Abstract
Table grapes (Vitis vinifera) are affected by botrytis bunch rot and summer bunch rot, the latter a complex disease caused by Botrytis cinerea, Aspergillus spp., Penicillium expansum and Rhizopus stolonifer. To search for biocontrol alternatives, a new bioproduct composed of Gluconobacter cerinus and Hanseniaspora osmophila, a consortium called PUCV-VBL, was developed for the control of fungal rots in table grapes. Since this consortium presents new biocontrol species, the effect of their VOCs (volatile organic compounds) was evaluated under in vitro and in vivo conditions. The VOCs produced by the PUCV-VBL consortium showed the highest mycelial inhibition against Botrytis cinerea (86%). Furthermore, H. osmophila was able to inhibit sporulation of A. tubingensis and P. expansum. VOCs’ effect in vivo was evaluated using berries from Red Globe, Thompson Seedless and Crimson Seedless grapes cultivars, demonstrating a mycelial inhibition by VOCs greater than 70% for all evaluated fungal species. The VOC identification of the PUCV-VBL consortium was analyzed by solid-phase microextraction coupled to gas chromatography-mass spectrometry (SPME-GCMS). A total 26 compounds were identified, including 1-butanol 3-methyl, propanoic acid ethyl ester, ethyl acetate, phenylethyl alcohol, isobutyl acetate and hexanoic acid ethyl ester. Our results show that VOCs are an important mode of action of the PUCV-VBL biological consortium.
1. Introduction
Summer bunch rot complex and botrytis bunch rot (“gray mold”) are responsible for the most significant reductions in table grape yield and quality worldwide [1]. In late summer, these microorganisms along with bacteria and yeasts, cause sour rot, a polymicrobial disease that attacks ripe, thin-skinned grapes [2]. In Chile, Botrytis cinerea pathologies have been shown to seriously affect susceptible cultivars, such as Thompson Seedless [3]. Besides, it was determined that the causal agents associated with summer bunch rot in cv. Red Globe is mainly due to Botrytis cinerea, Aspergillus spp., Rhizopus stolonifer and Penicillium expansum [4]. These pathogens are distributed worldwide, and infection can occur during the growing season, harvest, postharvest, storage, or transport [5,6].
Especially in postharvest, these diseases can cause significant economic losses: it is estimated that, in developed countries, 25% of fruit can be affected by such pathogens; and that, in developing countries, the percentage doubles [7], accounting for 80% of postharvest losses [8]. Chile, which is the most important table grape exporting country globally [9], these diseases can cause severe difficulties, especially when reaching the Far East destination market.
In this context, fungicides are the most common control method for these pathogens [10]; indeed, table grape and wine sectors represent 50% of the total market value for fungicides [10]. However, fungicide resistance is a growing problem [11,12,13,14,15]. Botrytis management’s overall expenses, including cultural measures, botrycides, broad-spectrum fungicides, and biocontrols, easily reach €1 billion/year in all countries; product and quality loss in the market chain, however, is probably much higher [10].
While Regulation (EU) 2015/408 [16] has proposed a chemical substitution list currently used in traditional agriculture, these alternative methods still require development and evaluation to demonstrate at least the same effectiveness in controlling postharvest diseases. With this goal, physical, chemical, and biological (biocontrol) control methods have been developed as alternatives to pesticides [14].
Of the above, biocontrol methods seek to decrease microbial pathogen inoculum or remit disease through one or more yeast, bacterial, and or fungal microorganisms [17]. Over the past 30 years, biocontrol research by multidisciplinary scientific teams, agri-food companies in the agri-sector and multinationals chemical companies [18] has produced phytosanitary compounds and contributed to its becoming an effective strategy to combat postharvest decay of fruits. Research into, e.g., yeast biocontrol methods have shown promising results, demonstrating ideal antagonistic properties and adaptability to adverse environmental conditions, few nutritional requirements, and prolonged half-life formulation [19,20]. Although numerous antifungal biocontrol procedures have been developed and patented in several countries [18,21,22,23], very few have been applied to agricultural use. This is likely due to their low antagonistic effectiveness not meeting the high quality and safety requirements, and trade regulations, of international markets [18].
In addressing the above, a promising area of biocontrol research in postharvest disease control is that of volatile organic compounds (VOCs), which have been shown to play a significant role in the control of several fungal pathogens [24,25,26]. Produced by microorganisms at very low concentrations [27], VOCs—which are biodegradable and do not leave toxic residues on fruit surfaces [28,29]—are hydrophobic, organic molecules with a low molecular weight (<300 Da) and high vapor pressure (≥0.01 kPa at 20 °C) [30]. The majority of VOCs, which can be created (refining, evaporation of organic solvents, unburned, etc.) or are naturally occurring (emissions by plants, animals, and microorganisms), belong to five chemical groups: terpenoids, fatty acid derivatives, benzenoid compounds, phenylpropanoids, and amino acid derivatives. Furthermore, VOCs produced naturally diffuse through biological fumigation (or “biofumigation”), which has shown promise against a wide range of storage pathogens and fungal decay [16,22].
To continue advances in the field of “clean technology” fungicide alternatives, the PUCV-VBL biological consortium research group developed a bioproduct with the ability to control table grape diseases, formulated with two antagonistic microorganisms isolated from table grapes bunches, Gluconobacter cerinus strain 515 and Hanseniaspora osmophila strain 337, for the control of fungi in grapes (WO2017088081A1).
Therefore, it is postulated that G. cerinus and H. osmophila possess VOCs as a mode of action capable of inhibiting the mycelial growth of pathogens that cause rot in table grapes. The objectives of this work were to evaluate the effect of the VOCs produced by the PUCV-VBL consortium; specifically, the following were studied: (i) the in vitro effects on Aspergillus, Botrytis, Penicillium and Rhizopus fungi; (ii) the in vivo effects against pathogens in Thompson Seedless, Crimson Seedless, and Red Globe cultivars; and (iii) the VOCs shown to control pathogenic fungi in table grapes in previous stages were characterized through Solid Phase Microextraction followed by Gas Chromatography-Mass Spectrometry (SPME-GC-MS).
2. Results
2.1. In Vitro Assay of VOC Production
In Figure 1, the in vitro effects of VOCs from bioantagonists G. cerinus and H. osmophila on the pathogens Botrytis cinerea, Aspergillus tubingensis, Penicillium expansum and Rhizopus stolonifer, as determined by double plate test (Scheme 1a), are shown. The VOCs emitted by the bioantagonists were shown to effectively inhibit mycelial growth of all pathogens evaluated. Indeed, inhibitory effects were greater in the PUCV-VBL biocontrol than those of each BCAs separately, demonstrating a synergistic effect among all the VOCs.
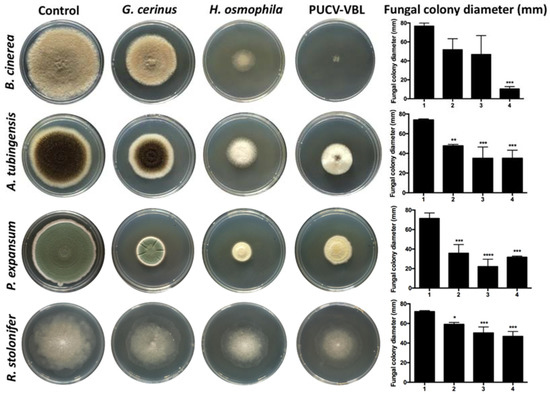
Figure 1.
Effect of VOCs of bioantagonists G. cerinus, H. osmophila and PUCV-VBL biological consortium on mycelial growth of pathogen B. cinerea, A. tubingensis, P. expansum and R. stolonifer under in vitro conditions, established by means of a double plate assay and through fungal colony diameter graph (mm) measurement, conducted using image analysis with the Image J® progam. 1: Control without biocontroller, 2: VOCs of G. cerinus, 3: VOCs of H. osmophila, 4: VOCs of PUCV-VBL biological consortium. Tukey’s multiple comparisons test * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
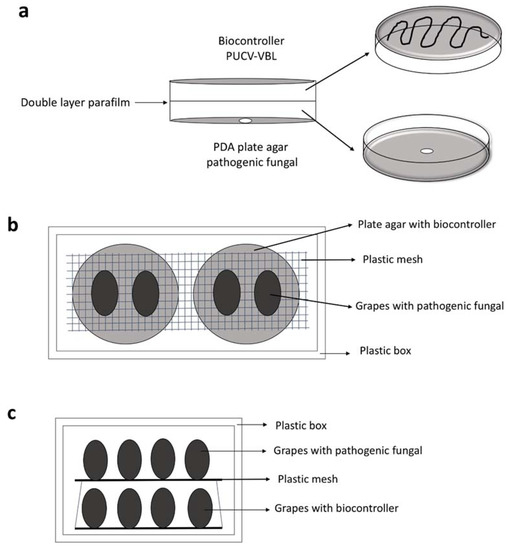
Scheme 1.
Schematic representation in vitro and in vivo tests. (a) Double plate test. (b) In vivo test berries inoculated with pathogens on Petri dishes with biocontroller. (c) In vivo test berries of grapes inoculated with pathogens on grape berries inoculated with biocontroller.
Botrytis cinerea was most affected, at 86% inhibition mycelial growth (Table 1). Aspergillus tubingensis was significantly inhibited as well, achieving 52%. This is likely due to H. osmophila VOCs preferentially inhibiting A. tubingensis sporulation instead of mycelial growth. Penicillium expansum also showed significant inhibition (68%), and similar to A. tubingensis, H. osmophila VOCs inhibited sporulation of P. expansum. While mycelia of Rhizopus stolonifer was the least inhibited (35%), it still had significant differences concerning control.

Table 1.
Mycelial growth inhibition (percentage) determined by in vitro assay by VOCs emitted by G. cerinus, H. osmophila and PUCV–VBL biological consortium. Means in rows followed by the same letters are not significantly different according to Tukey’s test (p = 0.05).
2.2. In Vivo Assays of VOC
Of the two in vivo trials conducted for VOC production, the results of the first BCAs in a Petri dish underneath grapes inoculated with pathogens (Scheme 1b) showed significant differences in mycelial growth of all pathogens, for the three grape cultivars evaluated, compared to control (Figure 2). For cv. Red Globe, the most significant effect was against A. tubingensis, at 93% mycelial inhibition (Table 2). There, G. cerinus was shown to inhibit its sporulation as well. Similar results were observed for cv. Thompson Seedless, at 79% mycelial inhibition for B. cinerea (statistically significant, Table 2). For cv. Crimson Seedless, the PUCV-VBL biocontrol VOCs presented the same level of significance against A. tubingensis, P. expansum and R. stolonifer. Only for R. stolonifer was the effect slightly less (Table 2).
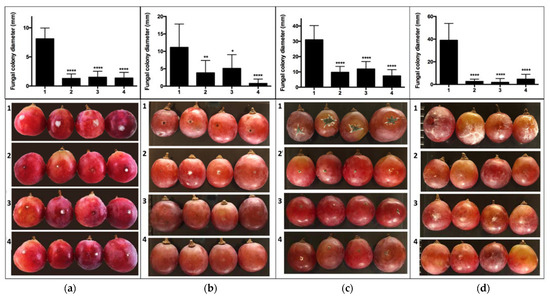
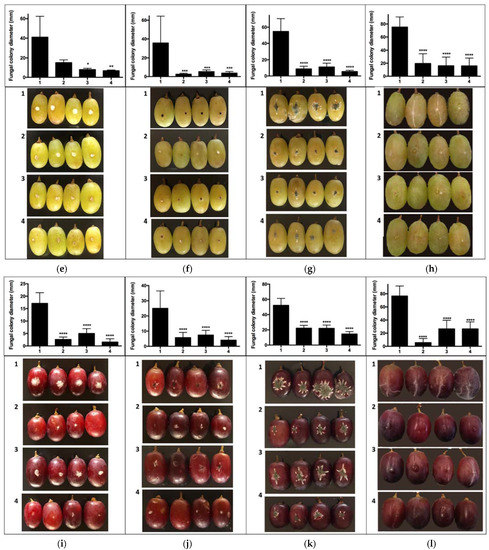
Figure 2.
In vivo test berries inoculated with pathogens on Petri dishes with biocontroller. Effect of VOCs produced by (1) control treatment without biocontroller; (2) H. osmophila biocontroller; (3) G. cerinus biocontroller; (4) Biological consortium PUCV-VBL biocontroller. (a) Percentage of mycelial inhibition of B. cinerea in cv Red Globe, (b) percentage of mycelial inhibition of A. tubingensis in cv Red Globe, (c) percentage of mycelial inhibition of P. expansum in cv Red Globe, (d) percentage of mycelial inhibition of R. stolonifer in cv Red Globe, (e) percentage of mycelial inhibition of B. cinerea in cv Thompson Seedless, (f) percentage of mycelial inhibition of A. tubingensis in cv Thompson Seedless, (g) percentage of mycelial inhibition of P. expansum in cv Thompson Seedless, (h) percentage of mycelial inhibition of R. stolonifer in cv Thompson Seedless, (i) percentage of mycelial inhibition of B. cinerea in cv Crimson Seedless, (j) percentage of mycelial inhibition of A. tubingensis in cv Crimson Seedless, (k) percentage of mycelial inhibition of P. expansum in cv Crimson Seedless, (l) percentage of mycelial inhibition of R. stolonifer in cv Crimson Seedless. Tukey’s multiple comparisons test * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.

Table 2.
Percentage of inhibition of mycelial growth in vitro of different causal agents with grape-plate test. Means in rows followed by the same letters are not significantly different according to Tukey’s test (p = 0.05).
The second in vivo trial results—BCAs in grapes placed underneath pathogen-inoculated berries (Scheme 1c)—showed significant differences for B. cinerea and P. expansum in cv. Red Globe (Figure 3a,c); and, for cvs. Thompson Seedless and Crimson Seedless, all treatments differed from control for all the pathogens evaluated (Figure 3e–l).
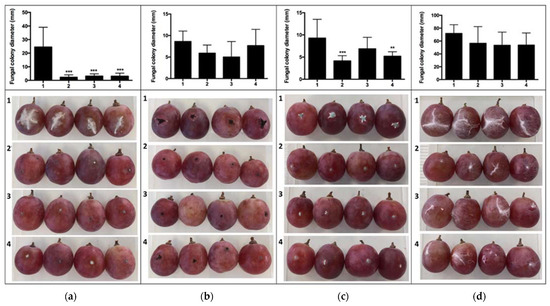
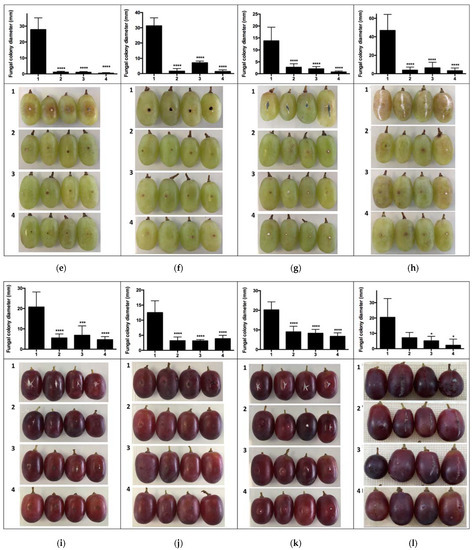
Figure 3.
In vivo test berries of grapes inoculated with pathogens on grape berries inoculated with biocontroller. Effect of VOCs produced by (1) control treatment without biocontroller; (2) H. osmophila biocontroller; (3) G. cerinus biocontroller; (4) Biological consortium PUCV-VBL biocontroller. (a) Percentage of mycelial inhibition of B. cinerea in cv Red Globe, (b) percentage of mycelial inhibition of A. tubingensis in cv Red Globe, (c) percentage of mycelial inhibition of P. expansum in cv Red Globe, (d) percentage of mycelial inhibition of R. stolonifer in cv Red Globe, (e) percentage of mycelial inhibition of B. cinerea in cv Thompson Seedless, (f) percentage of mycelial inhibition of A. tubingensis in cv Thompson Seedless, (g) percentage of mycelial inhibition of P. expansum in cv Thompson Seedless, (h) percentage of mycelial inhibition of R. stolonifer in cv Thompson Seedless, (i) percentage of mycelial inhibition of B. cinerea in cv Crimson Seedless, (j) percentage of mycelial inhibition of A. tubingensis in cv Crimson Seedless, (k) percentage of mycelial inhibition of P. expansum in cv Crimson Seedless, (l) percentage of mycelial inhibition of R. stolonifer in cv Crimson Seedless. Tukey’s multiple comparisons test * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
No statistical differences were observed for cv. Red Globe among the three separate treatments (Table 3); however, for cv. Thompson Seedless, treatment PUCV-VBL biocontrol VOCs was significantly more effective against A. tubingensis and P. expansum (over 90% inhibition) (Table 3). For cv. Crimson Seedless, the most considerable effect was for PUCV-VBL biocontroller VOCs, at 72% mycelial inhibition for B. cinerea (statistically significant, Table 3).

Table 3.
Percentage of mycelial inhibition of four causal agents in three different table grape cultivars tested in vivo with grapes-on-grapes test. Means in rows followed by the same letters are not significantly different according to Tukey’s test (p = 0.05).
2.3. Identification of VOCs Produced by BCAs (SPME GC-MS)
A total of 26 VOCs produced by bioantagonists G. cerinus, H. osmophila, and the combined PUCV-VBL biocontrol taken directly from grapes were identified in different concentrations by SPME GC-MS. Figure 4 shows the Principal Component Analysis (PCA) projection for biocontroller distributions regarding PC-1 with PC-2. BCAs metabolites are shown in four groups. H. osmophila metabolites are most represented in the PUCV-VBL biocontrol. Figure 5 shows a PCA biplot of VOCs correlated with the four treatments. Figure 6 shows a Euclidean distance heat map (Ward algorithm) of replicas for each treatment for the presence of all VOC compounds produced by bioantagonists, where dark brown indicates higher relative concentrations and dark blue, the lowest.
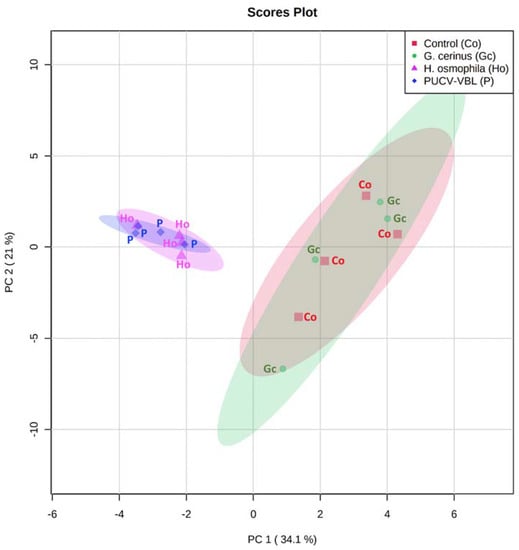
Figure 4.
Principal Component Analysis (PCA) of SPME-GCMS data. PCA score plot. Control (Co), grape without biocontroller, red; G. cerinus (Gc), grape with biocontroller G. cerinus, green; H. osmophila (Ho), grape with biocontroller H. osmophila, magenta; PUCV-VBL (P), grape with PUCV-VBL consortium, violet.
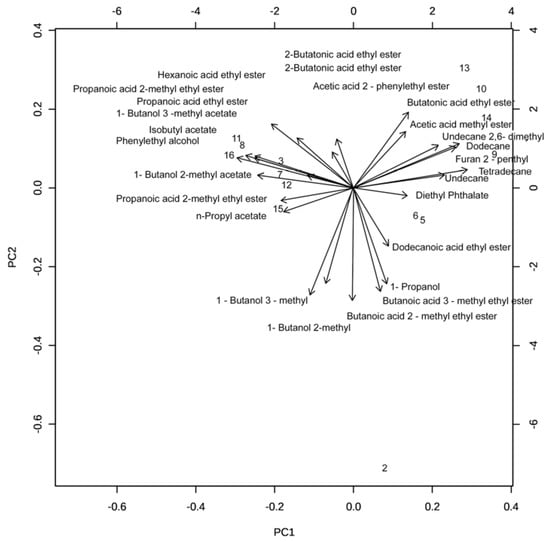
Figure 5.
Principal Component Analysis (PCA) Biplot of SPME-GCMS data, volatile organic compounds (VOCs) of biological consortium PUCV-VBL.
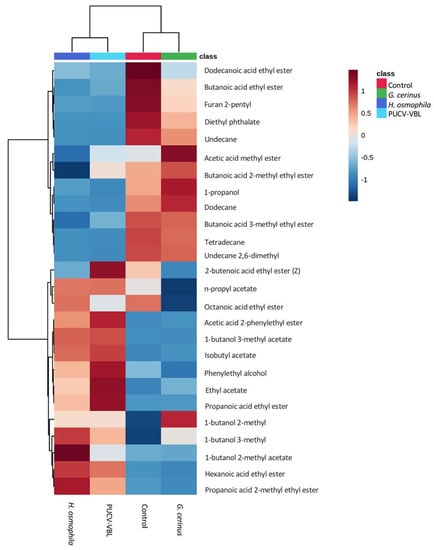
Figure 6.
Heat map based on Euclidean distance using Ward algorithm and showing group averages.. Each row in the color heat map indicates a single compound. Blue to reddish in the color code indicates down-to-up relative concentration of the compounds. Control, grape without biocontroller, red; G. cerinus grape with biocontroller G. cerinus, green; H. osmophila, grape with biocontroller H. osmophila, blue; PUCV-VBL, grape with PUCV-VBL consortium, light blue.
Results show that Hanseniaspora osmophila is most responsible for VOCs in grapes (1-butanol 3-methyl, 2-methyl butanoic acid, 2-methyl 1-butanol, hexanoic ethyl acid, 3-methyl 1-butanol, 2-phenyl acetic acid, propyl acetate, phenylethyl alcohol, ethyl acetate, 2-propanoic acid, isobutyl acetate, and propanoic acid); however, the same VOCs were present in the PUCV-VBL biocontrol. Although G. cerinus had fewer overall contributions to VOCs, it did overexpress 2-butenoic acid (also present in the PUCV-VBL biocontrol) and methyl acetic acid. There was some overlap in G. cerinus and control VOCs: 2-methyl-1-butanol, undecane, 1-propanol, dodecane, 2,6-dimethyl undecane, butanoic ethyl acid, 3-methyl butanoic acid, and tetradecane.
Finally, the VOCs significantly more present in both H. osmophila and the PUCV-VBL biocontrol were 1-butanol 3-methyl, propanoic acid ethyl ester, ethyl acetate, phenylethyl alcohol, isobutyl acetate, and hexanoic acid ethyl ester. Tetradecane was present only in control and G. cerinus treatments (Figure 7).
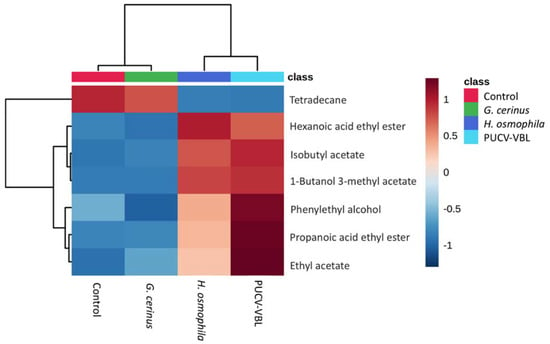
Figure 7.
Heat map with significant VOCs ANOVA (FDR = 0.05) FDR = False Discovery Rate. Control, grape without biocontroller, red; G. cerinus, grape with biocontroller G. cerinus, green; H. osmophila, grape with biocontroller H. osmophila blue; PUCV-VBL, grape with PUCV-VBL consortium, light blue. Blue to reddish in the color code indicates down- to up-regulation of the compounds.
3. Discussion
The PUCV-VBL biocontrol was shown to produce a wide range of VOCs. Desirable for their volatile characteristics and diffusibility in the air [31,32], VOCs are typically smaller than other BCAs secondary metabolites (up to C20) with low molecular mass (100-500 Daltons), high vapor pressure, low boiling point, and lipophilia [33]. These properties facilitate evaporation and diffusion through water- and gas-filled pores in soil and rhizosphere environments [34,35].
The present work shows that PUCV-VBL biocontrol VOCs limited the mycelial growth of fungal pathogens in table grapes. For in vitro, B. cinerea was most inhibited, at 86%. Next, VOCs emitted by component BCA H. osmophila were shown to inhibit sporulation of A. tubingensis and P. expansum (Figure 1). This is similar to the results obtained by Ul Hassan et al., [36] for Bacillus licheniformis VOCs, who indicated that, among them, 3-methyl-1-butanol was most responsible for inhibiting mycelial growth and sporulation. Our results also showed 3-methyl-1-butanol as the predominant VOC molecule produced by H. osmophila. Interestingly, some Bacillus spp.—including B. subtilis, B. amyloliquefaciens, and B. cereus—have been reported to produce 3-methyl-1-butanol and act as a robust antifungal compound [37].
SPME-GC-MS was used to identify VOCs from the PUCV-VBL biocontrol and its BCAs components, G. cerinus and H. osmophila. VOCs were detected after 72 h of bioantagonist incubation. Principal component analysis (PCA) of SPME-GC-MS VOC data revealed heterogeneity in component contribution to VOCs (Figure 4 and Figure 5). VOCs were similar between control and G. cerinus and between H. osmophila and the PUCV-VBL biocontrol. A Euclidean distance heat map based on the Ward algorithm for each treatment’s replicas provides an intuitive visualization of all compounds (Figure 6). Both component BCAs produced the same compounds but with different relative peak areas, and they are present in different concentrations. There are two distinguishable VOC clusters: that of the PUCV-VBL biocontrol and H. osmophila, in which VOC are overexpressed; and that of G. cerinus and the control, with lesser concentrations.
The VOCs significantly more present in H. osmophila and the PUCV-VBL biocontrol were 1-butanol 3-methyl, propanoic acid ethyl ester, ethyl acetate, phenylethyl alcohol, isobutyl acetate, and hexanoic acid ethyl ester (Figure 7). It is almost certain that their antipathogenic effects are due to this overexpression.
In total, the PUCV-VBL biocontrol VOCs were identified as ethyl acetate, propanoic acid ethyl ester, 1-butanol 3-methyl, 1-butanol, 2-methyl, propanoic acid 2-methyl ethyl ester, isobutyl acetate, butanoic acid ethyl ester, 2-butenoic acid ethyl ester (Z), butanoic acid 3-methyl ethyl ester, 1-butanol 3-methyl acetate, 1-butanol 3-methyl acetate, 1-butanol 2-methyl acetate, furan 2-pentyl, hexanoic acid ethyl ester, undecane, phenyl ethyl alcohol, octanoic acid ethyl ester, acetic acid 2-phenylethyl ester, tetradecane, and diethyl phthalate (Supplementary Material, Table S2). These compounds—mostly esters (65%), and to a lesser extent, alcohols (15%), alkanes (15%), and furan (5%)—have all been previously shown to have antifungal activity [28,29,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. The low proportion of furan is concordant with other studies of, e.g., Streptomyces albulus NJZJSA2 against S. sclerotiorum and Fusarium oxysporum [43].
Mainly, Kwasiborski et al., [52] reported that ethyl acetate might be involved in antimicrobial activity against B. cinerea [53]. Indeed, 2-phenylethanol—previously observed as the primary volatile produced by other yeasts, such as Saccharomyces cerevisiae—has been shown to control pathogen Sclerotinia sclerotiorum in vitro and bean seeds, to have a lethal effect against A. flavus and to inhibit the production of aflatoxin in sublethal doses. Furthermore, high concentrations of 2-phenylethanol can cause alterations in the biosynthesis of amino acids and proteins in the mitochondria and the nuclei of fungi and bacteria [17,45,54,55].
Additionally, 2-phenylethanol isolated from K. apiculata showed inhibitory activity against green and blue mold in citrus fruits caused by P. digitatum and P. italicum [19]. It was also shown to play a critical role in the antagonistic activity of A. pullulans against postharvest fruit pathogens both in vitro and in vivo [56,57].
Next, PUCV-VBL biocontrol VOCs 1-butanol-2-methyl, 1-butanol-3-methyl, 1-propanol-2-methyl, and phenylethyl alcohol have been previously reported in other fungi, such as M. albus [58], Trichoderma atroviride [59], P. expansum [27], Glomerella cingulata [60]; and yeasts, such as S. cerevisiae [61], S. pararoseus [62], C. intermedia [38] and A. pullulans [51].
Although various combinations of BCAs and or chemicals have been extensively investigated [63], there has to date been no description of bacteria Gluconobacter cerinus and yeast Hanseniaspora osmophila reported together, or their effects against bunch rot diseases affecting table grapes. Thus the VOC inhibition activity in this study adds to the results from previous research into BCAs.
For example, our study is supported by past results on Hanseniaspora uvarum, which was shown to be an effective BCA in, e.g., controlling fungal rot in strawberries by biofumigation using VOCs [64]. It reduces the natural development of decay in grapes and strawberries and maintains quality parameters [28,57,65,66]. It also exerts biocontrol against chili fruit rot [67] and green mold of postharvest oranges [68]; and inhibits the growth of B. cinerea with multiple modes of action, including competition for nutrients and space, host defense induction, morphology change and secondary metabolites [57,65,66,69,70]. Indeed, Moreira et al., [71] identified different VOCs produced by Hanseniaspora yeasts during red wine vinifications such as 3-methyl-1-butanol, ethyl acetate, phenylethyl alcohol, butanoic acid, and ethyl ester, similar to results from this study.
Furthermore, the findings in this study are further supported by the literature on alternative fungicides. Li et al. [72,73] found that volatile compounds of Streptomyces globisporus JK-1 inhibited spore germination and mycelial growth of B. cinerea and P. italicum in tomato and Citrus microcarpa, respectively. Zheng et al., [42] and Chen et al., [74] indicated that VOCs of Bacillus spp. were antagonistic to B. cinerea, C. gloeosporioides, P. digitatum, P. italicum, and P. crusto. Similar inhibitory effect on conidia germination by VOCs produced by two Aurebasidium pullulans yeast strains L1 and L8 with 2-methyl-1-butanol, 3-methyl-1-butanol, 2-phenethyl alcohol, and 2-methyl-1-propanol against five postharvest fruit pathogens in vitro and in vivo [51,56,57,75,76,77].
Notably, the antipathogenic action was shown to be highest for the combinatory PUCV-VBL biocontrol. This finding is similar to Li et al., [78], who attempted to reproduce the spectrum of naturally occurring VOCs of Ceratocystis fimbriata in proportions calculated using GC-MS analysis. The pure chemicals or several combinations (butyl, ethyl acetate, and ethanol) did not show any inhibitory effect. According to the authors, inhibition likely remains due to synergistic effects among all C. fimbriata VOCs, including molecules not detected using current identification methods. Indeed, VOCs have been known to rely on synergistic effects against phytopathogens [58].
In terms of its mode of action, the PUCV-VBL biocontrol VOCs are nearly a mesosystemic, quasi-systemic, or systemic surface mechanism [79]. While they move as a gas in the layer adjacent to the grapes’ surface, they may also enter to stop pathogenic fungi’ growth. Notwithstanding, there may be additional contributions to BCAs activities from, e.g., the production of diffusible compounds and additional mechanisms for the control of pathogenic fungi [80]. In summary, all the results obtained in these trials indicate that antibiosis is a mode of action of this new consortium [81].
4. Materials and Methods
4.1. Microorganism and Vegetable Materials
PUCV-VBL biocontrol microorganisms were isolated from the surface of table grapes harvested in commercial farms from the Central Valley of Chile. Bacterium Gluconobacter cerinus strain 515 (access code RGM2215) and yeast Hanseniaspora osmophila strain 337 (access code RGM2214) were obtained and deposited in the Chilean Collection of Microbial Genetic Resources (Patent WO2017088081A1). Microorganisms were identified visually, by morphology, and genetically, by genome amplification with GluF/R primer, for G. cerinus [82] and D1/2, for H. osmophila [83]. Cell concentration was adjusted to 1 × 106 UFC mL−1 for the bacterium using a spectrophotometer at an OD580 nm (S-300, BOECO, Germany) and 1 × 104 UFC mL−1 for the yeast using a Neubauer hemocytometer (8100204, Hirschmann, Germany).
Pathogenic fungi under study Botrytis cinerea, Aspergillus tubingensis, Penicillium expansum and Rhizopus stolonifer were amplified and sequenced with the following primers: ITS4/5 for the ITS region [84] and βt for ß-tubulin [85]. After identification, samples of each fungal pathogen were coded and stored in ceparium of PUCV Phytopathology Laboratory (Supplementary Material, Table S1).
Each fungal pathogen was adjusted to 1 × 105 conidia mL−1. Bunches from table grape cultivars—Thompson Seedless, Crimson Seedless, and Red Globe—were obtained from orchards located in Valparaíso Region (central valley of Chile). Total soluble solids (Brix) defined the harvest date: for cv. Red Globe and Crimson Seedless, 17°Brix; and for cv. Thompson Seedless, 16°Brix. Refrigerated at 1 °C until use, fruit sample surfaces were disinfected by dipping into 1% (v/v) of sodium hypochlorite (NaOCl) solution for 2 min, rinsed with sterilized water, and then air-dried.
4.2. In Vitro Assays of Biological Consortium VOCs
The VOCs of the PUCV-VBL biocontrol and those of its respective components were evaluated against the mycelial growth of summer bunch rot pathogens as follows. The double plate method (Scheme 1a) was used with Mannitol Yeast Peptone (MYP) culture medium (25 g/L mannitol, 5 g/L yeast extract, 3 g/L peptone, and 12 g/L agar) for bacteria; Honey Peptone Agar (HPA), modified (80 g/L honey, 20 g/L peptone, 20 g/L agar) for the yeast [86]; and Potato Dextrose Agar (PDA) (DifcoTM) for pathogens [27,28]. Petri dishes with PDA and MYP culture media were inoculated first with a suspension of each pathogen (20 µL) and then with 100 µL of each biocontroller at the concentrations described above. For each treatment, performed in triplicate and repeated twice, the two plates were sealed with parafilm and incubated for five days at 24 °C [38]. Fungus growth area was measured using image analysis with Image J® program (v. 1.50i, MD, USA). The percentage of inhibition was calculated as ((Control value-Treatment value)/(Control value)) × 100.
4.3. In Vivo Assays of Biological Consortium VOCs
Two trials determined in vivo biocontrol VOC production for the PUCV-VBL biocontrol and its components. Following Huang et al. [38], in the first trial (Scheme 1b), two Petri dishes with biocontrollers were placed at the bottom of a humid chamber (21 × 15 × 5 cm), underneath a plastic mesh rack. Six grapes were artificially wounded by a sterile needle, inoculated with pathogenic fungi, and placed on the mesh. In the second trial (Scheme 1c), Petri dishes were replaced with four berries inoculated with the biocontroller. Percent inhibition was calculated as indicated for in vitro assays (though in this case, concerning the berry). Both trials were repeated twice, and each treatment, performed in triplicate.
4.4. Identification of Biocontroller-Produced VOCs (SPME-GC-MS)
VOCs produced by biological control agents (BCAs) were identified by Solid-Phase Microextraction followed by Gas Chromatography-Mass Spectrometry. Briefly, samples of grape cultivars (2 g) were taken, deposited in 20 mL vials, and inoculated with 100 µL of each biocontroller at the concentrations described above; pre-incubated for one min at 30 °C with shaking; and then incubated at 24 °C for three days. Before testing, SPME fiber (SU57298U, 50/30 μm DVB/Carboxen/PDMS 23 Ga, Agilent Technologies, Santa Clara, CA, USA) was conditioned at 200 °C for 1 min. Fiber and samples were incubated for 1 h at 30 °C at 40 mm vial depth distance. Retained compounds were desorbed into the chromatograph injection port at 240 °C for 3 min in Splitless mode with a 50 mL min−1 purge flow to a Split valve for 0.5 min (Agilent 7890B-5977A single quadrupole MS and PAL3 autosampler) with 5190-4048 liner and 5182-3442 Merlin Microseal. The fiber was reconditioned at 200 °C for 5 min before proceeding with a new adsorption/desorption cycle.
Chromatography was performed following Qin et al. [28]. Helium was used as a carrier gas with a flow of 1 mL min−1 and an approximate pressure of 7.7 psi. The column used was a 122-5532 DB-5ms 30 m × 250 µm × 0.25 µm (Agilent Technologies, Santa Clara, CA, USA). The mass spectrometer transfer line was 280 °C, and the quadrupole temperature and the ionization source were set at 150 °C and 230 °C, respectively. The mass spectrometer was operated in SCAN mode. Mass spectra were generated in a range of 50–600 m/z and at a scan rate of 2.7 cycles per second. Peaks were identified using the NIST14 library in MassHunter Quantitative software.
4.5. Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA, Prism Software version 6.0) and means compared by Tukey’s test. Statistical significance was assessed at the level of p ≤ 0.05. Hierarchical clustering and principal component analyses (PCA) were performed using MetaboAnalyst 5.0 (http://www.metaboanalyst.ca) (accessed on 20 May 2021).
5. Conclusions
The results obtained in this work demonstrate the effectiveness of the PUCV-VBL biocontrol VOCs in controlling causal agents of diseases affecting table grapes, including gray and summer bunch rot, under both in vitro and in vivo conditions. The use of these BCAs as a sustainable alternative for the management of phytopathogenic diseases offers numerous advantages, including safe application methods, for the effective control and management of fungal diseases.
6. Patents
Patent N° 61580 February 07 2021, WO2017088081A1.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10060663/s1, Table S1: Molecular identification, percentage identity and access number of GenBank sequences of microorganism used, Table S2: Volatile organic compounds detected from PUCV-VBL consortium on grapes using SPME-GCMS.
Author Contributions
Conceptualization, N.D. and X.B.; methodology, N.D., M.O., F.C., C.F., R.P. and X.B.; software analysis, N.D., M.O., C.F. and R.P.; writing—original draft preparation, N.D., M.O., F.C., G.B., I.M., A.M., E.S., C.F., R.P. and X.B.; writing—review and editing, N.D., M.O., F.C., G.B., I.M., A.M., E.S., C.F., R.P. and X.B.; visualization, N.D. and X.B.; supervision, X.B.; funding acquisition, X.B., F.C. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project ID17AL0028, and funding was also received from FONDEF and ANASAC Chile S.A. through the Chile government.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The authors thank FONDEQUIP EQM140074 and EQM170194 from the government of Chile. The authors are grateful to Mason Taylor for the English revision of the manuscript, Excequel Ponce for SPME-GCMS and Rosario Gatica for figure design.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Fabiola Cádiz, Eduardo Salgado and Ximena Besoain are coinventors of Patent N° 61580 7 February 2021, WO2017088081A1.
References
- Stapleton, J.J. Leaf Removal for Nonchemical Control of the Summer Bunch Rot Complex of Wine Grapes in the San Joaquin Valley. Plant Dis. 1992, 76, 205. [Google Scholar] [CrossRef]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Toro, M.E.; Combina, M.; Castellanos de Figueroa, L.I.; Vazquez, F. Biocontrol of fungi isolated from sour rot infected table grapes by Saccharomyces and other yeast species. Postharvest Biol. Technol. 2013, 86, 456–462. [Google Scholar] [CrossRef]
- Esterio, M.; Muñoz, G.; Ramos, C.; Cofré, G.; Estévez, R.; Salinas, A.; Auger, J. Characterization of Botrytis cinerea isolates present in Thompson seedless table grapes in the Central Valley of Chile. Plant Dis. 2011, 95, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Besoain, X.A.; Araya, C.; Salgado, E.; Rendich, A.; Latorre, B.; Piontelli, E. Ethiology of Bunch Rot Complex of Red Globe Table Grapes Determined by Challenge Inoculations. XV Congreso Nacional de Fitopatología, Chile 2005. Available online: http://www.sochifit.cl/site/congreso/html/XV.html#Articulo_38 (accessed on 24 March 2021).
- Mari, M.; Martini, C.; Guidarelli, M.; Neri, F. Postharvest biocontrol of Monilinia laxa, Monilinia fructicola and Monilinia fructigena on stone fruit by two Aureobasidium pullulans strains. Biol. Control 2012, 60, 132–140. [Google Scholar] [CrossRef]
- Errampalli, D. Penicillium expansum (Blue Mold); Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124115682. [Google Scholar]
- Vico, I.; Duduk, N.; Vasic, M.; Nikolic, M. Identification of Penicillium expansum causing postharvest blue mold decay of apple fruit. Pestic. i fitomedicina 2014, 29, 257–266. [Google Scholar] [CrossRef]
- Blum, L.E.B.; Amarante, C.V.T.; Valdebenito-Sanhueza, R.M.; Guimarães, L.S.; Dezanet, A.; Hack Neto, P. Cryptococcus laurentii aplicado em pós-colheita reduz podridões em maçãs. Fitopatol. Bras. 2004, 29, 433–436. [Google Scholar] [CrossRef][Green Version]
- ASOEX Temporada 2019–2020: Exportaciones de uvas de mesa Chilena a China Crecen Potenciadas por Nuevas Variedades. Available online: https://www.asoex.cl/component/content/article/25-noticias/750-temporada-2019-2020-exportaciones-de-uvas-de-mesa-chilena-a-china-crecen-potenciadas-por-nuevas-variedades (accessed on 24 March 2021).
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Esterio, M.; Araneda, M.J.; Román, A.; Pizarro, L.; Copier, C.; Auger, J. First Report of Boscalid Resistant Botrytis cinerea Isolates Carrying the Mutations H272R, H272Y, P225L, and P225H from Table Grape in Chile. Plant Dis. 2015, 99, 891. [Google Scholar] [CrossRef]
- Esterio, M.; Ramos, C.; Walker, A.S.; Fillinger, S.; Leroux, P.; Auger, J. Phenotypic and genetic characterization of Chilean isolates of botrytis cinerea with different levels of sensitivity to fenhexamid. Phytopathol. Mediterr. 2011, 50, 414–420. [Google Scholar] [CrossRef]
- Latorre, B.A.; Flores, V.; Sara, A.M.; Roco, A. Dicarboximide-resistant isolates of Botrytis cinerea from table grape in Chile: Survey and characterization. Plant Dis. 1994, 78, 990–994. [Google Scholar] [CrossRef]
- Leroch, M.; Kretschmer, M.; Hahn, M. Fungicide resistance phenotypes of botrytis cinerea isolates from commercial vineyards in South West Germany. J. Phytopathol. 2011, 159, 63–65. [Google Scholar] [CrossRef]
- Esterio, M.; Auger, J.; Ramos, C.; García, H. First Report of Fenhexamid Resistant Isolates of Botrytis cinerea on Grapevine in Chile. Plant Dis. 2007, 91, 768. [Google Scholar] [CrossRef]
- Robin, D.C.; Marchand, P.A. Evolution of the biocontrol active substances in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2019, 75, 950–958. [Google Scholar] [CrossRef]
- Kevan, P.G.; Shipp, L. Biological Control as Biotechnological Amelioration and Ecosystem Intensification in Managed Ecosystems; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780128096338. [Google Scholar]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, Y.; Yang, M.; Liu, Y.; Chen, K.; Long, C.A.; Deng, X. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014, 14, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sundh, I.; Melin, P. Safety and regulation of yeasts used for biocontrol or biopreservation in the food or feed chain. Antonie van Leeuwenhoek 2011, 99, 113–119. [Google Scholar] [CrossRef]
- Salih, E.Y.A.; Fyhrquist, P.; Abdalla, A.M.A.; Abdelgadir, A.Y.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Fahmi, M.K.M.; Elamin, M.H.; Ali, H.A. LC-MS/MS tandem mass spectrometry for analysis of phenolic compounds and pentacyclic triterpenes in antifungal extracts of Terminalia brownii (Fresen). Antibiotics 2017, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; McDaniel, A.; Batziakas, K.G.; Yucel, U.; Nwadike, L.; Pliakoni, E. Antifungal packaging film to maintain quality and control postharvest diseases in strawberries. Antibiotics 2020, 9, 618. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Bravo, G.; Velásquez, A.; Cid, F.P.; Valenzuela, M.; Ramírez, I.; Vasconez, I.N.; Álvarez, I.; Jorquera, M.A.; Seeger, M. Microbial diversity of psychrotolerant bacteria isolated from wild flora of andes mountains and patagonia of chile towards the selection of plant growth-promoting bacterial consortia to alleviate cold stress in plants. Microorganisms 2021, 9, 538. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; du Jardin, P.; Fauconnier, M.L. Barley (Hordeum distichon L.) roots synthesise volatile aldehydes with a strong age-dependent pattern and release (E)-non-2-enal and (E,Z)-nona-2,6-dienal after mechanical injury. Plant Physiol. Biochem. 2016, 104, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Rouissi, W.; Ugolini, L.; Martini, C.; Lazzeri, L.; Mari, M. Control of postharvest fungal pathogens by antifungal compounds from penicillium expansum. J. Food Prot. 2013, 76, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xiao, H.; Cheng, X.; Zhou, H.; Si, L. Hanseniaspora uvarum prolongs shelf life of strawberry via volatile production. Food Microbiol. 2017, 63, 205–212. [Google Scholar] [CrossRef]
- Mercier, J.; Smilanick, J.L. Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biol. Control 2005, 32, 401–407. [Google Scholar] [CrossRef]
- Pagans, E.; Font, X.; Sánchez, A. Emission of volatile organic compounds from composting of different solid wastes: Abatement by biofiltration. J. Hazard. Mater. 2006, 131, 179–186. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Cheng, Z.; Ma, L.; Zhao, L.; Li, J. A comparison of electronic nose and gas chromatography–mass spectrometry on discrimination and prediction of ochratoxin A content in Aspergillus carbonarius cultured grape-based medium. Food Chem. 2019, 297, 124850. [Google Scholar] [CrossRef]
- Fischer, G.; Müller, T.; Schwalbe, R.; Ostrowski, R.; Dott, W. Exposure to airborne fungi, MVOC and mycotoxins in biowaste-handling facilities. Int. J. Hyg. Environ. Health 2000, 203, 97–104. [Google Scholar] [CrossRef]
- Werner, S.; Polle, A.; Brinkmann, N. Belowground communication: Impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl. Microbiol. Biotechnol. 2016, 100, 8651–8665. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; De Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Alnaimi, H.; Migheli, Q.; Jaoua, S. Investigation and Application of Bacillus licheniformis Volatile Compounds for the Biological Control of Toxigenic Aspergillus and Penicillium spp. ACS Omega 2019, 4, 17186–17193. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, G.Q.; Zhang, J.; Yang, L.; Che, H.J.; Jiang, D.H.; Huang, H.C. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869. [Google Scholar] [CrossRef]
- Fialho, M.B.; de Moraes, M.H.D.; Tremocoldi, A.R.; Pascholati, S.F. Potential of antimicrobial volatile organic compounds to control Sclerotinia sclerotiorum in bean seeds. Pesqui. Agropecu. Bras. 2011, 46, 137–142. [Google Scholar] [CrossRef]
- Singh, S.K.; Strobel, G.A.; Knighton, B.; Geary, B.; Sears, J.; Ezra, D. An Endophytic Phomopsis sp. Possessing Bioactivity and Fuel Potential with its Volatile Organic Compounds. Microb. Ecol. 2011, 61, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Kudalkar, P.; Strobel, G.; Riyaz-Ul-Hassan, S.; Geary, B.; Sears, J. Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience 2012, 53, 319–325. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, J.; Shi, J.; Wang, Q.; Li, Y. Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biol. Control 2013, 65, 200–206. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, J.; Yaoyao, E.; Raza, W.; Shen, Q.; Huang, Q. Effects of volatile organic compounds from Streptomyces albulus NJZJSA2 on growth of two fungal pathogens. J. Basic Microbiol. 2015, 55, 1104–1117. [Google Scholar] [CrossRef]
- Masoud, W.; Poll, L.; Jakobsen, M. Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Yeast 2005, 22, 1133–1142. [Google Scholar] [CrossRef]
- Jovanović, V.S.; Simonović, S.; Ilić, M.; Marković, M.; Mitić, V.; Djordjević, A.; Nikolić-Mandić, S. Chemical composition, antimicrobial and antioxidant activities of seseli pallasii besser. (syn seseli varium trev.) essential oils. Rec. Nat. Prod. 2016, 10, 277–286. [Google Scholar]
- Mayser, P. Medium chain fatty acid ethyl esters—Activation of antimicrobial effects by Malassezia enzymes. Mycoses 2015, 58, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Girija, S.; Duraipandiyan, V.; Kuppusamy, P.S.; Gajendran, H.; Rajagopal, R. Chromatographic Characterization and GC-MS Evaluation of the Bioactive Constituents with Antimicrobial Potential from the Pigmented Ink of Loligo duvauceli. Int. Sch. Res. Not. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Premjanu, N.; Jaynthy, C. Antimicrobial activity of diethyl phthalate: An insilico approach. Asian J. Pharm. Clin. Res. 2014, 7, 141–142. [Google Scholar]
- Naik, B.S. Volatile hydrocarbons from endophytic fungi and their efficacy in fuel production and disease control. Egypt. J. Biol. Pest Control 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Mo, E.K.; Sung, C.K. Phenylethyl alcohol (PEA) application slows fungal growth and maintains aroma in strawberry. Postharvest Biol. Technol. 2007, 45, 234–239. [Google Scholar] [CrossRef]
- Yala, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Kwasiborski, A.; Bajji, M.; Renaut, J.; Delaplace, P.; Haissam Jijakli, M. Identification of metabolic pathways expressed by pichia anomalakh6 in the presence of the pathogen botrytis cinereaon apple: New possible targets for biocontrol improvement. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Fredlund, E.; Blank, L.M.; Schnürer, J.; Sauer, U.; Passoth, V. Oxygen- and glucose-dependent regulation of central carbon metabolism in Pichia anomala. Appl. Environ. Microbiol. 2004, 70, 5905–5911. [Google Scholar] [CrossRef]
- Chang, P.-K.; Hua, S.S.T.; Sarreal, S.B.L.; Li, R.W. Suppression of Aflatoxin Biosynthesis in Aspergillus flavus by 2-Phenylethanol Is Associated with Stimulated Growth and Decreased Degradation of Branched-Chain Amino Acids. Toxins 2015, 7, 3887–3902. [Google Scholar] [CrossRef]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a Killer: Microbial Volatilome and Its Role in the Biological Control of Plant Pathogens. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Di Francesco, A.; Zajc, J.; Aprea, N.G.C.E.; Gasperi, F.; Baraldi, N.P.F.C.E. Bioactivity of volatile organic compounds by Aureobasidium species against gray mold of tomato and table grape. World J. Microbiol. Biotechnol. 2020, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Jiménez, J.I. Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biol. Technol. 2004, 31, 1–8. [Google Scholar] [CrossRef]
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 2010, 81, 187–193. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kimura, M.; Yabe, Y.; Tsukamoto, D.; Sakamoto, M.; Horibe, I.; Okuno, Y. Use of solid phase microextraction (SPME) for profiling the volatile metabolites produced by Glomerella cingulata. J. Oleo Sci. 2008, 57, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Fialho, M.B.; Toffano, L.; Pedroso, M.P.; Augusto, F.; Pascholati, S.F. Volatile organic compounds produced by Saccharomyces cerevisiae inhibit the in vitro development of Guignardia citricarpa, the causal agent of citrus black spot. World J. Microbiol. Biotechnol. 2010, 26, 925–932. [Google Scholar] [CrossRef]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Spadaro, D.; Gullino, M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004, 91, 185–194. [Google Scholar] [CrossRef]
- Wang, L.; Dou, G.; Guo, H.; Zhang, Q.; Qin, X.; Yu, W.; Jiang, C.; Xiao, H. Volatile organic compounds of Hanseniaspora uvarum increase strawberry fruit flavor and defense during cold storage. Food Sci. Nutr. 2019, 7, 2625–2635. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, H.; Xue, C.; Yu, Z.; Yang, R.; Cai, Z.; Si, L. Biocontrol of gray mold in grapes with the yeast Hanseniaspora uvarum alone and in combination with salicylic acid or sodium bicarbonate. Postharvest Biol. Technol. 2015, 100, 160–167. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, R.; Xiao, H.; Qin, X.; Si, L. Effect of preharvest application of Hanseniaspora uvarum on postharvest diseases in strawberries. Postharvest Biol. Technol. 2015, 100, 52–58. [Google Scholar] [CrossRef]
- Basha, H.; Ramanujam, B. Growth promotion effect of Pichia guilliermondii in chilli and biocontrol potential of Hanseniaspora uvarum against Colletotrichum capsici causing fruit rot. Biocontrol Sci. Technol. 2015, 25, 185–206. [Google Scholar] [CrossRef]
- Li, W.; Qin, Z.; Zhang, H. Extremal hexagonal chains with respect to the coefficients sum of the permanental polynomial. Appl. Math. Comput. 2016, 291, 30–38. [Google Scholar] [CrossRef]
- Liu, H.M.; Guo, J.H.; Luo, L.; Liu, P.; Wang, B.Q.; Cheng, Y.J.; Deng, B.X.; Long, C.A. Improvement of Hanseniaspora uvarum biocontrol activity against gray mold by the addition of ammonium molybdate and the possible mechanisms involved. Crop Prot. 2010, 29, 277–282. [Google Scholar] [CrossRef]
- Romanazzi, G.; Lichter, A.; Gabler, F.M.; Smilanick, J.L. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012, 63, 141–147. [Google Scholar] [CrossRef]
- Moreira, N.; Pina, C.; Mendes, F.; Couto, J.A.; Hogg, T.; Vasconcelos, I. Volatile compounds contribution of Hanseniaspora guilliermondii and Hanseniaspora uvarum during red wine vinifications. Food Control 2011, 22, 662–667. [Google Scholar] [CrossRef]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol. Control 2012, 61, 113–120. [Google Scholar] [CrossRef]
- Li, B.; Lai, T.; Qin, G.; Tian, S. Ambient pH stress inhibits spore germination of Penicillium expansum by impairing protein synthesis and folding: A proteomic-based study. J. Proteome Res. 2010, 9, 298–307. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, X.; Wang, J.; Wu, L.; Zheng, Z.; Yu, Z. Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol. Lett. 2008, 30, 919–923. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Performance evaluation of volatile organic compounds by antagonistic yeasts immobilized on hydrogel spheres against gray, green and blue postharvest decays. Food Microbiol. 2016, 63, 191–198. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2017, 265, 18–22. [Google Scholar] [CrossRef]
- Li, Q.; Wu, L.; Hao, J.; Luo, L.; Cao, Y.; Li, J. Biofumigation on post-harvest diseases of fruits using a new volatile-producing fungus of Ceratocystis fimbriata. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.P. Recent advances in crop protection. Recent Adv. Crop Prot. 2013, 1–268. [Google Scholar] [CrossRef]
- Olivera, M.; Delgado, N.; Cádiz, F.; Riquelme, N.; Montenegro, I.; Seeger, M.; Bravo, G.; Barros, W.; Pedreschi, R.; Besoain, X. Diffusible compounds produced by Hanseniaspora osmophila and Gluconobacter cerinus help to control the causal agents of gray rot and summer bunch rot of table grapes. 2021, unpublished; manuscript accepted. 2021; unpublished; manuscript accepted. [Google Scholar]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Trček, J.; Teuber, M. Genetic and restriction analysis of the 16S-23S rDNA internal transcribed spacer regions of the acetic acid bacteria. FEMS Microbiol. Lett. 2002, 208, 69–75. [Google Scholar] [CrossRef]
- Leaw, S.N.; Chang, H.C.; Sun, H.F.; Barton, R.; Bouchara, J.; Chang, T.C. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 2006, 44, 693–699. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods an Application Ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).