Cyclitols Content. Molecules 2019, 20, 34. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; te Velde, A.A.; de Boer, L.; Speijer, D. Vandenbroucke-Grauls CMJE, Zaat SAJ. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a Novel Glycoside, Leptosin, as a Chemical Marker of Manuka Honey. J. Agric. Food Chem. 2012, 60, 3418–3423. [Google Scholar] [CrossRef]
- Jenkins, R.; Cooper, R. Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS ONE 2012, 7, e45600. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between Medihoney and Rifampicin against Methicillin-Resistant Staphylococcus aureus (MRSA). PLoS ONE 2013, 8, e57679. [Google Scholar] [CrossRef]
- Gethin, G.; Cowman, S.; Conroy, R. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C. Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS ONE 2014, 9, e106967. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Bargul, J.L.; Irungu, J.W.; Lattorff, H.M.G. Bioactive constituents, in vitro radical scavenging and antibacterial activities of selected Apis mellifera honey from Kenya. Int. J. Food Sci. Technol. 2020, 55, 1246–1254. [Google Scholar] [CrossRef]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Lannigan, R. Mechanism of Honey Bacteriostatic Action Against MRSA and VRE Involves Hydroxyl Radicals Generated from Honey’s Hydrogen Peroxide. Front. Microbiol. 2012, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Header, E.; Hashish, A.; ElSawy, N.; Alkushi, A.; El-Boshy, M.; Elsawy, N. Gastroprotective effect of dietary honey against acetylsalicylate induced expermental ulcer in albino rat. Life Sci. J. 2016, 13, 42–47. [Google Scholar]
- Brown, H.; Metters, G.; Hitchings, M.; Wilkinson, T.; Sousa, L.; Cooper, J.; Dance, H.; Atterbury, R.; Jenkins, R. Antibacterial and anti-virulence activity of manuka honey against genetically diverse Staphylococcus pseudintermedius. Appl. Environ. Microbiol. 2020, 86, e01768-20. [Google Scholar] [CrossRef] [PubMed]
- Alnaqdy, A.; Al-Jabri, A.; Al Mahrooqi, Z.; Nzeako, B.; Nsanze, H. Inhibition effect of honey on the adherence of Salmonella to intestinal epithelial cells in vitro. Int. J. Food Microbiol. 2005, 103, 347–351. [Google Scholar] [CrossRef]
- Badet, C.; Quero, F. The in vitro effect of manuka honeys on growth and adherence of oral bacteria. Anaerobe 2011, 17, 19–22. [Google Scholar] [CrossRef]
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head Neck Surg. 2009, 141, 114–118. [Google Scholar] [CrossRef]
- Cooper, R.A.; Wigley, P.; Burton, N.F. Susceptibility of multiresistant strains of Burkholderia cepacia to honey. Lett. Appl. Microbiol. 2000, 31, 20–24. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Cavanagh, H.M.A. Antibacterial activity of 13 honeys against Escherichia coli and Pseudomonas aeruginosa. J. Med. Food. 2005, 8, 100–103. [Google Scholar] [CrossRef]
- Moussa, A.; Noureddine, D.; Mohamed, H.S.; Abdelmelek, M.; Saad, A. Antibacterial activity of various honey types of Algeria against Staphylococcus aureus and Streptococcus pyogenes. Asian Pac. J. Trop. Med. 2012, 5, 773–776. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef]
- Eick, S.; Schäfer, G.; Kwieciński, J.; Atrott, J.; Henle, T.; Pfister, W. Honey a potential agent against Porphyromonas gingivalis: An in vitro study. BMC Oral Health 2014, 25, 24. [Google Scholar] [CrossRef]
- Bucekova, M.; Bugarova, V.; Godocikova, J.; Majtan, J. Demanding New Honey Qualitative Standard Based on Antibacterial Activity. Foods 2020, 9, 1263. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.M.; Estevinho, L.; Teixeira-Santos, R.G.; Rodrigues, A.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Antibacterial Action Mechanisms of Honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 25, 1252. [Google Scholar] [CrossRef]
- Adeleke, O.E.; Olaitan, J.O.; Okpekpe, E.L. Comparative Antibacterial Activity of Honey and Gentamicin Against Escherichia Coli and Pseudomonas Aeruginosa. Ann. Burn. Fire Disasters 2006, 19, 201–204. [Google Scholar]
- Al-Jabri, A.A.; Al-Hosni, S.A.; Nzeako, B.C.; Al-Mahrooqi, Z.H.; Nsanze, H. Antibacterial activity of Omani honey alone and in combination with gentamicin. Saudi Med. J. 2005, 26, 767–771. [Google Scholar] [PubMed]
- Julianti, E.; Rajah, K.K.; Fidrianny, I. Antibacterial Activity of Ethanolic Extract of Cinnamon Bark, Honey, and Their Combination Effects against Acne-Causing Bacteria. Sci. Pharm. 2017, 85, 19. [Google Scholar] [CrossRef] [PubMed]
- Campeau, M.E.M.; Patel, R. Antibiofilm Activity of Manuka Honey in Combination with Antibiotics. Int. J. Bacteriol. 2014, 2014, 795281. [Google Scholar] [CrossRef]
- Liu, M.; Lu, J.; Müller, P.; Turnbull, L.; Burke, C.M.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Antibiotic-specific differences in the response of Staphylococcus aureus to treatment with antimicrobials combined with manuka honey. Front. Microbiol. 2014, 5, 779. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Cooper, R. Synergy between oxacillin and manuka honey Sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 2012, 67, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Blaser, G.; Santos, K.; Bode, U.; Vetter, H.; Simon, A. Effect of medical honey on wounds colonised or infected with MRSA. J. Wound Care 2007, 16, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Ilieva, J.; Gergova, G.; Vladimirov, B.; Nikolov, R.; Mitov, I. Honey and green/black tea consumption may reduce the risk of Helicobacter pylori infection. Diagn. Microbiol. Infect. Dis. 2015, 82, 85–86. [Google Scholar] [CrossRef]
- Yousaf, I.; Ishaq, I.; Hussain, M.B.; Inaam, S.; Saleem, S.; Qamar, M.U. Antibacterial activity of Pakistani Beri honey compared with silver sulfadiazine on infected wounds: A clinical trial. J. Wound Care 2019, 28, 291–296. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial Activity of Honey [Internet]. Honey Analysis. IntechOpen. 2017. Available online: https://www.intechopen.com/books/honey-analysis/antimicrobial-activity-of-honey (accessed on 5 June 2021).
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.M.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.; Cremers, N. Defining the standards for medical grade honey. J. Apic. Res. 2019, 59, 1–11. [Google Scholar] [CrossRef]
- Molan, P.C.; Allen, K.L. The Effect of Gamma-irradiation on the Antibacterial Activity of Honey. J. Pharm. Pharmacol. 1996, 48, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Francese, S.; Turillazzi, S. Beyond the antipredatory defence: Honey bee venom function as a component of social immunity. Toxicon 2011, 58, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Son, M.J.; Choi, J.; Yun, K.-J.; Jun, J.H.; Lee, M.S. Bee venom acupuncture for rheumatoid arthritis: A systematic review protocol. BMJ Open 2014, 4, e004602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Zhu, C.; Zhao, Y.; Liu, S.; Xia, X.; Liu, X.; Zhang, H.; Xu, Y.; Hang, B.; et al. The antimicrobial peptide MPX kills Actinobacillus pleuropneumoniae and reduces its pathogenicity in mice. Vet Microbiol. 2020, 243, 108634. [Google Scholar] [CrossRef]
- Owen, M.D.; Pfaff, L.A. Melittin synthesis in the venom system of the honey bee (Apis mellifera L.). Toxicon 1995, 33, 1181–1188. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharm. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First Characterization of The Venom from Apis mellifera syriaca, A Honeybee from The Middle East Region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Park, K.K.; Nicholls, Y.M.; Macfarlane, N.; Duncan, G. Effects of honeybee (Apis mellifera) venom on keratinocyte migration in vitro. Pharm. Mag. 2013, 9, 220–226. [Google Scholar] [CrossRef]
- Marques Pereira, A.F.; Albano, M.; Bérgamo Alves, F.C.; Murbach Teles Andrade, B.F.; Furlanetto, A.; Mores Rall, V.L.; Delazari, D.S.L.; de Oliveira, O.R.; Fernandes, J.A. Influence of apitoxin and melittin from Apis mellifera bee on Staphylococcus aureus strains. Microb. Pathog. 2020, 141, 104011. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Efimova, S.S.; Malev, V.V. Chapter Six Modifiers of Membrane Dipole Potentials as Tools for Investigating Ion Channel Formation and Functioning. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 245–297. [Google Scholar]
- Asthana, N.; Yadav, S.P.; Ghosh, J.K. Dissection of antibacterial and toxic activity of melittin: A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J. Biol. Chem. 2004, 279, 55042–55050. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, A.; Abdel-Rahman, E.H.; Alfattah, A. Antibacterial Activity of Bee Venom Collected from Apis Mellifera Carniolan Pure and Hybrid Races by Two Collection Methods. Undefined. 2015. Available online: /paper/Antibacterial-Activity-of-Bee-Venom-Collected-from-Hegazi-Abdel-Rahman/92bff1e2eff3d71ca4eb0ada4876e4b68d24d3fe (accessed on 8 April 2021).
- Socarras, K.; Theophilus, P.; Torres, J.; Gupta, K.; Sapi, E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.-G.; Lee, J.-A.; Park, S.-B.; Hyun, P.-M.; Park, J.-K.; Suh, G.-H.; Lee, B.G. Immunoprophylactic Effects of Administering Honeybee (Apis melifera) Venom Spray against Salmonella Gallinarum in Broiler Chicks. J. Vet Med. Sci. 2013, 75, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yeo, J.; Baek, H.; Lin, S.-M.; Meyer, S.; Molan, P. Postantibiotic effect of purified melittin from honeybee (Apis mellifera) venom against Escherichia coli and Staphylococcus aureus. J. Asian Nat. Prod. Res. 2009, 11, 796–804. [Google Scholar] [CrossRef]
- Picoli, T.; Peter, C.M.; Zani, J.L.; Waller, S.B.; Lopes, M.G.; Boesche, K.N.; Vargas, G.D.Á.; Hübner, S.O.; Fischer, G. Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb. Pathog. 2017, 112, 57–62. [Google Scholar] [CrossRef]
- Wu, X.; Singh, A.K.; Wu, X.; Lyu, Y.; Bhunia, A.K.; Narsimhan, G. Characterization of antimicrobial activity against Listeria and cytotoxicity of native melittin and its mutant variants. Colloids Surf. B Biointerfaces 2016, 143, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.F.; Mendes, C.A.; Casemiro, L.A.; Vinholis, A.H.C.; Cunha, W.R.; de Almeida, R.; Martin, C.H. Antimicrobial activity of apitoxin, melittin and phospholipase A₂ of honey bee (Apis mellifera) venom against oral pathogens. Acad. Bras. Cienc. 2015, 87, 147–155. [Google Scholar] [CrossRef]
- Bitar, L.; Jundi, D.; Rima, M.; Al Alam, J.; Sabatier, J.-M.; Fajloun, Z. Bee Venom PLA2 versus Snake Venom PLA2: Evaluation of Structural and Functional Properties. Venoms Toxins 2019, 1, 1–12. [Google Scholar]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef]
- Jamasbi, E.; Batinovic, S.; Sharples, R.A.; Sani, M.-A.; Robins-Browne, R.M.; Wade, J.D.; Separovic, F.; Hossain, M.A. Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 2014, 46, 2759–2766. [Google Scholar] [CrossRef]
- Park, D.; Jung, J.W.; Lee, M.O.; Lee, S.Y.; Kim, B.; Jin, H.J.; Kim, J.; Ahn, Y.J.; Lee, K.W.; Song, Y.S.; et al. Functional characterization of naturally occurring melittin peptide isoforms in two honey bee species, Apis mellifera and Apis cerana. Peptides 2014, 53, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Akbari, R.; Hakemi vala, M.; Hashemi, A.; Aghazadeh, H.; Sabatier, J.-M.; Pooshang Bagheri, K. Action mechanism of melittin-derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Fadl, A. Antibacterial and antibiofilm effects of bee venom from (Apis mellifera) on multidrug-resistant bacteria (MDRB). Al-Azhar J. Pharm. Sci. 2018, 58, 60–80. [Google Scholar] [CrossRef]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial Activity and Antibiotic-Enhancing Effects of Honeybee Venom against Methicillin-Resistant Staphylococcus aureus. Molecules 2016, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Al-Safar, M.; Salman, J. Antibacterial activity of bee venom against multidrug resistant Acinetobacter baumannii locally isolates. Int. J. Res. Pharm. Sci. 2018, 9, 1510–1514. [Google Scholar]
- Akbari, R.; Hakemi-Vala, M.; Pashaie, F.; Bevalian, P.; Hashemi, A.; Pooshang Bagheri, K. Highly Synergistic Effects of Melittin with Conventional Antibiotics Against Multidrug-Resistant Isolates of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Drug Resist. 2019, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; D’Amato, G.; Silvestri, C.; Del Prete, M.S.; Łukasiak, J.; Scalise, G. Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1-7)M(2-9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 2003, 24, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef]
- Cherniack, E.P.; Govorushko, S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon 2018, 154, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.M.; Olivo, T.E.T.; Mendes, R.P.; Barraviera, S.R.C.S.; Souza, L.; Martins, J.G.; Hashimoto, M.; Fabris, V.E.; Ferreira Junior, R.S.; Barraviera, B. Africanized honeybee stings: How to treat them. Rev. Soc. Bras. Med. Trop. 2011, 44, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Kragballe, K. Topical corticosteroids: Mechanisms of action. Acta Derm. Venereol. Suppl. Stockh. 1989, 151, 7–10. [Google Scholar] [PubMed]
- Fitzgerald, K.T.; Flood, A.A. Hymenoptera stings. Clin. Tech. Small Anim. Pr. 2006, 21, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.-Y.; Lee, G. Potential Therapeutic Applications of Bee Venom on Skin Disease and Its Mechanisms: A Literature Review. Toxins 2019, 11, 374. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Pak, S.C. Effects of cosmetics containing purified honeybee (Apis mellifera L.) venom on acne vulgaris. J. Integr. Med. 2013, 11, 320–326. [Google Scholar] [CrossRef]
- Park, J.H.; Yim, B.K.; Lee, J.-H.; Lee, S.; Kim, T.-H. Risk Associated with Bee Venom Therapy: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0126971. [Google Scholar] [CrossRef]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef]
- Crane, E. A short history of knowledge about honey bees (Apis) up to 1800. Bee World. 2015, 85, 6–11. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Pimenta, H.C.; Violante, I.M.P.; Musis, C.R.; de Borges, Á.H.; Aranha, A.M.F. In vitro effectiveness of Brazilian brown propolis against Enterococcus faecalis. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Hatano, A.; Yoshino, M.; Hosoya, T.; Shimamura, Y.; Masuda, S.; Ahn, M.R.; Tazawa, S.; Araki, Y.; Kumazawa, S. Identification of the phenolic compounds contributing to antibacterial activity in ethanol extracts of Brazilian red propolis. Nat. Prod. Res. 2014, 28, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Bastos, E.M.A.; Simone, M.; Jorge, D.M.; Soares, A.E.E.; Spivak, M. In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J. Invertebr. Pathol. 2008, 97, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.S.; Mendonça, S.D.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Netto, A.A.L.; Lira, I.S.; López, B.G.; Negrão, V.; Marcucci, M.C. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; dos Santos Almeida, J.C.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and Antibiofilm Activity against Streptococcus mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis. Biomed Res. Int. 2019, 2, 7602343. [Google Scholar] [CrossRef]
- Kharsany, K.; Viljoen, A.; Leonard, C.; van Vuuren, S. The new buzz: Investigating the antimicrobial interactions between bioactive compounds found in South African propolis. J. Ethnopharmacol. 2019, 238, 111867. [Google Scholar] [CrossRef]
- Okińczyc, P.; Paluch, E.; Franiczek, R.; Widelski, J.; Wojtanowski, K.K.; Mroczek, T.; Krzyżanowska, B.; Skalicka-Woźniak, K.; Sroka, Z. Antimicrobial activity of Apis mellifera L. and Trigona sp. propolis from Nepal and its phytochemical analysis. Biomed. Pharmacother. 2020, 129, 110435. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wąsik, T.J. Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother Res. 2008, 22, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Baysallar, M.; Besirbellioglu, B.; Salih, B.; Sorkun, K.; Tanyuksel, M. In Vitro Antimicrobial Activity of Propolis against Methicillin-Resistant Staphylococcus Aureus and Vancomycin-Resistant Enterococcus Faecium. 2005. Available online: https://avesis.hacettepe.edu.tr/yayin/3ef4193c-cc98-4711-bfca-d5a21f1fa7ee/in-vitro-antimicrobial-activity-of-propolis-against-methicillin-resistant-staphylococcus-aureus-and-vancomycin-resistant-enterococcus-faecium (accessed on 26 April 2021).
- Koru, O.; Toksoy, F.; Acikel, C.H.; Tunca, Y.M.; Baysallar, M.; Uskudar Guclu, A.; Akca, E.; Ozkok Tuylu, A.; Sorkun, K.; Tanyuksel, M.; et al. In vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe 2007, 13, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Rashid, M.; Tipu, H.N. Propolis, A Hope for the Future in Treating Resistant Periodontal Pathogens. Cureus 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Kolarov, R.; Gergova, G.; Mitov, I. In vitro activity of Bulgarian propolis against 94 clinical isolates of anaerobic bacteria. Anaerobe 2006, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Júnior, A.; Balestrin, E.C.; Betoni, J.E.C.; Orsi, R.; de Cunha, M.; Montelli, A.C. Propolis: Anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Mem. Inst. Oswaldo Cruz. 2005, 100, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella Typhi and their synergism with antibiotics acting on the ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Ali, B.M.M.; Ghoname, N.F.; Hodeib, A.; Elbadawy, M. Significance of topical propolis in the treatment of facial acne vulgaris. Egypt. J. Dermatol. Venerol. 2015, 35, 29. [Google Scholar]
- Darwita, R.R.; Finisha, A.; Nur Wahyuni, H.; Ghina, S.; Muhammad, R.; Satyanegara, A.; Setiawati, F.; Adiatman, M. The effectiveness of propolis fluoride application in inhibiting dental caries activity in school children age 6-9 years old. Int. J. Appl. Pharm. 2018, 9, 1. [Google Scholar] [CrossRef][Green Version]
- Gebara, E.C.E.; Pustiglioni, A.N. Propolis Extract as an Adjuvant to Periodontal Treatment. Oral Health 2003, 1, 7. [Google Scholar]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stričík, M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health B 2013, 48, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Bakour, M.; Fernandes, Â.; Barros, L.; Sokovic, M.; Ferreira, I.C.F.R.; Badiaa, L. Bee bread as a functional product: Chemical composition and bioactive properties. LWT 2019, 109, 276–282. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Schmickl, T.; Crailsheim, K. Cannibalism and early capping: Strategy of honeybee colonies in times of experimental pollen shortages. J. Comp. Physiol. A 2001, 187, 541–547. [Google Scholar]
- Taha, E.-K.A.; Al-Kahtani, S.; Taha, R. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 232–237. [Google Scholar] [CrossRef]
- Silva, G.R.; Natividade, T.B.; Camara, C.A.; Silva, E.M.S.; Santos, F.; Silva, T.M.S. Identification of Sugar, Amino Acids and Minerals from the Pollen of Jandaíra Stingless Bees (Melipona subnitida). Food Nutr. Sci. 2014. [Google Scholar] [CrossRef]
- Abouda, Z.; Zerdani, I.; Kalalou, I.; Faid, M.; Ahami, M.T. The Antibacterial Activity of Moroccan Bee Bread and Bee-Pollen (Fresh and Dried) against Pathogenic Bacteria. Res. J. Microbiol. 2011, 6, 376–384. [Google Scholar]
- Kroyer, G.; Hegedus, N. Evaluation of bioactive properties of pollen extracts as functional dietary food supplement. Innov. Food Sci. Emerg. Technol. 2001, 2, 171–174. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Erkmen, O.; Ozcan, M.M. Antimicrobial effects of Turkish propolis, pollen, and laurel on spoilage and pathogenic food-related microorganisms. J. Med. Food 2008, 11, 587–592. [Google Scholar] [CrossRef]
- Kacaniova, M. Antimicrobial Activity of Bee Collected Pollen against Clostridia. Sci. Pap. Anim. Sci. Biotechnol. 2015, 47, 362–365. [Google Scholar]
- Cabrera, C.; Montenegro, G. Pathogen control using a natural Chilean bee pollen extract of known botanical origin. Cienc. Investig. Agrar. 2013, 40, 223–230. [Google Scholar] [CrossRef]
- Karadal, F.; Onmaz, N.E.; Abay, S.; Yildirim, Y.; Al, S.; Tatyuz, I.; Ackay, A. A Study of Antibacterial and Antioxidant Activities of Bee Products: Propolis, Pollen and Honey Samples. Ethiop. J. Health Dev. 2018, 32, 2. [Google Scholar]
- Šimunović, K.; Abramovi, H.; Lilek, N.; Angelova, M.; Podržaj, L.; Smole Možina, S. Microbiological quality, antioxidantive and antimicrobial properties of Slovenian bee pollen. AGROFOR 2019, 4, 82–92. [Google Scholar] [CrossRef]
- Kacaniova, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dżugan, M.; PASTERNAKIEWICZ, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Campos, M.; Bogdanov, S.; Almeida-Muradian, L.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. Bee World 2008, 47, 156–163. [Google Scholar]
- Freire, K.R.L.; Lins, A.C.S.; Dórea, M.C.; Santos, F.A.R.; Camara, C.A.; Silva, T.M.S. Palynological Origin, Phenolic Content, and Antioxidant Properties of Honeybee-Collected Pollen from Bahia, Brazil. Molecules 2012, 17, 1652–1664. [Google Scholar] [CrossRef]
- Velásquez, P.; Rodríguez, K.; Retamal, M.; Giordano, A.; Valenzuela, L.; Montenegro, G. Relation between composition, antioxidant and antibacterial activities and botanical origin of multi-floral bee pollen. J. Appl. Bot. Food Qual. 2017, 90, 306–314. [Google Scholar]
- Khider, M.; Elbanna, K.; Mahmoud, A.; Owayss, A. Egyptian Honeybee Pollen as Antimicrobial, Antioxidant Agents, and Dietary Food Supplements. Food Sci. Biotechnol. 2013, 22, 2013. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.; Erler, S. More than royal food Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 72. [Google Scholar] [CrossRef]
- Fujita, T.; Kozuka-Hata, H.; Ao-Kondo, H.; Kunieda, T.; Oyama, M.; Kubo, T. Proteomic analysis of the royal jelly and characterization of the functions of its derivation glands in the honeybee. J. Proteome Res. 2013, 12, 404–411. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R. Preparation and functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Bíliková, K.; Hanes, J.; Nordhoff, E.; Saenger, W.; Klaudiny, J.; Simúth, J. Apisimin, a new serine-valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: Purification and molecular characterization. FEBS Lett. 2002, 528, 125–129. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. Camb. Philos. Soc. 2014, 89, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Rakwal, R.; Nam, H.W.; Shibato, J.; Agrawal, G.K.; Kim, Y.S.; Ogawa, Y.; Yoshida, Y.; Kouzuma, Y.; Masuo, Y.; et al. Comprehensive royal jelly (RJ) proteomics using one- and two-dimensional proteomics platforms reveals novel RJ proteins and potential phospho/glycoproteins. J. Proteome Res. 2008, 7, 3194–3229. [Google Scholar] [CrossRef]
- Scarselli, R.; Donadio, E.; Giuffrida, M.G.; Fortunato, D.; Conti, A.; Balestreri, E.; Felicioli, R.; Pinzauti, M.; Sabatini, A.G.; Felicioli, A. Towards royal jelly proteome. Proteomics 2005, 5, 769–776. [Google Scholar] [CrossRef]
- Hanes, J.; Šimuth, J. Identification and partial characterization of the major royal jelly protein of the honey bee (Apis mellifera L.). J. Apic. Res. 1992, 31, 22–26. [Google Scholar] [CrossRef]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- Šimúth, J. Some properties of the main protein of honeybee (Apis mellifera) royal jelly. Apidologie 2001, 32, 69–80. [Google Scholar] [CrossRef]
- Fontana, R.; Mendes, M.A.; de Souza, B.M.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Majtan, J. The MRJP1 honey glycoprotein does not contribute to the overall antibacterial activity of natural honey. Eur. Food Res. Technol. 2016, 242, 625–629. [Google Scholar] [CrossRef]
- Bíliková, K.; Mirgorodskaya, E.; Bukovská, G.; Gobom, J.; Lehrach, H.; Simúth, J. Towards functional proteomics of minority component of honeybee royal jelly: The effect of post-translational modifications on the antimicrobial activity of apalbumin2. Proteomics 2009, 9, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Rosmilah, M.; Shahnaz, M.; Patel, G.; Lock, J.; Rahman, D.; Masita, A.; Noormalin, A. Characterization of major allergens of royal jelly Apis mellifera. Trop Biomed. 2008, 25, 243–251. [Google Scholar] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef]
- Bilikova, K.; Wu, G.; Šimúth, J. Isolation of a peptide fraction from honeybee royal jelly as a potential antifoulbrood factor. Apidologie 2001, 32, 275–283. [Google Scholar] [CrossRef]
- Bachanov, K.; Klaudiny, J.; Kopernick, J.; Šimúth, J. Identification of honeybee peptide active against Paenibacillus larvae larvae through bacterial growth-inhibition assay on polyacrylamide gel. Apidologie 2002, 33, 259–269. [Google Scholar] [CrossRef]
- Klaudiny, J.; Bachanová, K.; Kohútová, L.; Dzúrová, M.; Kopernický, J.; Majtan, J. Expression of larval jelly antimicrobial peptide defensin1 in Apis mellifera colonies. Biologia 2012, 67, 200–211. [Google Scholar] [CrossRef]
- Shen, L.; Liu, D.; Li, M.; Jin, F.; Din, M.; Parnell, L.D.; Lai, C.Q. Mechanism of action of recombinant acc-royalisin from royal jelly of Asian honeybee against Gram-positive bacteria. PLoS ONE 2012, 7, e47194. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, X.; Li, Y.; Zhang, J.; Zhao, J. Optimized Determination Method for trans-10-Hydroxy-2-Decenoic Acid Content in Royal Jelly by High-Performance Liquid Chromatography with an Internal Standard. J. Aoac Int. 2007, 90, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Šedivá, M.; Laho, M.; Kohútová, L.; Mojžišová, A.; Majtán, J.; Klaudiny, J. 10-HDA, A Major Fatty Acid of Royal Jelly, Exhibits pH Dependent Growth-Inhibitory Activity Against Different Strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef] [PubMed]
- Bc, B.; Cs, H.; Ct, H.; Yo, B. Liquid chromatographic determination of trans-10-hydroxy-2-decenoic acid content of commercial products containing royal jelly. J. Aoac Int. 1995, 78, 1019–1023. [Google Scholar]
- Blum, M.S.; Novak, A.F.; Taber, S. 10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal jelly. Science 1959, 130, 452–453. [Google Scholar] [CrossRef]
- Moselhy, W.A.; Fawzy, A.M.; Kamel, A.A. An evaluation of the potent antimicrobial effects and unsaponifiable matter analysis of the royal jelly. Life Sci. J. 2013, 10, 290–296. [Google Scholar]
- Yousefi, B.; Ghaderi, S.; Rezapoor-Lactooyi, A.; Amiri, N.; Verdi, J.; Shoae-Hassani, A. Hydroxy decenoic acid down regulates gtfB and gtfC expression and prevents Streptococcus mutans adherence to the cell surfaces. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 21. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, s8987–s8992. [Google Scholar] [CrossRef]
- Celeste García, M.; Silvia Finola, M.; Miguel Marioli, J. Antibacterial activity of Royal Jelly against bacteria capable of infecting cutaneous wounds. Int. Bee Res. Assoc. 2021, 2, 93–99. [Google Scholar]
- Ratanavalachai, T.; Wongchai, V. Antibacterial Activitv of Intact Roval Jelly, Its Lipid Extract and Its Defatted Extract. Sci. Technol. Asia 2002, 7, 5–12. [Google Scholar]
- Khosla, A.; Gupta, S.J.; Jain, A.; Shetty, D.C.; Sharma, N. Evaluation and comparison of the antimicrobial activity of royal jelly A holistic healer against periodontopathic bacteria: An in vitro study. J. Indian Soc. Periodontol. 2020, 24, 221–226. [Google Scholar]
- Boukraâ, L.; Meslem, A.; Mokhtar, B.; Hammoudi, S. Synergistic Effect of Starch and Royal Jelly Against Staphylococcus aureus and Escherichia coli. J. Altern. Complement. Med. 2009, 15, 755–757. [Google Scholar] [CrossRef]
- Boukraa, L. Additive activity of royal jelly and honey against Pseudomonas aeruginosa. Altern. Med. Rev. 2008, 13, 330–333. [Google Scholar]
- Romanelli, A.; Moggio, L.; Montella, R.C.; Campiglia, P.; Iannaccone, M.; Capuano, F. Peptides from Royal Jelly: Studies on the antimicrobial activity of jelleins, jelleins analogs and synergy with temporins. J. Pept. Sci. 2011, 17, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Al-Masaudi, S.B.; Hussain, M.B.; Al-Maaqar, S.M.; Al Jaouni, S.; Harakeh, S. In vitro antibacterial activity of honey against multidrug-resistant Shigella sonnei. Complement.Ther. Clin. Pract. 2020, 41, 101257. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Khan, K.A.; Alshehri, A.M.A. Antibacterial potential of some Saudi honeys from Asir region against selected pathogenic bacteria. Saudi J. Biol. Sci. 2019, 26, 1278–1284. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.K.; Kim, M.S.; Lee, S.H. Antioxidant and Antibacterial Properties of Hovenia (Hovenia dulcis) Monofloral Honey Produced in South Korea. Food Sci. Anim. Resour. 2020, 40, 221–230. [Google Scholar] [CrossRef]
- Al-Jabri, A.A.; Nzeako, B.; Al Mahrooqi, Z.; Al Naqdy, A.; Nsanze, H. In vitro antibacterial activity of Omani and African honey. Br. J. Biomed. Sci. 2003, 60, 1–4. [Google Scholar] [CrossRef]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern Med. 2010, 10, 47. [Google Scholar] [CrossRef]
- Biological Activities and Chemical Composition of Three Honeys of Different Types from Anatolia ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0308814605008964 (accessed on 10 June 2021).
- Miorin, P.L.; Junior, N.C.L.; Custodio, A.R.; Bretz, W.A.; Marcucci, M.C. Antibacterial activity of honey and propolis from Apis mellifera and Tetragonisca angustula against Staphylococcus aureus. J. Appl. Microbiol. 2003, 95, 913–920. [Google Scholar] [CrossRef]
- French, V.M.; Cooper, R.A.; Molan, P.C. The antibacterial activity of honey against coagulase-negative staphylococci. J. Antimicrob. Chemother. 2005, 56, 228–231. [Google Scholar] [CrossRef]
- Lin, S.M.; Molan, P.C.; Cursons, R.T. The in vitro susceptibility of Campylobacter spp. to the antibacterial effect of manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 339–344. [Google Scholar] [CrossRef]
- Al-Kafaween, M.A.; Al-Jamal, H.A.N.; Hilmi, A.B.M.; Elsahoryi, N.A.; Jaffar, N.; Zahri, M.K. Antibacterial properties of selected Malaysian Tualang honey against Pseudomonas aeruginosa and Streptococcus pyogenes. Iran J. Microbiol. 2020, 12, 565–576. [Google Scholar]
- Cooper, R.A.; Halas, E.; Molan, P.C. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J. Burn. Care Rehabil. 2002, 23, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef]
| Honeybee Product | Bacterial Strains | MIC/MBC | Reference | ||
|---|---|---|---|---|---|
| Honey | Raw honey | Manuka honey | Shigella sonnei | MIC 9%(v/v) | [191] |
| Talah honey | MIC 20%(v/v) | ||||
| Saudi honey | S. flexneri | MIC 10–20%(v/v) | [192] | ||
| Canadian honey | E. coli | MIC 6.25% (w/v) MBC 6.25–12.5%(w/v) | [44] | ||
| Saudi honey | MIC 20–40%(v/v) | [192] | |||
| Korean honey | MIC 25–50%(w/v) | [193] | |||
| Omani and African honey | ND | [194] | |||
| Ulmo honey | MIC 12.5%(v/v) | [195] | |||
| Manuka honey | MRSA | MIC 9.98%(v/v) | [46] | ||
| Pasture honey | MIC 3.07%(v/v) | ||||
| Ulmo honey | MIC 3.1–6.3%(v/v) | [195] | |||
| Turkish honey | S. aureus | ND | [196] | ||
| Saudi honey | MIC 20–40%(v/v) | [192] | |||
| Brazilian honey | MIC 126.23–185.70 mg.mL−1 | [197] | |||
| Korean honey | MIC 25–50%(w/v) | [193] | |||
| Omani and African honey | ND | [194] | |||
| Algerian honey | MIC 12–95%(v/v) | [55] | |||
| Saudi honey | Staphylococcus epidermis | MIC 20–40%(v/v) | [192] | ||
| Manuka honey | vancomycin-sensitive Enterococci | MIC 4.92%(v/v) | [46] | ||
| Pasture honey | MIC 9.66%(v/v) | ||||
| Manuka honey | vancomycin-resistant Enterococci | MIC 4.61%(v/v) | |||
| Pasture honey | MIC 8.25%(v/v) | ||||
| Manuka honey | Coagulase-negative staphylococci | MIC 3.4%(v/v) | [198] | ||
| Pasture honey | MIC 3.6%(v/v) | ||||
| Manuka honey | Campylobacter spp. | MIC ≈ 1% (v/v) | [199] | ||
| Turkish honey | H. pylori | ND | [196] | ||
| Cider honey | [48] | ||||
| Turkish honey | B. subtilis | ND | [196] | ||
| Saudi honey | Proteus mirabilis | MIC 10–20%(v/v) | [192] | ||
| Korean honey | L. monocytogenes | MIC 25% (w/v) | [193] | ||
| Korean honey | Salmonella typhymurium | MIC 25–50% (w/v) | |||
| Omani honey | P. aeruginosa | ND | [194] | ||
| African honey | |||||
| Ulmo honey | MIC 12.5% (v/v) | [195] | |||
| Tualang honey | MIC 18.5% (w/v) MBC 25% (w/v) | [200] | |||
| Manuka honey | MIC 7.5%(v/v) MBC 9.71%(v/v) | [201] | |||
| Pasture honey | MIC 6.8%(v/v) MBC 9%(v/v) | ||||
| Tualang honey | S. pyogenes | MIC 13% (w/v) MBC 25% (w/v) | [200] | ||
| Algrian honey | 25–73%(v/v) | [55] | |||
| Manuka honey | S. mutans | MIC 100–200 μg/mL MBC 200–500 μg/mL | [51] | ||
| Manuka honey | B. cepacia | MIC 2.9%(v/v) | [53] | ||
| Pasture honey | MIC 3.6%(v/v) | ||||
| P. gingivalis | MIC 2–10% (w/v) | [57] | |||
| Hydrogen peroxide | Melilot honey (sample 5) | S. aureus | MIC 12.5% (w/v) | [32] | |
| Dutch Gold Honey | ND | [33] | |||
| Dutch Gold honey | S. sonnei | ND | [33] | ||
| Melilot honey (sample 5) | Salmonella spp. | MIC 12.5% (w/v) | [32] | ||
| Melilot honey (sample 5) | E. coli | MIC 12.5% (w/v) | [32] | ||
| Dutch Gold honey | L. monocytogenes | ND | [33] | ||
| Methylglyoxal | Manuka honey | B. subtilis | MIC 0.8 mM | [202] | |
| S. aureus | MIC 1.2 mM | [202] | |||
| P. aeruginosa | MIC 1.0 mM | [202] | |||
| E. coli | MIC 1.2 mM | [202] | |||
| Bee venom | Crude extract | MRSA CCARM 3366 | MIC 0.085 μg/mL MBC 0.106 μg/ mL | [102] | |
| MRSA ATCC 33591 | MIC90% 7.2 μg/mL MBC90% 28.7 μg/mL | [84] | |||
| S. aureus CCARM 3708 | MIC 0.11 μg/mL MBC 0.14 μg/mL | [102] | |||
| S. aureus enterotoxin ATCC 23235 | MIC 0.7 μg/mL | [84] | |||
| S. salivarius | MIC 20 µg/mL | [95] | |||
| S. sanguinis | MIC 30 µg/mL | ||||
| S. sobrinus | MIC 40 µg/mL | ||||
| S. mitis | MIC 40 µg/mL | ||||
| S. mutans | MIC 20 µg/mL | ||||
| K. pneumonia | MIC 30 µg/mL for 24 h | [87] | |||
| B. subtilis | |||||
| E. faecalis | MIC 20 µg/mL MIC 3.7 µg/mL | [95] | |||
| L. casei | MIC 20 µg/mL MIC 0.9 µg/mL | ||||
| Borrelia spirochetes | MIC 200 µg/mL MIC 10 µg/mL | [90] | |||
| E. coli | MIC 0.25 µg/mL | [92] | |||
| S. aureus | MIC 0.06 µg/mL | ||||
| S. salivarius | MIC 10 µg/mL | [95] | |||
| E. FAECALIS | MIC 6 µg/mL | ||||
| L. casei | MIC 4 µg/mL | ||||
| Melittin | S. sanguinis | MIC 10 µg/mL | |||
| S. sobrinus | |||||
| S. mitis | |||||
| S. mutans | MIC 40 µg/mL | ||||
| K. pneumonia | MIC 8 µg/mL throughout 24 h | [87] | |||
| B. subtilis | MIC 6 µg/mL for 24 h | ||||
| L. monocytogenes F4244 | MIC 0.315 µg/mL MBC 3.263 µg/mL | [94] | |||
| E. coli | MIC 0.125 µg/mL | [92] | |||
| S. aureus | MIC 0.06 µg/mL | ||||
| MRSA ATCC 33591 | MIC90% 6.7 μg/mL MBC90% 26 μg/mL | [84] | |||
| S. aureus enterotoxin ATCC 23235 | MIC 3.6 μg/mL | [84] | |||
| B. spirochetes | MIC 200 µg/mL | [90] | |||
| Mutant melittin I17K | L. monocytogenes F4244 | MIC 0.814 µg/mL MBC 7.412 µg/mL | [94] | ||
| Mutant melittin G1I | MIC 0.494 µg/mL MBC 5.366 µg/mL | ||||
| PLA2 | L. casei | MIC 400 µg/mL | [95] | ||
| Propolis | S. aureus | MIC90 246.3 μg/mL | [121] | ||
| P. gingivalis ATCC 33277 | MIC 64–128 μg/mL | [122] | |||
| S. aureus ATCC 25923 | MIC 6.2 mg/mL | [123] | |||
| L. monocytogenes | |||||
| E. faecalis ATCC 19433 | |||||
| S. saprophyticus ATCC 15305 | |||||
| S. aureus (ATCC 25923) | MIC 0.39 mg/mL | [125] | |||
| E. faecalis (ATCC 29212) | |||||
| E. coli (ATCC 25922) | |||||
| P. aeruginosa (ATCC 27853) | |||||
| L. monocytogenes (ATCC, 19111) | MIC 0.10 mg/mL | ||||
| K. pneumonia (ATCC 13883) | MIC 0.78 mg/mL | ||||
| S. aureus | MIC50 0.39 mg/mL MIC90 0.78 mg/mL MBC 0.78 to 3.13 mg/mL | [127] | |||
| S. typhi | MIC 9.90% v/v MIC 10.0% v/v | [134] | |||
| Royal jelly | S. aureus | MIC 3.4–9 mg/mL MBC < 250 mg/mL | [185] | ||
| MIC 12.5 mg/mL | [186] | ||||
| MIC 1.7% (v/v) | [188] | ||||
| MRSA | MIC 8–14.5 mg/mL MBC < 250 mg/mL | [185] | |||
| E. coli | MIC 13.5 mg/mL | [186] | |||
| MIC 2% (v/v) | [188] | ||||
| S. epidermis | MIC 8.7–10.3 mg/mL MBC 125 mg/mL | [185] | |||
| M. luteus | MIC 7.5–11.8 mg/mL MBC 125 mg/mL | ||||
| E. faecalis | MIC 3.7–17.7 mg/mL MBC < 250mg/mL | ||||
| P. aeruginosa | MIC 3.3–14.4 mg/mL | ||||
| MIC 15.5 mg/mL | [186] | ||||
| P. vulgaris | MIC 15.5 mg/mL | ||||
| S. typhi | MIC 14.5 mg/mL | ||||
| B. cereus | MIC 12.5 mg/mL | ||||
| Sarcina lutia | MIC 0.30 mg/mL | ||||
| S. flexneri | MIC 14.5 mg/mL | ||||
| Pollen | B. cereus | MIC 0.17% (w/v) | [147] | ||
| S. aureus | MIC 0.21% (w/v) | ||||
| E. coli | MIC 82.4 mg/mL | [150] | |||
| P. aeruginosa | MIC 41.2 mg/mL | ||||
| S. aureus pyogenes | MIC 20.6 mg/mL | ||||
| E. coli | MIC 2.68 mg/mL | [152] | |||
| C. jejuni | MIC 9.93 mg/mL | ||||
| L. monocytogenes | MIC > 6.25 mg/m | ||||
| MIC 320 µg/mL | [157] | ||||
| E. coli | |||||
| S. enteritidis | |||||
| S.aureus | |||||
| S. pyogenes | MIC 0.78–6.25 mg/mL | [14] | |||
| P. aeruginosa | MIC 640 µg/mL | [157] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nader, R.A.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive Products as Antibacterial Agents: A Review. Antibiotics 2021, 10, 717. https://doi.org/10.3390/antibiotics10060717
Nader RA, Mackieh R, Wehbe R, El Obeid D, Sabatier JM, Fajloun Z. Beehive Products as Antibacterial Agents: A Review. Antibiotics. 2021; 10(6):717. https://doi.org/10.3390/antibiotics10060717
Chicago/Turabian StyleNader, Rita Abou, Rawan Mackieh, Rim Wehbe, Dany El Obeid, Jean Marc Sabatier, and Ziad Fajloun. 2021. "Beehive Products as Antibacterial Agents: A Review" Antibiotics 10, no. 6: 717. https://doi.org/10.3390/antibiotics10060717
APA StyleNader, R. A., Mackieh, R., Wehbe, R., El Obeid, D., Sabatier, J. M., & Fajloun, Z. (2021). Beehive Products as Antibacterial Agents: A Review. Antibiotics, 10(6), 717. https://doi.org/10.3390/antibiotics10060717







