Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing
Abstract
:1. Introduction
2. Results
2.1. Therapeutic Exposure
2.2. Predictors for Clinical Outcome
3. Discussion
4. Material and Methods
4.1. Study Design and Population
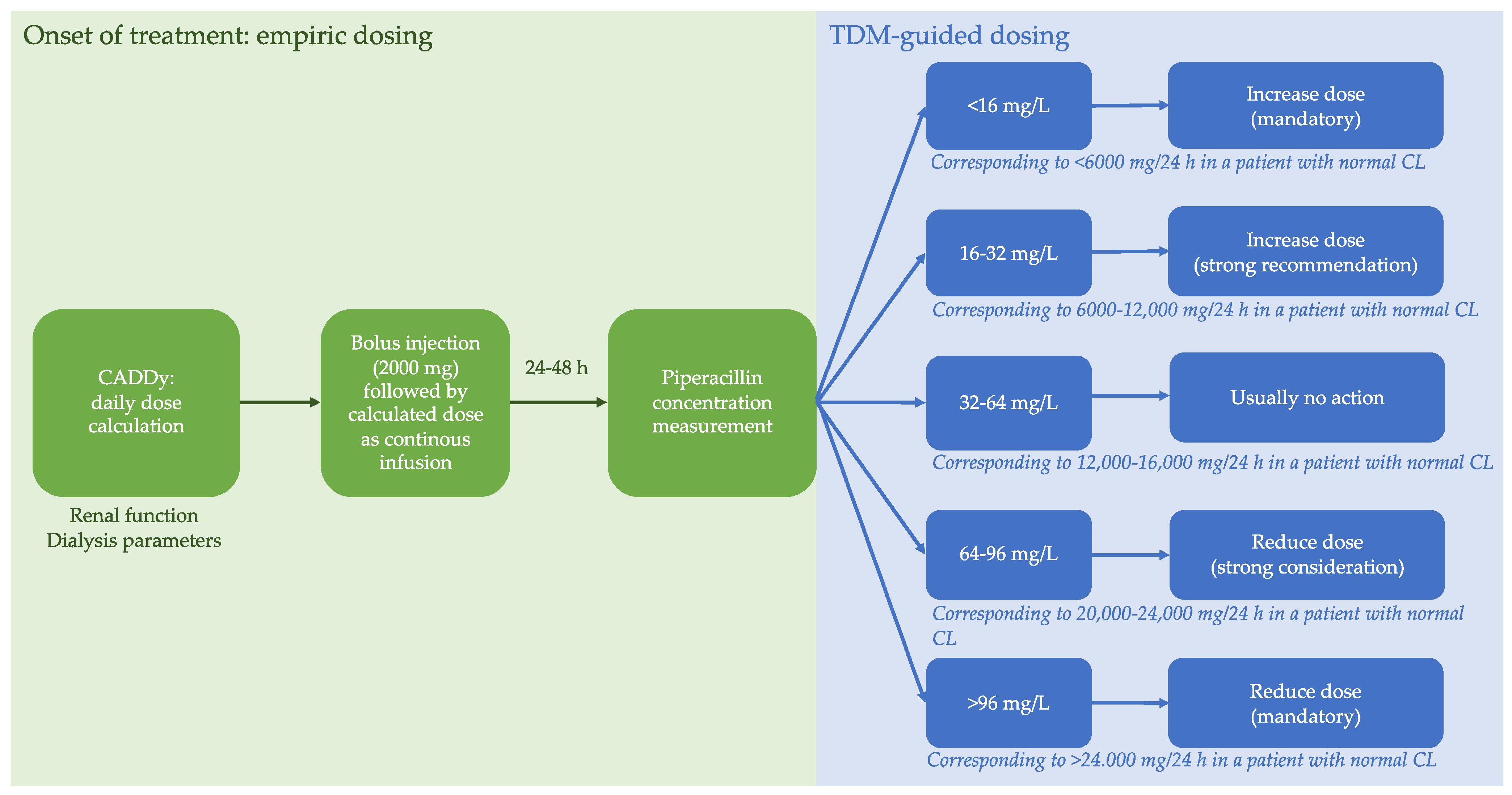
4.2. Study Procedures
4.3. Assessment of Therapeutic Drug Exposure
4.4. Statistical Analysis
5. Conclusions
Key Messages
- -
- Piperacillin clearance in critically ill patients with septic patients shows high variability;
- -
- Recommended personalized dosing strategy of piperacillin, including dosing software and TDM, ensures adequate serum concentrations;
- -
- CADDy is a useful and reliable dosing software for empiric dose calculations.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloos, F.; Ruddel, H.; Thomas-Ruddel, D.; Schwarzkopf, D.; Pausch, C.; Harbarth, S.; Schreiber, T.; Grundling, M.; Marshall, J.; Simon, P.; et al. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: A cluster randomized trial. Intensive Care Med. 2017, 43, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Antibacterial dosing in intensive care: Pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin. Pharm. 2006, 45, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, A.; Röhr, A.C.; Köberer, A.; Fuchs, T.; Preisenberger, J.; Krüger, W.A.; Frey, O.R. Therapeutic drug monitoring and individual dosing of antibiotics during sepsis: Modern or just “trendy”? Med. Klin. Intensivmed. Notf. 2018, 113, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Sukarnjanaset, W.; Jaruratanasirikul, S.; Wattanavijitkul, T. Population pharmacokinetics and pharmacodynamics of piperacillin in critically ill patients during the early phase of sepsis. J. Pharmacokinet. Pharm. 2019, 46, 251–261. [Google Scholar] [CrossRef]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining Antibiotic Levels in Intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van, G.T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Felton, T.W.; Ogungbenro, K.; Boselli, E.; Hope, W.W.; Rodvold, K.A. Comparison of piperacillin exposure in the lungs of critically ill patients and healthy volunteers. J. Antimicrob. Chemother. 2018, 73, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.W.; McCalman, K.; Malagon, I.; Isalska, B.; Whalley, S.; Goodwin, J.; Bentley, A.M.; Hope, W.W. Pulmonary penetration of piperacillin and tazobactam in critically ill patients. Clin. Pharm. 2014, 96, 438–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhaese, S.A.M.; Thooft, A.D.J.; Farkas, A.; Lipman, J.; Verstraete, A.G.; Stove, V.; Roberts, J.A.; De Waele, J.J. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: A prospective observational study. J. Crit. Care 2019, 52, 75–79. [Google Scholar] [CrossRef]
- Richter, D.C.; Frey, O.; Röhr, A.; Roberts, J.A.; Köberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinton, M.C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e0065417. [Google Scholar] [CrossRef] [Green Version]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of beta-lactam concentration-toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Lodise, T.P. Saving lives with optimal antimicrobial chemotherapy. Clin. Infect. Dis. 2013, 56, 245–247. [Google Scholar] [CrossRef]
- Roberts, J.A.; Roger, C.; De Waele, J.J. Personalized antibiotic dosing for the critically ill. Intensive Care Med. 2019, 45, 715–718. [Google Scholar] [CrossRef] [Green Version]
- Aardema, H.; Bult, W.; van Hateren, K.; Dieperink, W.; Touw, D.J.; Alffenaar, J.C.; Zijlstra, J.G. Continuous versus intermittent infusion of cefotaxime in critically ill patients: A randomized controlled trial comparing plasma concentrations. J. Antimicrob. Chemother. 2020, 75, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; bdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef]
- Roberts, J.A.; bdul-Aziz, M.H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent beta-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Taccone, F.; Villois, P.; Scheetz, M.H.; Rhodes, N.J.; Briscoe, S.; McWhinney, B.; Nunez-Nunez, M.; Ungerer, J.; Lipman, J.; et al. beta-Lactam pharmacodynamics in Gram-negative bloodstream infections in the critically ill. J. Antimicrob. Chemother. 2020, 75, 429–433. [Google Scholar] [PubMed]
- Scharf, C.; Liebchen, U.; Paal, M.; Taubert, M.; Vogeser, M.; Irlbeck, M.; Zoller, M.; Schröder, I. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J. Intensive Care 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Wong, G.; Brinkmann, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; El, H.N.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of beta-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.; Beall, G.; Cotta, M.O.; Roberts, J.A. Antimicrobial dosing in critical care: A pragmatic adult dosing nomogram. Int. J. Antimicrob. Agents 2020, 55, 105837. [Google Scholar] [CrossRef]
- Minichmayr, I.K.; Roberts, J.A.; Frey, O.R.; Röhr, A.C.; Kloft, C.; Brinkmann, A. Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J. Antimicrob. Chemother. 2018, 73, 1330–1339. [Google Scholar] [CrossRef]
- Roberts, J.A.; Joynt, G.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S.; et al. The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: Data from the multinational SMARRT Study. Clin. Infect. Dis. 2020, 72, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; hyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit. Care 2019, 23, 1–10. [Google Scholar]
- Liebchen, U.; Paal, M.; Scharf, C.; Schröder, I.; Grabein, B.; Zander, J.; Siebers, C.; Zoller, M. The ONTAI study—A survey on antimicrobial dosing and the practice of therapeutic drug monitoring in German intensive care units. J. Crit. Care 2020, 60, 260–266. [Google Scholar] [CrossRef]
- Brinkmann, A.; Röhr, A.C.; Frey, O.R.; Krüger, W.A.; Brenner, T.; Richter, D.C.; Bodmann, K.F.; Kresken, M.; Grabein, B. S2k-Leitlinie der PEG zur kalkulierten parenteralen Initialtherapie bakterieller Erkrankungen bei Erwachsenen Fokussierte Zusammenfassung und ergänzende Informationen zur Antibiotikatherapie kritisch kranker Patienten [S2k guidelines of the PEG on calculated parenteral initial treatment of bacterial diseases in adults: Focussed summary and supplementary information on antibiotic treatment of critically ill patients]. Anaesthesist 2018, 67, 936–949. [Google Scholar]
- Derendorf, H.; Heinrichs, T.; Reimers, T.; Lebert, C.; Brinkmann, A. Calculated initial parenteral treatment of bacterial infections: Pharmacokinetics and pharmacodynamics. GMS Infect. Dis. 2020, 8. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; SepNet Critical Care Trials, G.; Brinkmann, A. Incidence of severe sepsis and septic shock in German intensive care units: The prospective, multicentre INSEP study. Intensive Care Med. 2016, 42, 1980–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Vincent, J.L.; Matos, R.; Mendonca, A.; Cantraine, F.; Thijs, L.; Takala, J.; Sprung, C.; Antonelli, M.; Bruining, H.; et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999, 25, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Thomas-Rueddel, D.O.; Hartmann, M.; Hartog, C.S.; Welte, T.; Heublein, S.; Heublein, S.; Dennler, U.; Reinhart, K. Hospital Incidence and Mortality Rates of Sepsis. Dtsch. Arztebl Int. 2016, 113, 159–166. [Google Scholar]
- Levy, M.M.; Artigas, A.; Phillips, G.S.; Rhodes, A.; Beale, R.; Osborn, T.; Vincent, J.L.; Townsend, S.; Lemeshow, S.; Dellinger, R.P. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: A prospective cohort study. Lancet Infect. Dis. 2012, 12, 919–924. [Google Scholar] [CrossRef]
- Beale, R.; Janes, J.M.; Brunkhorst, F.M.; Dobb, G.; Levy, M.M.; Martin, G.S.; Ramsay, G.; Silva, E.; Sprung, C.L.; Vallet, B.; et al. Global utilization of low-dose corticosteroids in severe sepsis and septic shock: A report from the PROGRESS registry. Crit. Care 2010, 14, R102. [Google Scholar] [CrossRef] [Green Version]
- Bellos, I.; Karageorgiou, V.; Pergialiotis, V.; Perrea, D.N. Acute kidney injury following the concurrent administration of antipseudomonal beta-lactams and vancomycin: A network meta-analysis. Clin. Microbiol. Infect. 2020, 26, 696–705. [Google Scholar] [CrossRef]
- Kadomura, S.; Takekuma, Y.; Sato, Y.; Sumi, M.; Kawamoto, K.; Itoh, T.; Sugawara, M. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: A single-center retrospective cohort study. J. Pharm. Health Care Sci. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Zelenitsky, S.; Nash, J.; Weber, Z.; Iacovides, H.; Ariano, R. Targeted benefits of prolonged-infusion piperacillin-tazobactam in an in vitro infection model of Pseudomonas aeruginosa. J. Chemother. 2016, 28, 390–394. [Google Scholar] [CrossRef]
- Leone, M.; Roberts, J.A.; Bassetti, M.; Bougle, A.; Lavigne, J.P.; Legrand, M.; Neely, M.; Paiva, J.A.; Payen, D.; Rello, J.; et al. Update in Antibiotic Therapy in Intensive Care Unit Report from the 2019 Nimes International Symposium. Anaesth. Crit. Care Pain Med. 2019, 38, 647–656. [Google Scholar] [CrossRef]
- Crass, R.L.; Williams, P.; Roberts, J.A. The challenge of quantifying and managing pharmacokinetic variability of beta-lactams in the critically ill. Anaesth. Crit. Care Pain Med. 2020, 39, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Carrette, S.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int. J. Antimicrob. Agents 2014, 43, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Scheetz, M.H.; Drusano, G.L.; Lodise, T.P. Determination of antibiotic dosage adjustments in patients with renal impairment: Elements for success. J. Antimicrob. Chemother. 2010, 65, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Scheetz, M.H.; Drusano, G.L.; Lodise, T.P. Identification of optimal renal dosage adjustments for traditional and extended-infusion piperacillin-tazobactam dosing regimens in hospitalized patients. Antimicrob. Agents Chemother. 2010, 54, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Hagel, S.; Fiedler, S.; Hohn, A.; Brinkmann, A.; Frey, O.R.; Hoyer, H.; Schlattmann, P.; Kiehntopf, M.; Roberts, J.A.; Pletz, M.W. Therapeutic drug monitoring-based dose optimisation of piperacillin/tazobactam to improve outcome in patients with sepsis (TARGET): A prospective, multi-centre, randomised controlled trial. Trials 2019, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Udy, A.A.; Lipman, J.; Jarrett, P.; Klein, K.; Wallis, S.C.; Patel, K.; Kirkpatrick, C.M.; Krüger, P.S.; Paterson, D.L.; Roberts, M.S.; et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care 2015, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dillinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Röhr, A.C.; Köberer, A.; Fuchs, T.; von Freyberg, P.; Frey, O.R.; Brinkmann, A. Individuelle Dosierung und Applikation von Antiinfektiva auf der Intensivstation. Intensive Up2date 2018, 14, 238–243. [Google Scholar]
- Röhr, A.C.; Frey, O.R.; Köberer, A.; Fuchs, T.; Roberts, J.A.; Brinkmann, A. Anti-infective drugs during continuous hemodialysis—Using the bench to learn what to do at the bedside. Int. J. Artif. Organs 2015, 38, 17–22. [Google Scholar] [CrossRef]
- De Waele, J.J.; Lipman, J.; Akova, M.; Bassetti, M.; Dimopoulos, G.; Kaukonen, M.; Koulenti, D.; Martin, C.; Montravers, P.; Rello, J.; et al. Risk factors for target non-attainment during empirical treatment with β-lactam antibiotics in critically ill patients. Intensive Care Med. 2014, 40, 1340–1351. [Google Scholar] [CrossRef]
- Imani, S.; Buscher, H.; Day, R.; Gentili, S.; Jones, G.R.D.; Marriott, D.; Norris, R.; Sandaradura, I. An evaluation of risk factors to predict target concentration non-attainment in critically ill patients prior to empiric β-lactam therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Woksepp, H.; Hällgren, A.; Borgström, S.; Kullberg, F.; Wimmerstedt, A.; Oscarsson, A.; Nordlund, P.; Lindholm, M.L.; Bonnedahl, J.; Brudin, L.; et al. High target attainment for β-lactam antibiotics in intensive care unit patients when actual minimum inhibitory concentrations are applied. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 553–563. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Beal, S.L. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 1981, 9, 503–512. [Google Scholar] [CrossRef] [PubMed]
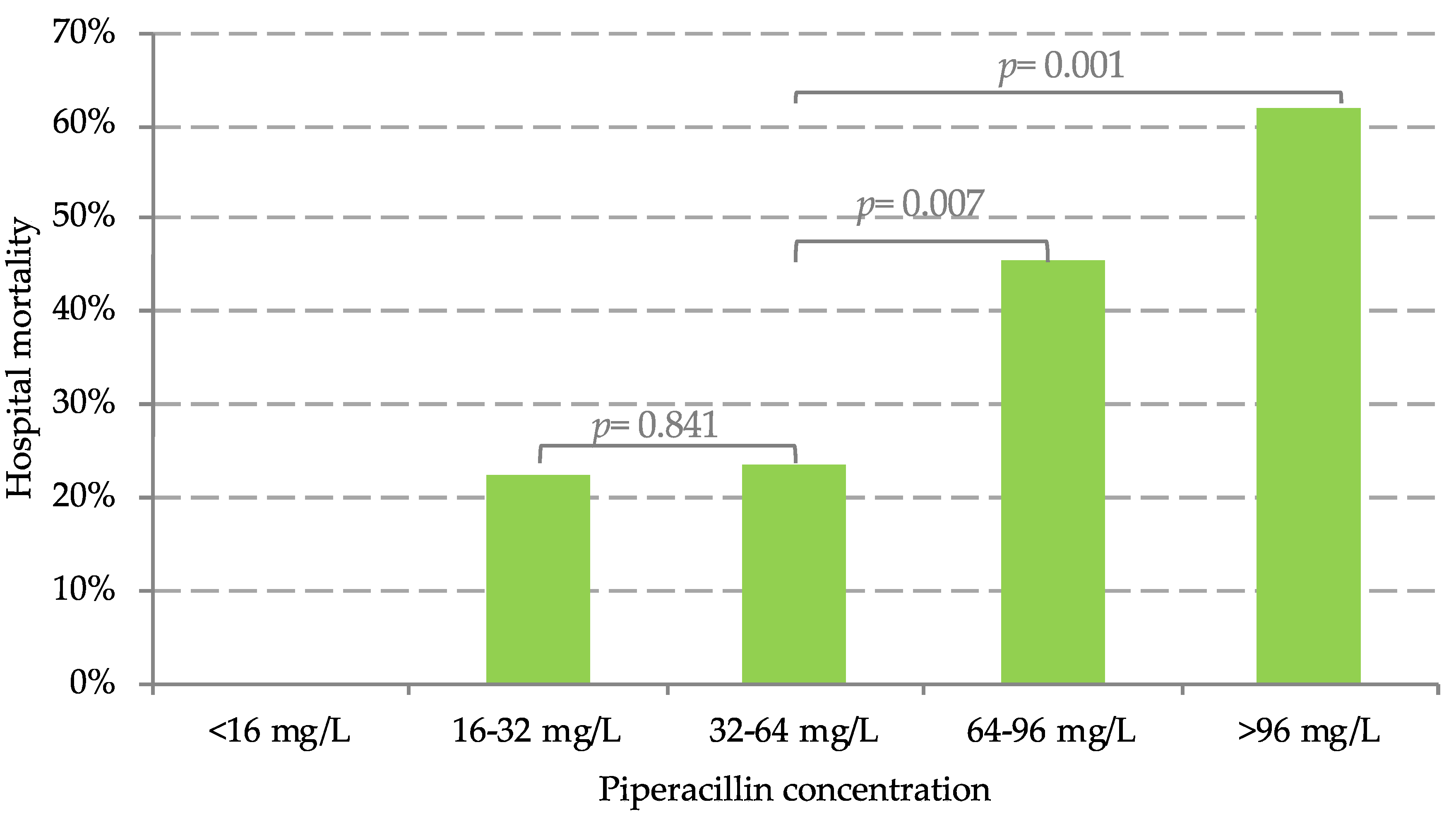


| Median (IQR), n (%) | |
|---|---|
| Age, years | 75 (15) |
| Weight, kg | 78 (18) |
| Height, cm | 170 (13) |
| BMI, kg/m2 | 26 (7) |
| Sex male | 115 (64%) |
| Creatinine, mg/dL | 1.18 (1.13) |
| CrCL, mL/min | 47 (50) |
| RRT | 33 (19%) |
| CRRT | 18 (10%) |
| iHD | 15 (8%) |
| Mechanical ventilation | 105 (59%) |
| SOFA | 6 (6) |
| SAPS | 37 (18) |
| APACHE II * | 22 (14) |
| ICU mortality | 56 (31%) |
| Hospital mortality | 64 (36%) |
| Length of hospital stay, days | 17 (18) |
| Antimicrobial treatment, days | 6 (4) |
| Diagnosis | n (%) |
|---|---|
| Sepsis/Severe sepsis | 126 (70%) |
| Septic shock | 53 (30%) |
| Site of infection | |
| Pneumonia | 96 (54%) |
| Abdominal infection, peritonitis | 37 (23%) |
| Soft tissue/bone infection | 10 (6%) |
| Urinary tract infection | 11 (7%) |
| Endocarditis, blood stream infection | 7 (4%) |
| Cholecystitis, cholangitis | 6 (4%) |
| Diverse | 10 (6%) |
| cPIP (mg/L) | <16 | 16–32 | 32–64 | 64–96 | >96 |
|---|---|---|---|---|---|
| Predicted based on standard dosing | 0 (0%) | 3 (1.7%) | 35 (19.6%) | 63 (35.2%) | 78 (43.6%) |
| Software-guided empiric dosing | 1 (0.6%) | 18 (10.1%) | 72 (40.2%) | 66 (36.9%) | 22 (12.3%) |
| cPIP (mg/L) | <16 | 16–32 | 32–64 | 64–96 | >96 |
|---|---|---|---|---|---|
| Hospital mortality (%) | 0% | 22% | 24% | 45% * | 62% * |
| Median SOFA score (IQR) | 2 (1) * | 3 (7) * | 6 (6) | 7 (8) | 6 (5) |
| Median SAPS (IQR) | 26 (7) * | 30 (23) * | 37 (19) | 38 (20) | 37 (7) |
| Median CLPIP (L/h) (IQR) | 11.5 (8.8) | 12.8 (10.6) | 6.9 (5.0) * | 5.0 (3.2) * | 2.8 (1.7) * |
| Median CrCL (mL/min) (IQR) | 71.6 (12.6) | 88.5 (91.4) | 42.1 (54.1) * | 38.7 (38.8) * | 23.2 (19.1) * |
| Median age (years) (IQR) | 77 (0) | 61 (24) | 72 (16) | 77(13) | 79 (11) |
| Median BMI (kg/m2) (IQR) | 30 (0) | 29 (8) | 27 (7) | 25 (7) | 25 (5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, U.; Richter, D.C.; Frey, O.R.; Röhr, A.C.; Helbig, S.; Preisenberger, J.; Hagel, S.; Roberts, J.A.; Weigand, M.A.; Brinkmann, A. Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing. Antibiotics 2021, 10, 667. https://doi.org/10.3390/antibiotics10060667
Chiriac U, Richter DC, Frey OR, Röhr AC, Helbig S, Preisenberger J, Hagel S, Roberts JA, Weigand MA, Brinkmann A. Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing. Antibiotics. 2021; 10(6):667. https://doi.org/10.3390/antibiotics10060667
Chicago/Turabian StyleChiriac, Ute, Daniel C. Richter, Otto R. Frey, Anka C. Röhr, Sophia Helbig, Judit Preisenberger, Stefan Hagel, Jason A. Roberts, Markus A. Weigand, and Alexander Brinkmann. 2021. "Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing" Antibiotics 10, no. 6: 667. https://doi.org/10.3390/antibiotics10060667






