Etiology and Antimicrobial Susceptibility of Pathogens Associated with Urinary Tract Infections among Women Attending Antenatal Care in Four South African Tertiary-Level Facilities, 2015–2019
Abstract
1. Introduction
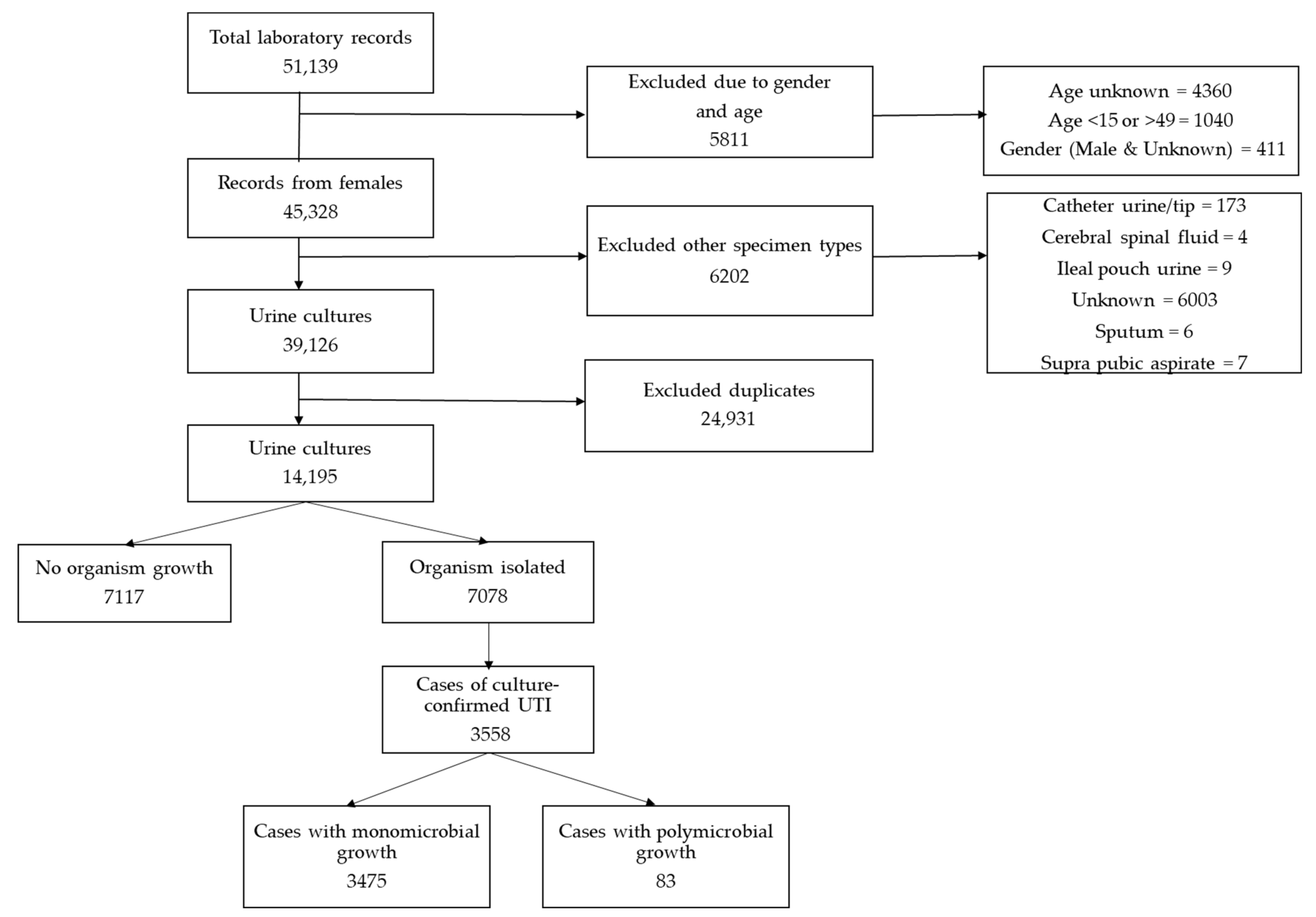
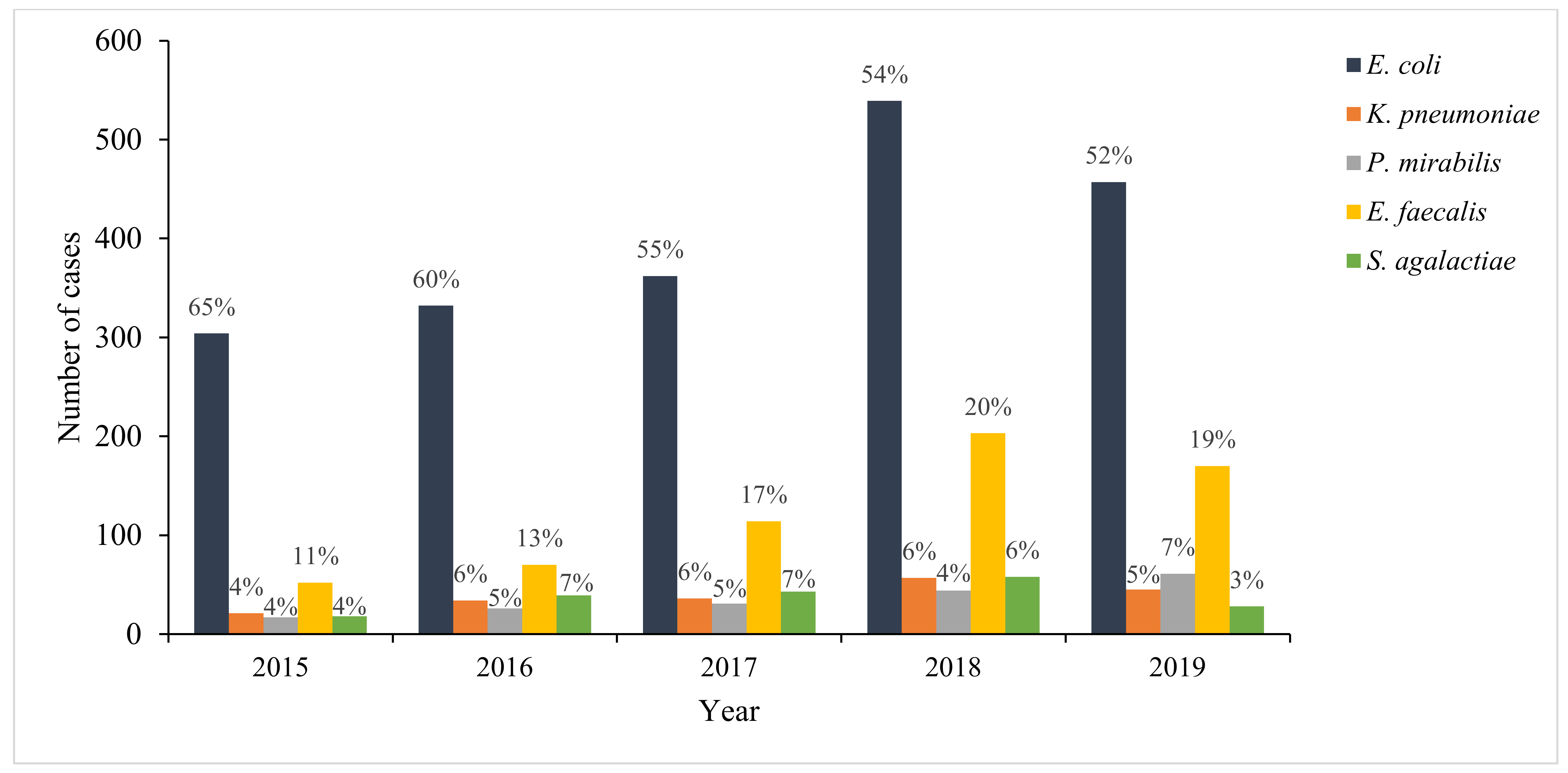
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Definitions
4.3. Exclusion Criteria
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jindal, A.K.; Pandya, K.; Khan, I.D. Antimicrobial resistance: A public health challenge. Med. J. Armed Forces India 2015, 71, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Shariq, A.; Alsalloom, A.A.; Babikir, I.H.; Alhomoud, B.N. Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int. J. Health Sci. 2019, 13, 48–55. [Google Scholar]
- Davenport, M.; Mach, K.E.; Dairiki Shortliffe, L.M.; Banaei, N.; Wang, T.-H.; Liao, J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 2017, 14, 296–310. [Google Scholar] [CrossRef] [PubMed]
- McQuiston, H.J.; Rosborg, D.M.; Sternhagen, N.A.B.; Llor, C.; Bjerrum, L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand. J. Prim. Health Care 2013, 31, 235–240. [Google Scholar] [CrossRef]
- Jhang, J.-F.; Kuo, H.-C. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Tzu Chi Med. J. 2017, 29, 131–137. [Google Scholar]
- Al-Badr, A.; Al-Shaikh, G. Recurrent Urinary Tract Infections Management in Women. Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Kabugo, D.; Kizito, S.; Kitizo, S.; Ashok, D.D.; Graham, K.A.; Nabimba, R.; Namunana, S.; Kabaka, M.R.; Achan, B.; Najjuka, F.C. Factors associated with community-acquired urinary tract infections among adults attending assessment centre, Mulago Hospital Uganda. Afr. Health Sci. 2016, 16, 1131–1142. [Google Scholar] [CrossRef]
- Habak, P.J.; Griggs, J. Urinary Tract Infection in Pregnancy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537047 (accessed on 31 May 2021).
- Managing Urinary Tract Infections in Pregnancy. Available online: https://bpac.org.nz/bpj/2011/april/pregnant-uti.aspx (accessed on 31 May 2021).
- John, E.; Delzell, J.; LeFevre, M. Urinary Tract Infections during Pregnancy. AFP 2000, 61, 713–720. [Google Scholar]
- Widmer, T.A.; Theron, G.; Grove, D. Prevalence and risks of asymptomatic bacteriuria among HIV-positive pregnant women. S. Afr. Med. J. Epidemiol. Infect. 2010, 25, 28–32. [Google Scholar] [CrossRef]
- Ayoyi, A.O.; Kikuvi, G.; Bii, C.; Kariuki, S. Prevalence, aetiology and antibiotic sensitivity profile of asymptomatic bacteriuria isolates from pregnant women in selected antenatal clinic from Nairobi, Kenya. Pan Afr. Med. J. 2017, 26. [Google Scholar] [CrossRef]
- Lewis, D.A.; Gumede, L.Y.E.; Van der, H.L.A.; De Gita, G.N.; De Kock, E.J.E.; De Lange, T.; Maseko, V.; Kekana, V.; Smuts, F.P.; Perovic, O. Antimicrobial susceptibility of organisms causing community-acquired urinary tract infections in Gauteng Province. S. Afr. Med. J. 2013, 103, 377. [Google Scholar] [CrossRef]
- Belete, M.A.; Saravanan, M. A Systematic Review on Drug Resistant Urinary Tract Infection Among Pregnant Women in Developing Countries in Africa and Asia; 2005–2016. Infect. Drug Resist. 2020, 13, 65–77. [Google Scholar] [CrossRef]
- Kamgang, F.d.P.S.; Maise, H.C.; Moodley, J. Pregnant women admitted with urinary tract infections to a public sector hospital in South Africa: Are there lessons to learn? S. Afr. J. Infect. Dis. 2016, 31, 79–83. [Google Scholar] [CrossRef]
- Hay, A.D.; Birnie, K.; Busby, J.; Delaney, B.; Downing, H.; Dudley, J. Determinants of Urinary Contamination. NIHR Journals Library. 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK373496/ (accessed on 31 May 2021).
- South African Standard Treatment Guidelines and Essential Medicines List: Primary Healthcare Level. 2020. Available online: https://www.idealhealthfacility.org.za/docs/guidelines/STG%20and%20EML%20PHC%202018.pdf (accessed on 31 May 2021).
- Erdem, I.; Ail, R.K.; Ardic, E.; Omar, E.S.; Mutlu, R.; Topkaya, A.E. Community-acquired Lower Urinary Tract Infections: Etiology, Antimicrobial Resistance, and Treatment Results in Female Patients. J. Glob. Infect. Dis 2018, 10, 129–132. [Google Scholar] [CrossRef]
- Bhola, P.; Mvelase, N.R.; Balakrishna, Y.; Mlisana, K.P.; Swe-Han, K.S. Antimicrobial susceptibility patterns of uropathogens isolated from pregnant women in KwaZulu-Natal Province: 2011–2016. S. Afr. Med. J. 2020, 110, 872–876. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Mothibi, L.M.; Bosman, N.N.; Nana, T. Fosfomycin susceptibility of uropathogens at Charlotte Maxeke Johannesburg Academic Hospital. S. Afr. Med. J. Infect. Dis. 2020, 35, 8. [Google Scholar] [CrossRef]
- Rosana, Y.; Ocviyanti, D.; Karuniawati, A.; Akhmad, S.R.P. Comparison of Microbial Pattern Causing Urinary Tract Infection in Female Out- and Hospitalized Patients in Jakarta. Microbiol. Indones 2016, 10, 5. [Google Scholar] [CrossRef]
- Dube, S.; Habte, T.; Ismail, N.; Hoosen, A.A. Hospital and community isolates of uropathogens at a tertiary hospital in South Africa. S. Afr. Med. J. 2009, 99, 584. [Google Scholar]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children: Executive Summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef]
- Cunha, B.A.; Schoch, P.E.; Hage, J.R. Nitrofurantoin: Preferred Empiric Therapy for Community-Acquired Lower Urinary Tract Infections. Mayo Clin. Proc. 2011, 86, 1243–1244. [Google Scholar] [CrossRef]
- Li, G.; Zhao, S.; Wang, S.; Sun, Y.; Zhou, Y.; Pan, X. A 7-year surveillance of the drug resistance in Klebsiella pneumoniae from a primary health care center. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 34. [Google Scholar] [CrossRef]
- Storberg, V. ESBL-producing Enterobacteriaceae in Africa–a non-systematic literature review of research published 2008–2012. Infect. Ecol. Epidemiol. 2014, 4. [Google Scholar] [CrossRef]
- Peirano, G.; van Greune, C.H.J.; Pitout, J.D.D. Characteristics of infections caused by extended-spectrum β-lactamase–producing Escherichia coli from community hospitals in South Africa. Diagn. Microbiol. Infect. Dis. 2011, 69, 449–453. [Google Scholar] [CrossRef]
- van Schoor, J. Urinary tract infections in women. S. Afr. Fam. Pract. 2016, 58, 6–10. [Google Scholar]
- Gardiner, B.J.; Stewardson, A.J.; Abbott, I.J.; Peleg, A.Y. Nitrofurantoin and fosfomycin for resistant urinary tract infections: Old drugs for emerging problems. Aust. Prescr. 2019, 42, 14–19. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D. Multidrug Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; Available online: https://file.qums.ac.ir/repository/mmrc/clsi%202017.pdf (accessed on 31 May 2021).
- Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Patient Safety Component Manual. 2020. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf (accessed on 31 May 2021).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbath, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
| Antibiotic | E. coli | K. pneumoniae | P. mirabilis | E. faecalis | S. agalactiae | All |
|---|---|---|---|---|---|---|
| n * (% [95% CI]) | ||||||
| Ciprofloxacin | 1626 (88% (86–89)) | 172 (96% (91–98)) | 157 (99% (96–100)) | 243 (96% (92–98)) | 16 (100% (79–100)) | 2483 (90% (89–91)) |
| Ampicillin/amoxicillin | 470 (24% (22–26)) | 0 (0% (0–1)) | 89 (51% (43–58)) | 612 (99% (99–100)) | 63 (100% (94–100)) | 1215 (38% (37–40)) |
| Trimethoprim/sulfamethoxazole | 742 (38% (36–40)) | 151 (75% (68–81)) | 85 (49% (41–56)) | 20 (37% (24–51)) | 16 (94% (71–100)) | 1234 (35% (33–36)) |
| Nitrofurantoin | 1827 (95% (94–96)) | 80 (40% (34–48)) | - | 301 (99% (97–100)) | - | 2374 (83% (82–85)) |
| Amoxicillin/clavulanic acid | 1594 (82% (81–84)) | 169 (85% (80–90)) | 165 (98% (94–99)) | - | - | 2034 (81% (79–82)) |
| Gentamicin | 1835 (93% (92–94)) | 178 (95% (90–97)) | 170 (94% (90–97)) | - | - | 2482 (93% (92–94)) |
| Cefepime | 1880 (95% (93–95)) | 185 (92% (87–95)) | 180 (99% (97–100)) | - | - | 2514 (94% (93–95)) |
| Cefotaxime/ceftriaxone | 1805 (93% (91–94)) | 177 (91% (86–95)) | 177 (99% (96–100)) | - | 84 (100% (96–100)) | 2489 (93% (92–94)) |
| Cefuroxime | 904 (86% (84–88)) | 165 (89% (83–92)) | 103 (99% (95–100)) | - | - | 1225 (85% (83–87)) |
| Cefazolin | 1122 (88% (86–90)) | 31 (97% (84–100)) | 86 (99% (94–100)) | - | - | 1367 (86% (84–88)) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwane, T.; Shuping, L.; Perovic, O. Etiology and Antimicrobial Susceptibility of Pathogens Associated with Urinary Tract Infections among Women Attending Antenatal Care in Four South African Tertiary-Level Facilities, 2015–2019. Antibiotics 2021, 10, 669. https://doi.org/10.3390/antibiotics10060669
Zwane T, Shuping L, Perovic O. Etiology and Antimicrobial Susceptibility of Pathogens Associated with Urinary Tract Infections among Women Attending Antenatal Care in Four South African Tertiary-Level Facilities, 2015–2019. Antibiotics. 2021; 10(6):669. https://doi.org/10.3390/antibiotics10060669
Chicago/Turabian StyleZwane, Thembekile, Liliwe Shuping, and Olga Perovic. 2021. "Etiology and Antimicrobial Susceptibility of Pathogens Associated with Urinary Tract Infections among Women Attending Antenatal Care in Four South African Tertiary-Level Facilities, 2015–2019" Antibiotics 10, no. 6: 669. https://doi.org/10.3390/antibiotics10060669
APA StyleZwane, T., Shuping, L., & Perovic, O. (2021). Etiology and Antimicrobial Susceptibility of Pathogens Associated with Urinary Tract Infections among Women Attending Antenatal Care in Four South African Tertiary-Level Facilities, 2015–2019. Antibiotics, 10(6), 669. https://doi.org/10.3390/antibiotics10060669






