The Potential Role of Bacteriophages in the Treatment of Recalcitrant Chronic Rhinosinusitis
Abstract
1. Background
1.1. Pathophysiology of Chronic Rhinosinusitis
1.2. Role of Bacteriophages or Phage-Derived Endolysins in the Treatment of Recalcitrant CRS
2. Preclinical Studies
2.1. In Vitro Studies Using Ex Vivo Bacterial Strains from CRS Patients
2.1.1. Activity of Bacteriophages and Phage-Derived Enzymes against S. aureus
2.1.2. Activity of Bacteriophages against P. aeruginosa
2.2. Animal Studies
3. Clinical Trials
3.1. Safety of Phage Therapy in Recalcitrant CRS
3.2. Efficacy of Phage Therapy in Recalcitrant CRS
4. Interpretation of the Available (Pre)Clinical Data
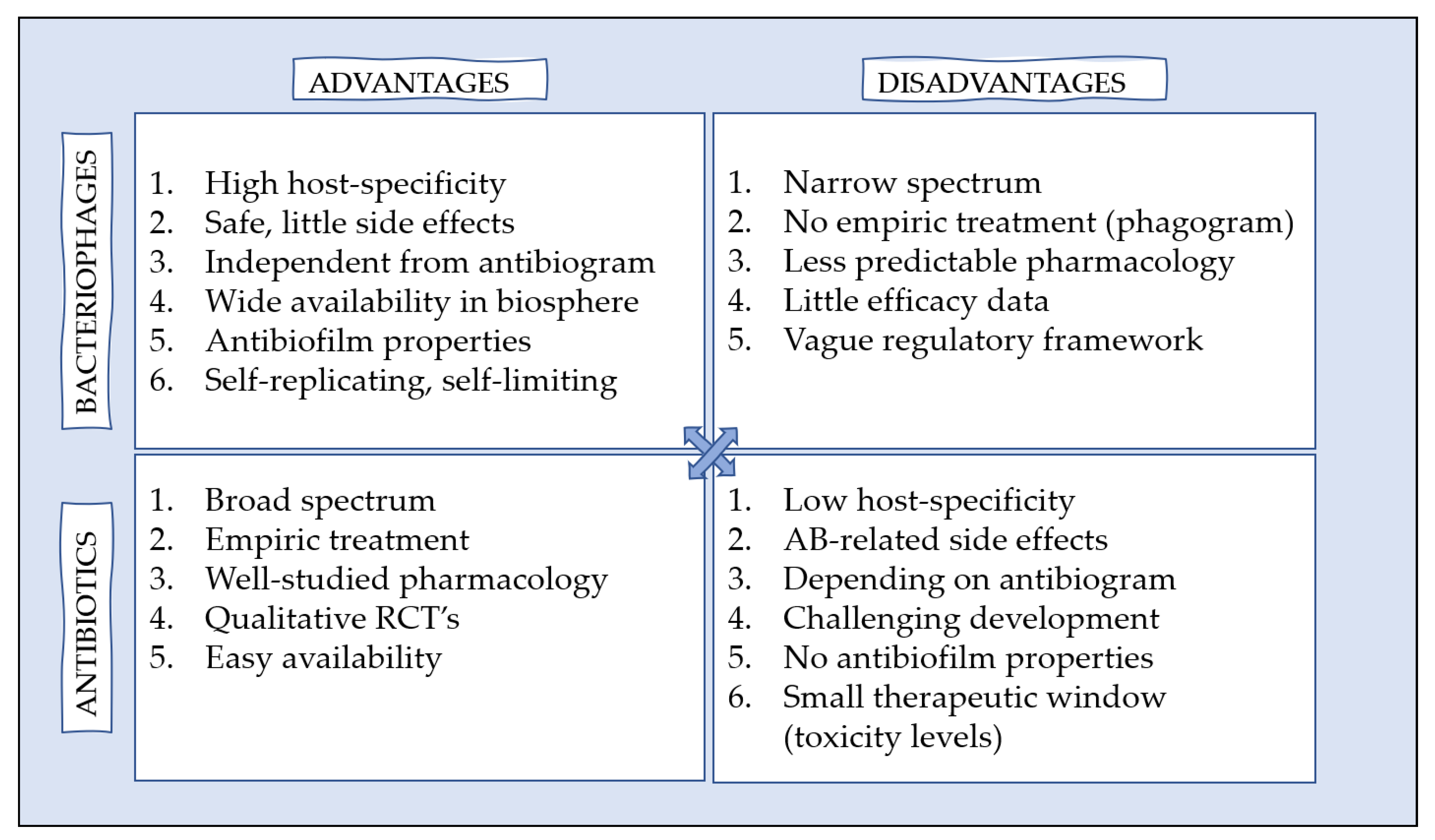
5. (Dis)Advantages of Phage Therapy versus Antibiotics
6. Considerations before Implementation of Phage Therapy in Recalcitrant Chronic Rhinosinusitis
6.1. Vision on Phage Therapy in Clinical Practice
6.2. Regulatory Framework
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hastan, D.; Fokkens, W.J.; Bachert, C.; Newson, R.B.; Bislimovska, J.; Bockelbrink, A.; Bousquet, P.J.; Brozek, G.; Bruno, A.; Dahlén, S.E.; et al. Chronic rhinosinusitis in Europe—An underestimated disease. A GA2LEN study. Allergy 2011, 66, 1216–1223. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Szaleniec, J.; Górski, A.; Szaleniec, M.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Stręk, P.; Składzień, J. Can phage therapy solve the problem of recalcitrant chronic rhinosinusitis? Future Microbiol. 2017, 12, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Alt, J.A. The relationship of chronic rhinosinusitis and depression. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 1–5. [Google Scholar] [CrossRef]
- Bachert, C.; Holtappels, G.; Merabishvili, M.; Meyer, T.; Murr, A.; Zhang, N.; Van Crombruggen, K.; Gevaert, E.; Völker, U.; Bröker, B.; et al. Staphylococcus aureus controls interleukin-5 release in upper airway inflammation. J. Proteom. 2018, 180, 53–60. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.; Morales, S.; Cornet, M.E.; Woodworth, B.A.; Fokkens, W.J.; Psaltis, A.J.; Vreugde, S.; Wormald, P.-J. Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients. Front. Cell. Infect. Microbiol. 2017, 7, 418. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Y.; Paramasivan, S.; Richter, K.; Morales, S.; Wormald, P.-J.; Vreugde, S. Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2018, 8, 406–414. [Google Scholar] [CrossRef]
- Drilling, A.J.; Ooi, M.L.; Miljkovic, D.; James, C.; Speck, P.; Vreugde, S.; Clark, J.; Wormald, P.-J. Long-Term Safety of Topical Bacteriophage Application to the Frontal Sinus Region. Front. Cell. Infect. Microbiol. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Drilling, A.; Morales, S.; Boase, S.; Jervis-Bardy, J.; James, C.; Jardeleza, C.; Tan, N.C.-W.; Cleland, E.; Speck, P.; Vreugde, S.; et al. Safety and efficacy of topical bacteriophage and ethylenediaminetetraacetic acid treatment of Staphylococcus aureus infection in a sheep model of sinusitis. Int. Forum Allergy Rhinol. 2014, 4, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.L.; Drilling, A.J.; Morales, S.; Fong, S.; Moraitis, S.; Macias-Valle, L.; Vreugde, S.; Psaltis, A.J.; Wormald, P.-J. Safety and Tolerability of Bacteriophage Therapy for Chronic Rhinosinusitis Due to Staphylococcus aureus. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.M.; Bleier, B.S. Future topical medications in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, S32–S46. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/332081/9789240005587-eng.pdf?ua=1 (accessed on 17 February 2021).
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef]
- Chaudhry, W.N.; Concepción-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef] [PubMed]
- Drilling, A.; Morales, S.; Jardeleza, C.; Vreugde, S.; Speck, P.; Wormald, P.-J. Bacteriophage Reduces Biofilm of Staphylococcus Aureus Ex Vivo Isolates from Chronic Rhinosinusitis Patients. Am. J. Rhinol. Allergy 2014, 28, 3–11. [Google Scholar] [CrossRef]
- Drilling, A.J.; Cooksley, C.; Chan, C.; Wormald, P.J.; Vreugde, S. Fighting sinus-derived Staphylococcus aureus biofilms in vitro with a bacteriophage-derived muralytic enzyme. Int. Forum Allergy Rhinol. 2016, 6, 349–355. [Google Scholar] [CrossRef]
- Szaleniec, M.; Gibała, A.; Pobiega, M.; Parasion, S.; Składzień, J.; Stręk, P.; Gosiewski, T. Exacerbations of Chronic Rhinosinusitis—Microbiology and Perspectives of Phage Therapy. Antibiotics 2019, 8, 175. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.J.; Ooi, M.L.; Paramasivan, S.; Finnie, J.W.; Morales, S.; Psaltis, A.J.; Vreugde, S.; Wormald, P.-J. Safety and efficacy of a bacteriophage cocktail in an in vivo model of Pseudomonas aeruginosa sinusitis. Transl. Res. 2019, 206, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Casey, P.G.; Hill, C.; Gahan, C.G.M.; Ross, R.P.; Mcauliffe, O.; O’Mahony, J.; Maher, F.; Coffey, A. The truncated phage lysin CHAPk eliminates Staphylococcus aureus in the nares of mice. Bioeng. Bugs 2010, 1, 404–407. [Google Scholar] [CrossRef]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brüssow, H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef]
- Mills, E.A. Staphylococcus bacteriophage lysate aerosol therapy of sinusitis. Laryngoscope 1956, 66, 846–858. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Mulczyk, M.; Górski, A. Bacteriophage therapy of bacterial infections: An update of our institute’s experience. Arch. Immunol. Ther. Exp. 2000, 48, 547–551. Available online: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L32108401 (accessed on 17 February 2021).
- Łusiak-Szelachowska, M.; Międzybrodzki, R.; Fortuna, W.; Borysowski, J.; Górski, A. Anti-phage serum antibody responses and the outcome of phage therapy. Folia Microbiol. 2021, 66, 127–131. [Google Scholar] [CrossRef]
- Kryukov, A.I.; Gurov, A.V.; Izotova, G.N.; Lapenko, E.G. Results of the observational (non-interventional) research ‘Analysis of therapeutic efficiency of the polyvalent pyobacteriophage (Secstaphag) in the treatment of acute sinusitis’. Vestn. Otorinolaringol. 2019, 84, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Knoll, B.M.; Mylonakis, E. Antibacterial Bioagents Based on Principles of Bacteriophage Biology: An Overview. Clin. Infect. Dis. 2014, 58, 1–7. [Google Scholar] [CrossRef]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage Therapy in Clinical Practice: Treatment of Human Infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Bukhari, S.M.A.U.S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef]
- Burrowes, B.; Harper, D.R.; Anderson, J.; McConville, M.; Enright, M.C. Bacteriophage therapy: Potential uses in the control of antibiotic-resistant pathogens. Expert Rev. Anti-Infect. Ther. 2011, 9, 775–785. [Google Scholar] [CrossRef]
- Rolain, J.-M.; Hraiech, S.; Brégeon, F. Bacteriophage-based therapy in cystic fibrosis-associated Pseudomonas aeruginosa infections: Rationale and current status. Drug Des. Dev. Ther. 2015, 9, 3653–3663. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage Neutralization by Sera of Patients Receiving Phage Therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Letkiewicz, S.; Fortuna, W.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; Olchawa, E.; et al. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol. 2017, 12, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
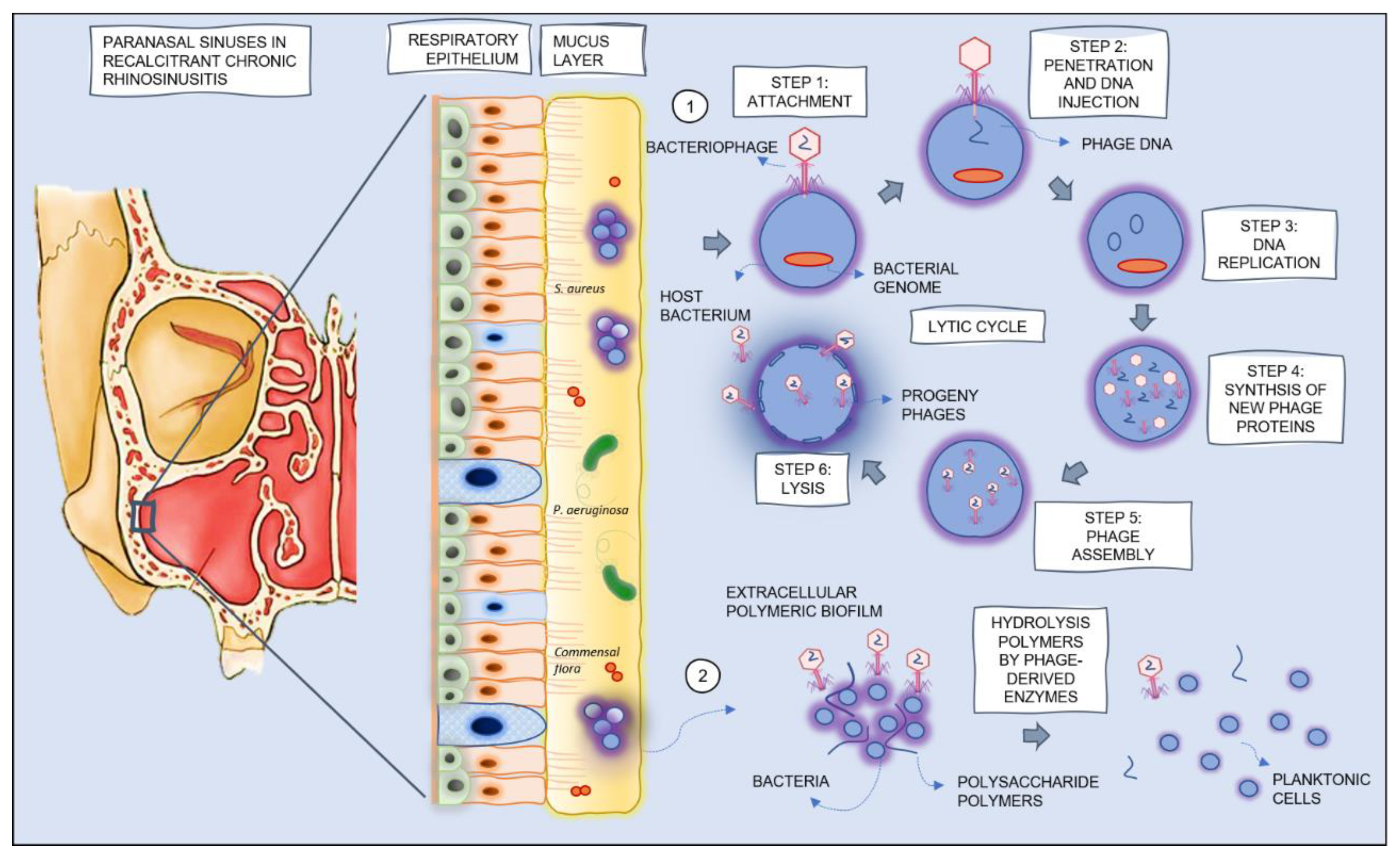
| Chronic rhinosinusitis [2] | Presence of two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or anterior/posterior nasal drip: ±facial pain/pressure, ±reduction or loss of smell; for >12 weeks |
| Recalcitrant chronic rhinosinusitis [2] | Patients who have persisting symptoms of rhinosinusitis despite appropriate treatment (recommended medication and surgery) |
| Authors (Year) | Isolates Sinonasal Swabs | Included CRS Patients | Phage | Phage Sensitivity | Efficacy |
|---|---|---|---|---|---|
| Drilling A. et al. (2014) [16] | S. aureus | 66 | CT-SA, cocktail SA1, single phage | 94% 90% | Biofilm mass reduction: 80% after CT-SA application |
| Drilling A. et al. (2016) [17] | S. aureus | NS | P128, bacteriophage derived muralytic enzyme | NS | Biofilm mass reduction: 95.5% |
| Fong S. et al. (2017) [6] | P. aeruginosa | 47 1 | Pa193, single phage Pa204, single phage Pa222, single phage Pa223, single phage CT-PA, cocktail | 73% 53% 73% 71% 85% | Significant biofilm mass reduction after CT-PA, Pa222 and Pa223 |
| Drilling A. et al. (2017) [8] | S. aureus | 61 | P68, single phage K710, single phage NOV012, cocktail | 74% 59% 85% | NS |
| Bachert C. et al. (2018) [5] | S. aureus | 9 2 | ISP, single phage | NS | Reduced IL-5 levels after 24 and 72 h, no significant changes compared to antibiotics |
| Zhang G. et al. (2018) [7] | S. aureus | 65 | Sa83, single phage Sa87, single phage | 69% 71% | NS |
| Szaleniec J. et al. (2019) [18] | Different pathogen 3 | 50 | Sensitive phage from collection Biophage Pharma, NS | 80% | NS |
| Ooi M. et al. (2019) [10] | S. aureus | 15 | AB-SA01, cocktail | 80% | NS |
| Authors (Year) | Pathogen | Animal Model + Application Method | Included Subjects | Phage | Safety | Efficacy |
|---|---|---|---|---|---|---|
| Fenton M. et al. (2010) [20] | S. aureus | Mice, intranasal instillation | 14 | Phage lysin CHAPk from bacteriophage K | NS | Two-log reduction in S. aureus cells 1 h after single application |
| Drilling A. et al. (2014) [9] | S. aureus | Sheep, frontal rinsing via mini-trephinations | 27 | CT-SA cocktail | No histological changes to frontal sinus mucosa | Significant reduction of biofilm mass |
| Drilling A. et al. (2017) [8] | S. aureus | Sheep, frontal rinsing via mini-trephinations | 21 | NOV012 cocktail | No histological changes to frontal sinus mucosa | NS |
| Fong S. et al. (2019) [19] | P. aeruginosa | Sheep, frontal rinsing via mini-trephinations | 32 | CT-PA cocktail | No histological changes to frontal sinus mucosa | Significant reduction of biofilm mass |
| Authors (Year) | Study Type | Participants | Pathogen | Therapeutic Regimen | Phage | Safety | Efficacy |
|---|---|---|---|---|---|---|---|
| Mills E. et al. (1956) [22] | OBS | Recalcitrant CRS (n = 60) | S. aureus | Nasal nebulizer | Phage lysate A-1 and B-7 | No reported AE | Clinical improvement: Excellent: 45%, Good: 33%, Fair: 17%, Poor: 5% |
| Weber-Dabrowska et al. (2000) [23] | OBS | Supparative sinusitis (n = 46) | Different pathogen 1 | Oral or nasal drops, NS | Sensitive phage from collection, NS | NS | Clinical improvement: Full recovery: 83%, Marked: 7%, No effect: 11% |
| McCallin S. et al. (2018) [21] | OBS | Healthy carriers (n = 21) | S. aureus | Oral (n = 10) or nasal application (n = 11) | Staphylococcal monophage (n = 21), Pyophage cocktail (n = 21), Placebo (n = 21) | No reported AE after nasal therapy, mild AE 2 in 4 subjects after oral therapy. No changes in blood values. | NS |
| Kryukov A. et al. (2019) [25] | RCT | Acute maxillary sinusitis (n = 58) | Different pathogen 3 | Peroperative nasal rinsing, followed by oral treatment (n = 38); Second generation cephalosporin (n = 20) | Polyvalent Pyophage | No reported AE | Clinical improvement: No significant changes between both groups after 10 days of treatment |
| Ooi M. et al. (2019) [10] | OBS | Recalcitrant CRS (n = 9) | S. aureus | Intranasal high-volume irrigations | AB-SA01, cocktail | Mild AE 4 in 3 patients, all of them resolved by the end of the trial. No changes in vital signs or blood values. | Improved LKS in all patients. Reduction bacterial load: 100%. Eradication bacteria: 22% |
| Lusiak M. et al. (2020) [24] | OBS | CRS (n = 25) | Different pathogen 5 | Nasal (n = 4) or nasal + oral application (n = 21) | Sensitive phage from collection | NS | Clinical response: Positive: 32%, inadequate: 68% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyttebroek, S.; Onsea, J.; Metsemakers, W.-J.; Dupont, L.; Devolder, D.; Wagemans, J.; Lavigne, R.; Spriet, I.; Van Gerven, L. The Potential Role of Bacteriophages in the Treatment of Recalcitrant Chronic Rhinosinusitis. Antibiotics 2021, 10, 675. https://doi.org/10.3390/antibiotics10060675
Uyttebroek S, Onsea J, Metsemakers W-J, Dupont L, Devolder D, Wagemans J, Lavigne R, Spriet I, Van Gerven L. The Potential Role of Bacteriophages in the Treatment of Recalcitrant Chronic Rhinosinusitis. Antibiotics. 2021; 10(6):675. https://doi.org/10.3390/antibiotics10060675
Chicago/Turabian StyleUyttebroek, Saartje, Jolien Onsea, Willem-Jan Metsemakers, Lieven Dupont, David Devolder, Jeroen Wagemans, Rob Lavigne, Isabel Spriet, and Laura Van Gerven. 2021. "The Potential Role of Bacteriophages in the Treatment of Recalcitrant Chronic Rhinosinusitis" Antibiotics 10, no. 6: 675. https://doi.org/10.3390/antibiotics10060675
APA StyleUyttebroek, S., Onsea, J., Metsemakers, W.-J., Dupont, L., Devolder, D., Wagemans, J., Lavigne, R., Spriet, I., & Van Gerven, L. (2021). The Potential Role of Bacteriophages in the Treatment of Recalcitrant Chronic Rhinosinusitis. Antibiotics, 10(6), 675. https://doi.org/10.3390/antibiotics10060675








