Abstract
The need for easily biodegradable and less toxic chemicals in drug development and pest control continues to fuel the exploration and discovery of new natural molecules. Like certain plants, some insects can also respond rapidly to microbial infections by producing a plethora of immune-induced molecules that include antibacterial and antifungal peptides/polypeptides (AMPs), among other structurally diverse small molecules. The recent recognition that new natural product-derived scaffolds are urgently needed to tackle life-threatening pathogenic infections has been prompted by the health threats posed by multidrug resistance. Although many researchers have concentrated on the discovery of AMPs, surprisingly, edible insect-produced AMPs/small molecules have received little attention. This review will discuss the recent advances in the identification and bioactivity analysis of insect AMPs, with a focus on small molecules associated with the microbiota of selected African edible insects. These molecules could be used as templates for developing next-generation drugs to combat multidrug-resistant pathogens.
1. Introduction
Plants are the primary sources of natural products, having contributed immensely to drug discovery for the treatment of a variety of human diseases. However, the arsenal of secondary metabolites has recently expanded to include those of microbial origin associated with insects [1]. The early regime of antibiotics was boosted by the “accidental” discovery of penicillin (β-lactam), isolated from the fungus Penicillium sp., whose mode of action entailed targeting bacterial cell wall synthesis. Subsequent investigations in the field of antibiotic metabolites led to the discovery of many other classes of antimicrobial compounds through conventional fermentation procedures. However, the systematic emergence of resistance towards the available classes of antibiotics has increasingly posed a major challenge [2]. This has continued to justify the search for new antimicrobial metabolites, with potentially new modes of action, towards countering the problem of multi-drug resistance.
Bacteria and fungi, like many other organisms, must compete for resources by using a range of strategies, including those inflicted directly by toxins and indirectly through the activity of host immune responses, which results in changes in pathogenicity [3]. Bacterial and fungal interactions with their hosts including insects are crucial for many biological processes in agriculture, waste management, food production, and medicine [4,5].
Reviews on the nutritional value of certain insects, such as black soldier fly larvae, housefly maggot and pupae, mealworms, silkworm pupae, locusts, beetles, grasshoppers, and crickets, show that they have the potential to be used as an alternative protein source in various livestock feeds [6,7]. Additionally, insect meals can be used effectively in rearing of animals, such as poultry, to enhance animal health, intestinal morphology, and the composition of the intestinal microbiota [8], with an effect close to that of plant bioactive substances [9]. These insects’ gut microbiota comprises a diverse range of microorganisms that produce bioactive compounds that, among other things, protect their host from pathogenic assault [10]. However, little is known about these bioactive compounds, often rich in peptides and other classes of natural products.
Antimicrobial peptides/polypeptides (AMPs), for example, are an innate component of insect immunity found in their hemolymph and have been shown to have significant biological activity against fungi, viruses, parasites, and, most importantly, antibiotic-resistant bacteria [11,12]. Antimicrobial peptides (AMPs) are small molecules that range in size from 10 to 100 amino acid (AA) residues and are produced by all living organisms. The rich diversity of insects makes them strong candidates to screen for novel sources of AMPs. The black soldier fly (BSF) Hermetia illucens L. (Diptera: Stratiomyidae), in particular, has been shown to have an extraordinary ability to live in hostile environments rich in microbial colonies, making it one of the most promising sources of AMPs [13]. Some of the AMPs identified from the BSF larvae and adult transcriptomes include defensin (44%), cecropins and lysozymes (18%), attacins (7%), and other AMPs (<5%) [13]. Structural examples of the described defensin-like peptide (DLP4) and cecropin (CLP1) from BSF are shown in Figure 1. Many other AMPs found in insects have been compiled in a mini-review by Wu et al. [14], which sheds light on their biological effects [14]. In their analysis, these authors delved mainly on the various modes of action suggested for insect AMPs. However, there is paucity of evidence to support the structure–activity relationships (SARs) and mechanisms that underpin AMP activity. There is also no information on other antimicrobial chemicals produced by insects, which is the subject of the current review, with special emphasis on edible insects.
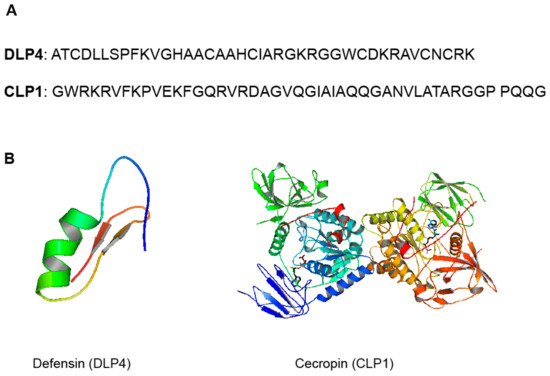
Figure 1.
(A) Sequence of DLP4 (40-amino acid sequence, 4267 Da), [15] and CLP1 (46_amino acid sequence, 4840 Da) [16]. (B) Structural representation and molecular modeling of DLP4 (pdb code: 2nz3) and CLP1 (pdb code: 2npi). Molecular models were generated with PyMOL 2.4.1.
Infections caused by resistant bacteria, on the other hand, are more difficult to treat, jeopardizing the efficacy of first-line antibiotics. As such, drugs that are more effective, more widely available, and have less toxic side effects are in high demand to treat these infections [17,18]. Thus, this review will discuss the recent advances in the identification and bioactivity of antimicrobial compounds derived from selected African edible insects and opportunities for their use as templates for developing next-generation drugs to combat multidrug-resistant pathogens. Although this review targets edible insects from Africa, in some sections, references are drawn from other parts of the world to buttress the growing demand for antibiotics.
2. Edible Insects in Africa and Their Microbiota
The consumption of insects (entomophagy) is an ancient and traditional practice, which has been recognized as one of the ways to alleviate hunger around the world, particularly in Latin America, Africa, and Asia. In sub-Saharan Africa, edible insects have been used as food because they are a good source of protein and essential fatty acids [19,20,21]. They are also high in micronutrients such as copper, iron, magnesium, manganese, phosphorus, selenium, and zinc, as well as vitamins such as riboflavin, pantothenic acid, biotin, folic acid, vitamin A, B complex, and C [22,23].
Africa has the most diverse collection of edible insects, with over 500 species including caterpillars (Lepidoptera), termites (Isoptera), locusts, grasshoppers and crickets (Orthoptera), ants, wasps and bees (Hymenoptera), bugs (Heteroptera and Homoptera), dragonflies (Odonata), flies (Diptera), and beetles (Coleoptera) [24]. As such, these different insect orders may associate with a diversity of microorganisms including bacteria and fungi.
Spore-forming bacteria and Enterobacteriaceae, for example, have previously been detected in crushed mealworms and crickets and were believed to be released from the gut [25]. Furthermore, research on cricket (Gryllotalpa Africana), weevil (Rhynchophorus phoenicis), and butterfly (Bematistes alcinoe) species showed that the majority of microorganisms belonged to the two bacterial genera Bacillus and Staphylococcus, with saprophytes accounting for the remainder [26].
Additionally, a recent study profiled the black soldier fly larval gut microbiota, which included bacterial and fungal communities from four different substrates (brewers’ spent grain, kitchen food waste, poultry manure, and rabbit manure). Metagenomic analysis revealed 21 bacterial and 20 fungal genera. These findings further indicated that the composition and abundance of the identified microbes differed depending upon the substrate [27]. The highly represented bacterial population in BSF larvae reared on all substrates except rabbit waste, for example, belonged to the Dysgonomonas genus. In contrast, the Campylobacter genus was more abundant in BSF larvae reared on rabbit waste than in larvae raised on the other three substrates [27]. Pichia, Cyberlindnera, and Saccharomycecodes were found in high concentrations in brewers’ spent grain, kitchen food waste, and rabbit manure, respectively, in the fungal community. These findings suggest that there is a large population of microorganisms that is yet to be identified, including those found in edible insects, from which new scaffolds of drugs with potentially new modes of action could be developed to target multidrug-resistant bacteria.
3. Multi-Drug Resistance (MDR)
The development of antibiotic resistance has continued to warrant the search for novel bioactive metabolites in the field of natural products. A contributing factor to the problem of multi-drug resistance is the widespread and uncontrolled usage of antibiotics to treat bacterial infections. Scientific research has shown that some disease-causing microbes have developed resistance against certain available classes of antibiotics. These include the “ESKAPE” organisms, i.e., Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter sp. [28]. Methicillin-resistant Staphylococcus aureus (MRSA) is a classic example of a notorious case of multi-drug resistance development. It has developed resistance against the major classes of antibiotics such as aminoglycosides, macrolides, tetracycline, chloramphenicol, and lincosamides [29].
Multi-drug resistance (MDR) is therefore linked to several disease treatment challenges, including prolonged time of infection in patients due to the increased spread of resistant pathogens as a result of first-line drug efficacy, high treatment costs that may lead to high morbidity and mortality rates, and the exposure of immune-compromised patients as an easy target due to decreased drug efficacy [30]. These reports encourage the exploration of alternative sources of antibiotics to overcome MDR. That begs the question of whether insects could serve as a potential source for identifying novel antibiotics.
4. Insects as Potential Antibiotic Producers
Insects not only perform a variety of roles in the environment, but also host a diverse community of microorganisms. The multifaceted cellular and humoral mechanisms comprise the innate immune system of an insect [31,32]. The cellular mechanism is based on phagocytosis being activated by enzymes and invading microorganisms being encapsulated by the hemolymph. The humoral response, on the other hand, is involved in the production of broad-spectrum antimicrobial peptides (AMPs), reactive oxygen or nitrogen intermediates, and complex enzymatic cascades that help to regulate hemolymph coagulation or melanization [33,34]. The presence of microorganisms invading insects causes the fat body to rapidly synthesize AMPs, which are then secreted into the hemolymph [35,36].
Previous research indicates that each insect species produces a distinct antimicrobial peptide that acts against specific microorganisms, as shown in the review by Yi et al. [11]. However, in order to boost the insect’s defense system against other pathogens, some of the peptides are expressed concurrently, encouraging synergism [36,37,38]. For example, when a cecropin (LSer-Cec6) and a defensin (LSer-Def4) from the wound maggot Lucilia sericata were examined together, they showed significantly increased antibacterial activity [39]. Furthermore, the amount of AMPs produced by insects varies greatly depending on the species. As such, AMPs have distinct modes of action, such as altering the electrochemical gradient at the membrane, producing reactive oxygen/nitrogen species (ROS/RNS) that cause cell death, inhibiting protein synthesis, and permeabilizing the cell membrane [38,40]. Antimicrobial peptides (AMPs) have pharmacological properties such as low molecular weight, high water solubility, broad-spectrum antimicrobial activity, and low levels of cytotoxicity [41].
5. The Chemistry of Microorganisms from Selected Edible Insects
In this section, chemical compounds identified in microbes found in the six selected edible insects (black soldier fly, termites, beetles, locusts, caterpillars, and crickets) are highlighted. Some of these chemicals have been reported to have antibacterial, antifungal, antimalarial, anti-inflammatory, and cytotoxic activities.
5.1. Black Soldier Fly Hermetia Illucens (Diptera: Stratiomyidae)
The gut of the black soldier fly (BSF) larvae has been shown to harbor beneficial microbes and fungi that also control pathogens [42]. Thus, the microbes linked to it are a good target for the discovery of new antimicrobial compounds of pharmaceutical relevance. In some studies, the Pichia genus was found to be related to the larvae fed on vegetable waste, whereas Trichosporon, Rhodotorula, and Geotrichum were the most abundant genera in the larvae fed on chicken feed only [42]. Regarding the production of AMPs, it is highly conserved between insects and may only vary between species depending on their respective habitats [43]. In BSF larvae, AMPs have been classified as defensins (cysteine-rich peptides), cecropins (α-helical peptides), attacins (glycine-rich peptides/proteins), and diptericins (a family of related glycine-rich antibacterial peptides), as well as lipids (hexanedioic acid) [43,44]. In a previous analysis, scores that predicted the biological activity of unknown peptides in the transcriptomes of BSF larvae and adults were determined using different algorithm in in silico tests. Biological activities that were predicted included antimicrobial, anticancer, antiviral, and antifungal properties [13]. Follow-up in vitro studies are yet to be carried out to validate these findings.
The general mechanisms of action of AMPs have been investigated in a few studies [45,46]. For example, Park et al. [47], demonstrated that H. illucens larvae extracts have antibacterial properties against Gram-positive S. aureus, methicillin-resistant S. aureus (MRSA), and Gram-negative Pseudomonas aeruginosa [47]. The methanol extract of BSF was found to inhibit the growth of K. pneumoniae, Neisseria gonorrhoeae, and Shigella sonnei bacteria, whereas the extract elicited no antibacterial effects against the bacteria B. subtilis, Streptococcus mutans, and Sarcina lutea [48].
The expression and characterization of stomoxynZH1 (encoded by a 189-basepair gene) with antimicrobial activity against Escherichia coli and S. aureus is one of two recent promising results involving AMPs from H. illucens [49]. In addition, DLP4, a novel AMP isolated and characterized from H. illucens hemolymph larvae, showed potent activity against MRSA and methicillin-susceptible S. aureus (MSSA) [15].
Furthermore, secondary metabolites from Chrysosporium multifidum broth extract, a fungus isolated from the midgut of BSF larvae fed on fresh unsterilized chicken guano, have been described in the literature. As shown below, these molecules include six pyrone derivatives (1–6) and one diketopiperazine (7) (Figure 2). The epoxy moiety containing α-pyrone (5) was found to have moderate antibacterial activity against 43,300 ATCC strains of methicillin-resistant Staphylococcus aureus (MRSA) [10]. Thus, AMPs and small molecules from H. illucens are still understudied, despite the fact that they have strong antibacterial properties. So far, only one fungal strain has been targeted within the rich microbiota of H. illucens, with no reports on the antifungal and cytotoxic effects of the identified molecules.
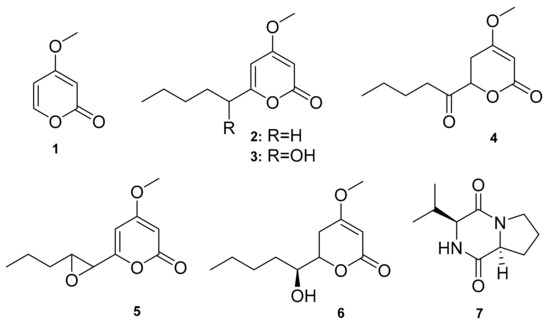
Figure 2.
Small molecules isolated from the midgut of BSF larvae.
5.2. Termites (Isoptera)
Termites are recognized as beneficial insects in agriculture, entomotherapy, and the environment. Their ability to act as mediators of the process of decomposing plant organic matter and as influential agents in soil formation, particularly in tropical forests, is one of their important ecological roles [50]. The diversity of termite gut communities is astounding, but the function of each group of symbionts is poorly understood. Hemicellulose-degrading bacteria, lignolytic bacteria, cellulolytic bacteria, aromatic compound-degrading bacteria, and nitrogen-fixing bacteria are among the primary termite intestinal microorganisms [51,52,53,54,55,56,57,58,59]. According to research, the most abundant bacteria found in both higher and lower termites are strict aerobes or facultative anaerobes. There is also evidence that the most abundant bacteria in termite guts belong to the Staphylococcus and Bacillus genera [60,61]. Moreover, research has previously discovered a link between the major gut bacteria and the termite family (see Table 1) [62].

Table 1.
Classification of microorganisms found in termites.
Additional studies have shown that Streptomyces strains isolated from termites had significantly higher inhibition activity against Gram-negative bacteria than soil isolates [64]. Chemical analysis of termite-associating microbes, in particular fungi from O. formosanus, revealed three small molecules named 5-hydroxyramulosin (8), biatriosporin M (9) from the Pleosporales sp. BYCDW4, and 1-(2,5-dihydroxyphenyl)-3-hydroxybutan-1-one (10) from the Microdiplodia sp. BYCDW8. Investigation of their biological activity indicated that compound (10) had moderate inhibitory activity against B. subtilis and S. aureus [65]. Streptomyces davaonensis YH01, isolated from the body surface of the queen of the termite O. formosanus, demonstrated antibacterial activity. Roseoflavin (11) and 8-methylamino-8-demethyl-d-riboflavin (12), which exhibited antibacterial activities, were discovered in a subsequent study on the same strain [66]. Their absolute stereochemistry, however, is yet to be assigned (Figure 3).
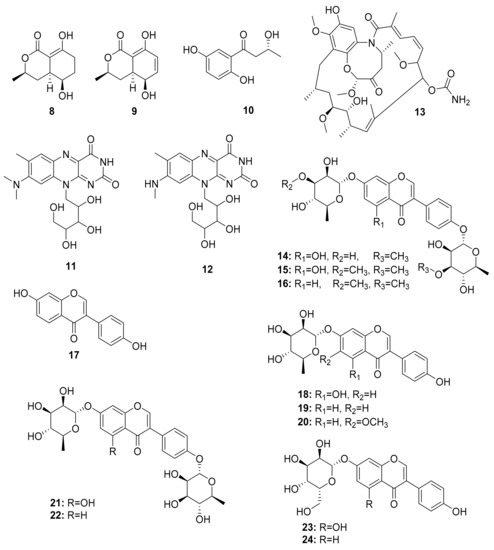
Figure 3.
Small molecules isolated from fungi related to Odontotermes formosanus and Streptomyces strains.
Several other studies have shown that Actinobacteria in association with Macrotermes natalensis (a fungus-growing termite species) led to the isolation of natalamycin (13) from Streptomyces sp. M56, a new antifungal geldanamycin derivative [67]. Termisoflavones A–C (14–16) and eight isoflavonoid molecules (17–24) have been reported from Streptomyces sp. RB1 [68]. In addition, the structures of the complex nonribosomal peptide synthetase–polyketide synthase (NRPS/PKS) hybrid depsipeptides dentigerumycins B–D (25–27) were characterized from Streptomyces sp. M41 [69]; actinomycin D (28) was isolated from Streptomyces sp. RB94 [70]. The glycosylated polyketide macrolactams macrotermycin A–D (29–32) have also been described in Amycolatopsis sp. M39 [71], and a group of tropolone derivatives, rubterolone A–F (33–38,) were found in Actinomadura sp. RB29/5-2 [70,72]. Co-culture studies of Streptomyces sp. RB108 with Pleosporales sp. also yielded the PKS-derived barceloneic acid A (39), which acts as a farnesyl-protein transferase inhibitor (Figure 4 and Figure 5) [70,73]. Other than (19) and (24), no antimicrobial activity was found for termisoflavones and isoflavanoid compounds (see Table 2). At a cisplatin dose of 25 μM, the two compounds reduced cisplatin-induced kidney cell damage to 80% of the control value [68].
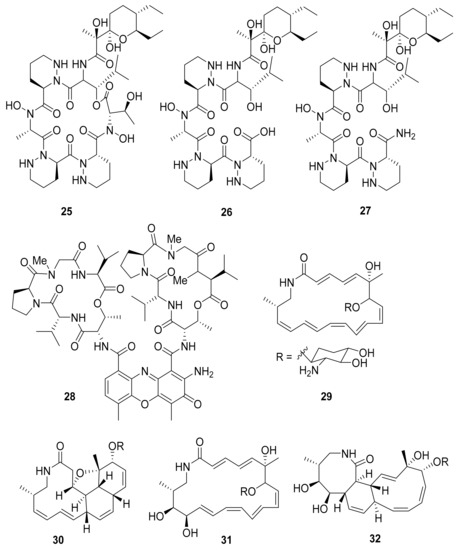
Figure 4.
Peptides from Streptomyces strains and macrolactams from Amycolatopsis sp. associated with Macrotermes natalensis termites.
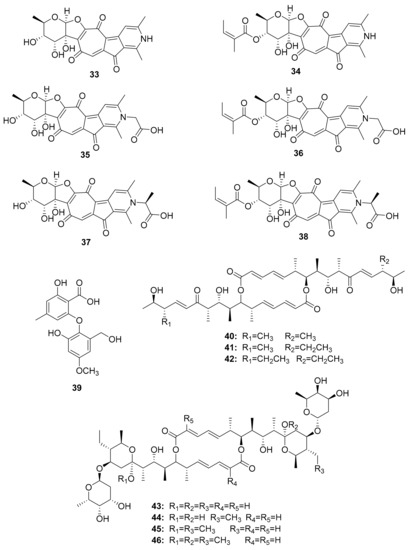
Figure 5.
Tropolone derivatives, small molecules obtained after co-culture experiments, and efomycin-related compounds.

Table 2.
Small molecules from selected edible insects.
Further research on Streptomyces sp. M56 resulted in the identification of two new compounds with an unsaturated enone moiety, named efomycins K (40) and L (41), in addition to the well-known efomycin M (42), a potent and specific inhibitor of selectin [74,75,76]. Subsequently, five known and structurally related hemiketal derivatives—efomycin G (43) [77], elaiophylin (44) [78], 11-O-methylelaiophylin (45) [78], and 11,11′-O-dimethylelaiophylin (46)—were isolated [79,80].
The investigation of Streptomyces sp. MspM5 found in a South African fungus-growing termite, Microtermes species, led to the discovery of two novel PKS/NRPS pathway peptides. The peptides, known as microtermolide A (47) and B (48), exhibited no antibacterial or antifungal activity [84]. Pseudoxylaria sp. X802, which evolved from Microtermes sp., produces a number of antimicrobial compounds. Pseudoxyallemycin B (49) is one of the isolated antibacterial peptides, with a rare and chemically accessible allene moiety (Figure 6) [85].
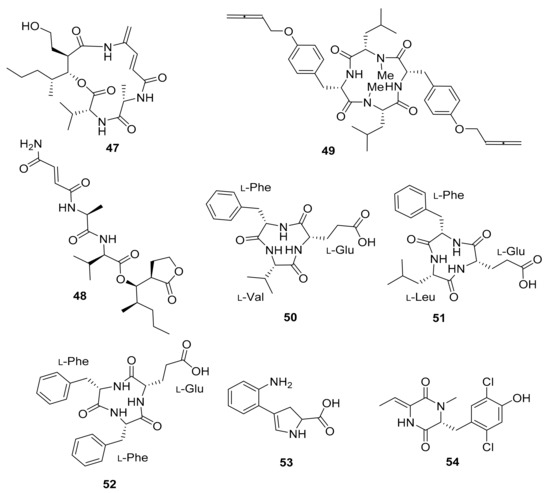
Figure 6.
Peptides isolated from the microbiota of Microtermes sp. and Macrotermes natalensis termites.
Three new cyclic tripeptides named natalenamides A–C (50–52) were isolated from the termite-associated Actinomadura sp. RB99 isolated from the fungus-growing termite Macrotermes natalensis. Compounds 50 and 51 exhibited weak cytotoxicity in HepG2 and HeLa/A549 cells, whereas compound 52 inhibited IBMX-mediated melanin synthesis in a dose-dependent manner [86]. Furthermore, co-cultivation experiments with Actinomadura sp. RB29 and Trichoderma sp. led to the discovery of antifungal compounds such as banegasine (53) and cyclo(NMe-l-3,5-dichlorotyrosine-Dhb) (54) [70].
In silico analysis of the genome of Actinomadura sp. RB29 from Macrotermes natalensis, using antiSMASH and tandem MS2 data submitted to Global Natural Product Social Molecular Networking (GNPS), and subsequent RiPPquest processing revealed the presence of two lanthipeptides with proposed structures of rubrominin A (55) and B (56) [70]. Their gene cluster resembles that of cinnamycin (Figure 7) [100,101,102]. Except for the Actinobacteria microorganisms, the termite family has a large range of microbiota that has not been thoroughly investigated in terms of their chemical capacity as presented here. The bulk of their chemical structures consists of peptides and isoflavonoids, which makes them peculiar.
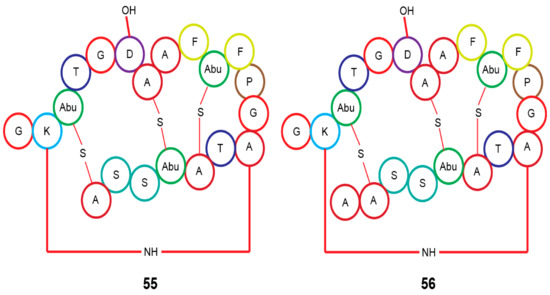
Figure 7.
Proposed lanthipeptides from Actinomadura sp. RB29 linked to Macrotermes natalensis termites. Abu, 2-aminobutyric acid.
5.3. Beetles (Coleoptera)
Beetles are generally known to benefit the environment (nutrient recyclers, pollinators), but a significant portion of them are pests of economically important crops and storage products [103]. Bark beetles, in particular the southern pine beetle (Dendroctonus frontalis), are damaging to trees [104]. They have a symbiotic relationship with the fungus Entomocorticium sp. A, which serves as food for the beetle larvae [105].
Recent research has revealed a link between various beetle species and four bacterial phyla, Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes, as well as three fungal phyla, Ascomycota, Zygomycota, and Basidiomycota. These microbial communities were discovered to differ depending on the beetle host, individual organism, and environment [106].
Actinobacteria, particularly Streptomyces, have been described as having a chemical defense mechanism that produces antimicrobial compounds that aid in the fight against infectious disease. Mycangimycin (57), frontalamide A (58), and frontalamide B (59), for example, were isolated from a Streptomyces strain that was symbiotically associated with the southern pine beetle (Dendroctonus frontalis). Mycangimycin inhibits the beetles’ antagonistic fungus Ophiostoma minus and has potent antimalarial activity. Frontalamide A and frontalamide B have antifungal properties [87,88].
Nakashima et al. studied the fungal strain Fusarium sp. from the ambrosia beetle Euwalecea validus in the early 1980s. Its culture extract contained two antifungal compounds, cerulenin (60) and the nortriterpenoid helvolic acid (61), which inhibited the growth of mold fungi and are thought to suppress bacterial contaminations [89]. Chemical analysis of rove beetles (Paederus sp.) yielded a complex polyketide pederin (62) molecule from an endosymbiotic Pseudomonas sp. [49,50,51,52] that showed toxicity against predators such as wolf spiders in a study by Kellner and Dettner [90].
The soil-dwelling Korean dung beetle (Copris tripartitus) has previously been chemically studied and found to contain a diverse array of Actinobacteria. Their distinct metabolomic profiles resulted in the isolation of tripartilactam (63), a new tricyclic macrolactam that lacks antimicrobial activity but acts as a Na+/K+ ATPase inhibitor [91,92]. Follow-up studies by the same group revealed phenylpyridines, coprismycin A–B (64–65) exhibiting neuroprotective effects, dipyridines, collismycin A (66), SF2738D (67), and a dichlorinated indanone tripartin (68), an inhibitor of the histone H3 lysine 9 demethylase KDM4 in HeLa cells [91,93]. More recently, new cyclic heptapeptides named coprisamides A–B (69–70) were isolated from a Streptomyces strain found in the gut of C. tripartitus, demonstrating significant activity for the induction of quinone reductase (Figure 8) [94]. About a year later, the naphthoquinone–oxindole alkaloids Coprisidins A (71) and B (72) were isolated from the same Streptomyces strain previously studied by Um et al. [95]. Coprisidin A (71) was shown to inhibit the action of Na+/K+ ATPase, whereas Coprisidin B (72) showed induction of NAD(P)H:quinone oxidoreductase 1 (Figure 9) [95].
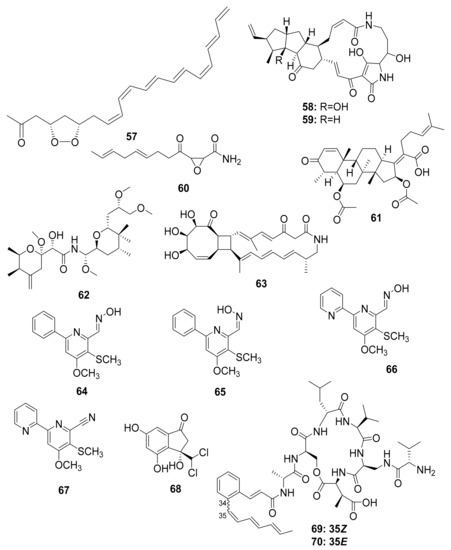
Figure 8.
Chemical diversity in the microbiota of certain beetles.
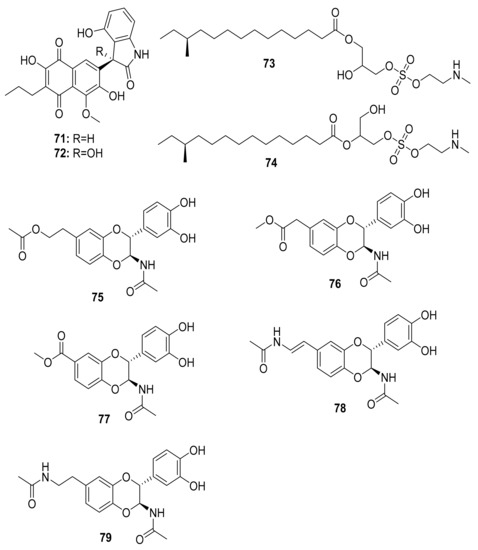
Figure 9.
Chemical structures of compounds isolated from microorganisms linked to the dung beetles.
The antibacterial molecules lenzimycins A (73) and B (74) were isolated from the Brevibacillus sp. PTH23, associating with the dung beetle Onthophagus lenzii. These molecules were also discovered to be effective in activating a reporter system designed to detect bacterial cell envelope stress [96]. Furthermore, the study of biological agents from the entire body of the dung beetle Catharsius molossus resulted in the discovery of three new N-acetyldopamine dimers, molossusamide A–C (75–77), in addition to other known compounds, i.e., cis-2-(3′,4′-dihydroxyphenyl)-3-acetylamino-7-(N-acetyl-2″-amino-ethylene)-1,4-benzodioxane (78) and trans-2-(3′,4′-dihydroxyphenyl)-3-acetyl-amino-7-(N-acetyl-2″-aminoethyl)-1,4-benzodioxane (79) [97]. With the exception of 78, which exhibited inhibitory effects against COX-1 and COX-2, these molecules lacked biological activity against the tested organisms (see Table 2 and Figure 9).
5.4. Locusts (Orthoptera: Acrididae)
Locusts, like other insects, have bacterial cells both inside and outside their bodies, such as on their cuticles. With respect to other locust species, the bacterial composition of the desert locust (Schistocerca gregaria) gut has been extensively described [107]. For example, the bacteria in the hindgut of the desert locust were discovered to be relatively simple, consisting of members of the families Enterobacteriaceae (including Enterobacter and Klebsiella) and Enterocococeaea [108]. This study followed previous research that found gut bacterial flora in S. gregaria that included Escherichia coli, Enterobacter liquefaciens, Klebsiella, Pneumoniae, and Enterobacter cloacae [109,110]. The lack of complexity in the desert locust’s gut microbiota could be attributed to its simple structure and short throughput time.
Despite numerous studies on the desert locust, there is little documented knowledge about the antimicrobial compounds it produces. Using gas chromatography–mass spectrometry (GC–MS), researchers recently investigated how ingested phytosterols are metabolized and biotransformed into other derivatives such as desmosterol, (3β, 5α) cholesta-8, 14, 24-trien-3-ol, 4, 4-dimethyl, (3β, 20R) cholesta-5, 24-dien-3, 20-diol, present as the dealkylated products of lanosterol in the gut [111]. However, the biological activity of these sterols against multidrug-resistant pathogens is yet to be investigated.
5.5. Caterpillars (Lepidoptera)
Caterpillars are among the most popular, consumed, and economically valuable edible insects in the tropics, because they have high protein and fat content [21]. They are mostly found in tropical rainforests. There are over 130 edible caterpillar species consumed in Africa alone, compared to nearly 400 species worldwide [112]. Despite their enormous contribution to global food security, little is known about their ecological relevance [113], associated microbiota, and antimicrobial potential. Caterpillars, unlike other living organisms, do not have a gut microbiota, demonstrating their freedom from symbionts [114].
So far, volicitin (80), N-(17-hydroxy-linoleoyl)-l-glutamine (81), N-linolenoyl-l-glutamine (82), N-linoleoyl-l-glutamine (83) and volicitin-related compounds (84), linolenic acid (85), and the 17-hydroxylinolenic acid (86) (Figure 10) have been discovered in the oral secretions of three Noctuidae species, i.e., Helicoverpa armigera, Mythimna separata, and Spodoptera litura, as well as one Sphingidae species, Agrius convolvuli [98]. The structure–activity relationships of volicitin-related compounds were investigated to determine the elicitor activity of volatiles from corn seedlings, revealing that chirality at C-17 in the linolenic acid chain had no effect on bioactivity. However, it was discovered that the l-glutamine moiety was more important than the hydroxyl moiety [115]. Derivatizing these molecules can lead to a wide variety of agricultural applications, such as identifying volatile components that can be used in pest control. Interestingly, there is no evidence of these compounds being associated with the gut microbiota of caterpillars.
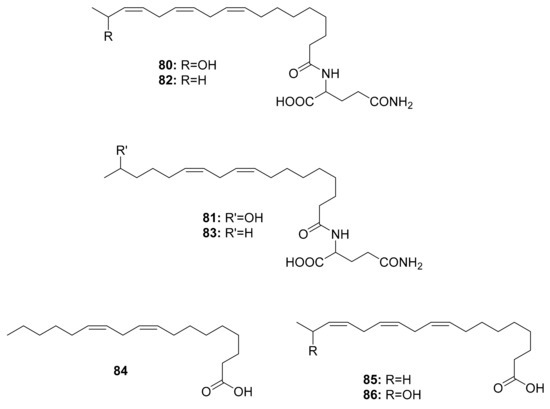
Figure 10.
Compounds isolated from different caterpillar species.
5.6. Crickets (Orthoptera)
Crickets have traditionally been used as a source of medicine for a variety of ailments. In Korea, for example, the mole cricket Gryllotalpa Africana (Gryllotalpidae) is used to treat urine retention, urolithiasis, edema, lymphangitis, and furuncles [116]. In Latin America, the house cricket Acheta domesticus (Gryllidae) is used to treat scabies, asthma, eczema, lithiasis, earache, oliguresis, rheumatism, urine retention, urinary incontinence, and ophthalmological problems [117]. The crickets Paragryllus temulentus (Gryllidae) and Gryllus assimilis (Gryllidae) are both useful in the treatment of rheumatism and warts. Interestingly, bacteria from the Citrobacter, Klebsiella, Yersinia, Bacteroides, and Fusobacterium genera were found in the gastrointestinal tract of the house cricket Acheta domestica [118]. The Photorhabdus asymbiotica bacterium is known to have a two-part life cycle that colonizes the intestines of entomophagous nematodes and necessitates adaptation to both symbiotic and pathogenic phases [119]. The infection of live crickets with the Photorhabdus asymbiotica bacterium resulted in the isolation and characterization of glidobactin A (87), luminmycin A (88), and luminmycin D (89). Only compound (89) was found to be cytotoxic to human pancreatic cells and inhibited proteasome activity (Figure 11) [99]. The macrolactam center of these molecules consists of nonribosomal peptide synthetase modules catalyzing the condensation of 4-hydroxylysine, l-alanine, and malonyl-CoA [120]. Additionally, there is a threonine moiety connecting it to the fatty acid side chain. In this regard, from the perspective of structure–activity relationships, the hydroxyl group on the threonine side chain and the R1 positions can be targeted as accessible sites for modifying the molecule to potentially enhance its biological activities.
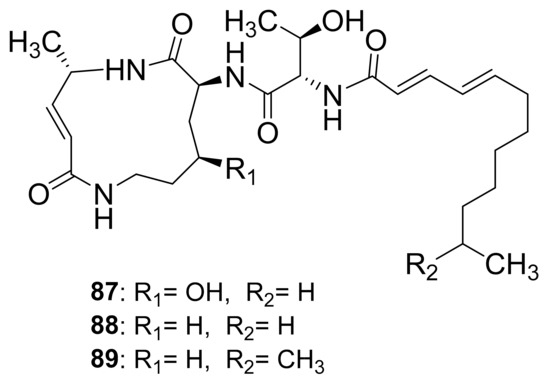
Figure 11.
Compounds obtained from crickets infected with the Photorhabdus asymbiotica bacterium.
6. Future Perspectives
Insects thrive in Africa’s tropical climate. However, this review reveals that very little research has been done on the chemistry of edible insects and their microbiota in Africa. With the current knowledge, it is imperative to advance studies to a higher level, by developing various methods to isolate and characterize new AMPs and small molecules. This may involve varying the habitats in which the insects are reared to stimulate different microbiota, which may increase the likelihood of discovering new pharmacologically relevant metabolites. It is also possible to screen different life stages because insects may live in different habitats, aquatic and/or terrestrial; hence, their potential association with different microorganisms. Moreover, derivatization can be facilitated by using known chemical structures as a template. Aside from that, the identified molecules can be tested in binary or ternary mixtures to increase their synergistic/additive effectiveness against multidrug-resistant species. Bioengineering and biotransformation of species and scaffolds, respectively, may also enhance the observed biological activity. In addition, insights into how these molecules are synthesized by various organisms would be useful to identify the enzymes involved. These studies should therefore help to clarify what function these microorganisms play in their hosts and pave the way for the production of antimicrobial agents.
7. Concluding Remarks
This review has shown that edible insects are a good source of novel antimicrobial peptides and compounds that can be screened against multidrug-resistant pathogens. StomoxynZH1 and DLP4 are among the AMPs found in the BSF with potent antibacterial activity. The majority of isolated microorganisms from which small molecules have been identified were sourced from the insect’s gut. Overall, termite-related microorganisms are the most researched in terms of chemical diversity. In comparison to fungal strains, bacterial strains, especially Actinobacteria, have been extensively studied. As it is evident, Streptomyces yielded 25 (52%) of the 48 molecules isolated from termites, while 13 (27%) were isolated from Actinomadura species. In terms of the chemistry of BSF-associated microorganisms, only one fungal strain has been investigated so far and identified to contain primarily antibacterial α-pyrone molecules. In addition, about 29% of the small molecules characterized herein were obtained from the gut microbiota of beetles. Apart from sterols that have been identified in locusts, there is little knowledge on antimicrobial compounds that they may produce. Caterpillars, on the other hand, contain mainly linolenic acid-derived molecules in their oral secretions. Although the number of studies documenting antimicrobial peptides and small molecules is on the rise, the microbiota of edible insects still remains an understudied topic.
Author Contributions
C.M.M. wrote the manuscript; B.T., S.K., and C.M.T. reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Norwegian Agency for Development Cooperation, the Section for research, innovation, and higher education grant number RAF-3058 KEN-18/0005 (CAP-Africa), Bioinnovate Africa Programme (INSBIZ—Contribution ID No. 51050076), Canadian International Development Research Centre (IDRC), and the Australian Centre for International Agricultural Research (ACIAR) (INSFEED–Phase 2: Cultivate Grant No: 108866-001), The Netherlands Organization for Scientific Research, WOTRO Science for Global Development (NWO-WOTRO) (ILIPA–W 08.250.202), and the Rockefeller Foundation (SiPFeed–Grant No: 2018 FOD 009) through the International Centre of Insect Physiology and Ecology (icipe). We also gratefully acknowledge the support of icipe’s core funding provided by United Kingdom’s Foreign, Commonwealth & Development Office (FCDO); the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Therefore, the views expressed herein do not necessarily reflect the official opinion of the donors.
Acknowledgments
The authors wish to thank Christine Oryema for her great ideas and contribution during discussions.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Seabrooks, L.; Hu, L. Insects: An underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef]
- Waglechner, N.; Wright, G.D. Antibiotic resistance: It’s bad, but why isn’t it worse? BMC Biol. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef] [PubMed]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-Fungal Interactions: Hyphens between Agricultural, Clinical, Environmental, and Food Microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Costa-Neto, E.M. Entomotherapy, or the Medicinal Use of Insects. J. Ethnobiol. 2005, 25, 93–114. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Józefiak, D.; Józefiak, A.; Kierończyk, B.; Rawski, M.; Świątkiewicz, S.; Długosz, J.; Engberg, R.M. Insects—A Natural Nutrient Source for Poultry—A Review. Ann. Anim. Sci. 2016, 16, 297–313. [Google Scholar] [CrossRef]
- Ognik, K.; Kozłowski, K.; Stępniowska, A.; Listos, P.; Józefiak, D.; Zduńczyk, Z.; Jankowski, J. Antioxidant status and liver function of young Turkeys receiving a diet with full-fat insect meal from Hermetia illucens. Animals 2020, 10, 1339. [Google Scholar] [CrossRef]
- Krauze, M.; Cendrowska-Pinkosz, M.; Matuseviĉius, P.; Stępniowska, A.; Jurczak, P.; Ognik, K. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Correa, Y.; Cabanillas, B.; Jullian, V.; Álvarez, D.; Castillo, D.; Dufloer, C.; Bustamante, B.; Roncal, E.; Neyra, E.; Sheen, P.; et al. Identification and characterization of compounds from Chrysosporium multifidum, a fungus with moderate antimicrobial activity isolated from Hermetia illucens gut microbiota. PLoS ONE 2019, 14, e0218837. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-I.; Yoe, S.M. A novel cecropin-like peptide from black soldier fly, Hermetia illucens: Isolation, structural and functional characterization. Entomol. Res. 2017, 47, 115–124. [Google Scholar] [CrossRef]
- Gould, I.M.; Bal, A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence 2013, 4, 185–191. [Google Scholar] [CrossRef]
- Wright, G.D. Something old, something new: Revisiting natural products in Antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Netshiheni, R.K. Selected Edible Insects and Their Products in Traditional Medicine, Food and Pharmaceutical Industries in Africa: Utilisation and Prospects. In Future Foods; InTech: London, UK, 2017. [Google Scholar]
- Yang, H. Nonvesicular sterol transport: Two protein families and a sterol sensor? Trends Cell Biol. 2006, 16, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Roy, S. Edible Insects: Future of Human Food - A Review. Int. Lett. Nat. Sci. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Flores, D.R.; Casados, L.E.; Velasco, S.F.; Ramírez, A.C.; Velázquez, G. Comparative study of composition, antioxidant and antimicrobial activity of two adult edible insects from Tenebrionidae family. BMC Chem. 2020, 14, 55. [Google Scholar] [CrossRef]
- Mutungi, C.; Irungu, F.G.; Nduko, J.; Mutua, F.; Affognon, H.; Nakimbugwe, D.; Ekesi, S.; Fiaboe, K.K.M. Postharvest processes of edible insects in Africa: A review of processing methods, and the implications for nutrition, safety and new products development. Crit. Rev. Food Sci. Nutr. 2019, 59, 276–298. [Google Scholar] [CrossRef]
- Klunder, H.C.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.J.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Amadi, E.N.; Kiin-Kabari, D.B. Nutritional Composition and Microbiology of Some Edible Insects Commonly Eaten in Africa, Hurdles and Future Prospects: A Critical Review. J. Food Microbiol. Saf. Hyg. 2016, 1, 107. [Google Scholar] [CrossRef]
- Tanga, C.M.; Waweru, J.W.; Tola, Y.H.; Onyoni, A.A.; Khamis, F.M.; Ekesi, S.; Paredes, J.C. Organic Waste Substrates Induce Important Shifts in Gut Microbiota of Black Soldier Fly (Hermetia illucens L.): Coexistence of Conserved, Variable, and Potential Pathogenic Microbes. Front. Microbiol. 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H.; Yu, X.Q. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 2004, 198, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.; Hultmark, D. Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol. 1999, 29, 965–972. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Ali, A.M.; Rauf, A. Insects as producers of antimicrobial polypeptides: A short review. GSC Biol. Pharm. Sci. 2020, 2020, 102–107. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Reichhart, J.M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Rees, J.A.; Moniatte, M.; Bulet, P. Novel antibacterial peptides isolated from a European bumblebee, Bombus pascuorum (Hymenoptera, apoidea). Insect Biochem. Mol. Biol. 1997, 27, 413–422. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Cytryńska, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Wiesner, J.; Twyman, R.M.; Zuchner, T.; Sadd, B.M.; Regoes, R.R.; Schmid-Hempel, P.; et al. Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteria. Proc. R. Soc. B 2015, 282, 20150293. [Google Scholar] [CrossRef] [PubMed]
- Pöppel, A.K.; Vogel, H.; Wiesner, J.; Vilcinskas, A. Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob. Agents Chemother. 2015, 59, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Warnecke, D.C.; François, I.E.J.A.; Leipelt, M.; Heinz, E.; Ott, C.; Zähringer, U.; Thomma, B.P.H.J.; Ferket, K.K.A.; Cammue, B.P.A. Defensins from Insects and Plants Interact with Fungal Glucosylceramides. J. Biol. Chem. 2004, 279, 3900–3905. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Boccazzi, I.V.; Ottoboni, M.; Martin, E.; Comandatore, F.; Vallone, L.; Spranghers, T.; Eeckhout, M.; Mereghetti, V.; Pinotti, L.; Epis, S. A survey of the mycobiota associated with larvae of the black soldier fly (Hermetia illucens) reared for feed production. PLoS ONE 2017, 12, e0182533. [Google Scholar] [CrossRef]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch. Sect. C J. Biosci. 2017, 72, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Jiang, M. Evaluation of antibacterial activity of hexanedioic acid isolated from Hermetia illucens larvae. J. Appl. Biomed. 2014, 12, 179–189. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed]
- Akbari, R.; Hakemi Vala, M.; Hashemi, A.; Aghazadeh, H.; Sabatier, J.M.; Bagheri, K.P. Action mechanism of melittin-derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef]
- Park, S.-I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Choi, W.-H.; Yun, J.-H.; Chu, J.-P.; Chu, K.-B. Antibacterial effect of extracts of Hermetia illucens (Diptera: Stratiomyidae) larvae against Gram-negative bacteria. Entomol. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, Expression, Purification and Functional Characterization of Novel Antimicrobial Peptide Genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef]
- Vasconcellos, A.; Moura, F.M.D.S. Wood litter consumption by three species of Nasutitermes termites in an area of the Atlantic Coastal Forest in northeastern Brazil. J. Insect Sci. 2010, 10, 1–9. [Google Scholar] [CrossRef]
- Schäfer, A.; Konrad, R.; Kuhnigk, T.; Kämpfer, P.; Hertel, H.; König, H. Hemicellulose-degrading bacteria and yeasts from the termite gut. J. Appl. Bacteriol. 1996, 80, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, D.; Vijayarani, K.; Kumanan, K. 6S rRNA typing of cellulolytic bacteria from the termite Odontotermes formosanus. J. Vet. Anim. Sci. Res. 2014, 43, 359–368. [Google Scholar]
- Wenzel, M.; Schonig, I.; Berchtold, M.; Kampfer, P.; Konig, H. Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. J. Appl. Microbiol. 2002, 92, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Butera, G.; Ferraro, C.; Alonzo, G.; Colazza, S.; Quatrini, P. The gut microbiota of the wood-feeding termite Reticulitermes lucifugus (Isoptera; Rhinotermitidae). Ann. Microbiol. 2016, 66, 253–260. [Google Scholar] [CrossRef]
- Borji, M.; Rahimi, S.; Ghorbani, G.R.; Vandyousefi, J.; Fazaeli, H. Isolation and identification of some bacteria from termites gut capable in degrading straw lignin and polysaccharides. J. Vet. Res. 2003, 58, 249–256. [Google Scholar]
- Fröhlich, J.; Koustiane, C.; Kämpfer, P.; Rosselló-Mora, R.; Valens, M.; Berchtold, M.; Kuhnigk, T.; Hertel, H.; Maheshwari, D.K.; König, H. Occurrence of rhizobia in the gut of the higher termite Nasutitermes nigriceps. Syst. Appl. Microbiol. 2007, 30, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Harazono, K.; Yamashita, N.; Shinzato, N.; Watanabe, Y.; Fukatsu, T.; Kurane, R. Isolation and Characterization of Aromatics-degrading Microorganisms from the Gut of the Lower Termite Coptotermes formosanus. Biosci. Biotechnol. Biochem. 2003, 67, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Van Dexter, S.; Boopathy, R. Biodegradation of phenol by Acinetobacter tandoii isolated from the gut of the termite. Environ. Sci. Pollut. Res. 2019, 26, 34067–34072. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Isolation and Characterization of Novel Lignolytic, Cellulolytic, and Hemicellulolytic Bacteria from Wood-Feeding Termite Cryptotermes brevis. Int. Microbiol. 2019, 22, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Konig, H. Bacillus species in the intestine of termites and other soil invertebrates. J. Appl. Microbiol. 2006, 101, 620–627. [Google Scholar] [CrossRef]
- Breznak, J.A. Intestinal Microbiota of Termites and other Xylophagous Insects. Annu. Rev. Microbiol. 1982, 36, 323. [Google Scholar] [CrossRef]
- Eutick, M.L.; O’Brien, R.W.; Slaytor, M. Bacteria from the Gut of Australian Termites. Appl. Environ. Microbiol. 1978, 35, 823–828. [Google Scholar] [CrossRef]
- Adams, L.; Boopathy, R. Isolation and characterization of enteric bacteria from the hindgut of Formosan termite. Bioresour. Technol. 2005, 96, 1592–1598. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Xu, X.; Shao, M.; Yin, C.; Mao, Z.; Shi, J.; Yu, X.; Wang, Y.; Sun, F.; Zhang, Y. Diversity, Bacterial Symbionts, and Antimicrobial Potential of Termite-Associated Fungi. Front. Microbiol. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-F.; Wu, J.; Li, S.; Li, Q.; Jin, L.-P.; Yin, C.-P.; Zhang, Y.-L. Antibacterial Potential of Termite-Associated Streptomyces sp. ACS Omega 2021, 6, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ramadhar, T.R.; Beemelmanns, C.; Cao, S.; Poulsen, M.; Currie, C.R.; Clardy, J. Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci. 2014, 5, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Lee, D.; Benndorf, R.; Jung, W.H.; Beemelmanns, C.; Kang, K.S.; Kim, K.H. Termisoflavones A–C, Isoflavonoid Glycosides from Termite-Associated Streptomyces sp. RB1. J. Nat. Prod. 2016, 79, 3072–3078. [Google Scholar] [CrossRef]
- Wyche, T.P.; Ruzzini, A.C.; Beemelmanns, C.; Kim, K.H.; Klassen, J.L.; Cao, S.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; Clardy, J. Linear Peptides Are the Major Products of a Biosynthetic Pathway That Encodes for Cyclic Depsipeptides. Org. Lett. 2017, 19, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Benndorf, R.; Guo, H.; Sommerwerk, E.; Weigel, C.; Garcia-Altares, M.; Martin, K.; Hu, H.; Küfner, M.; de Beer, Z.W.; Poulsen, M.; et al. Natural products from actinobacteria associated with fungus-growing termites. Antibiotics 2018, 7, 83. [Google Scholar] [CrossRef]
- Beemelmanns, C.; Ramadhar, T.R.; Kim, K.H.; Klassen, J.L.; Cao, S.; Wyche, T.P.; Hou, Y.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; et al. Macrotermycins A-D, Glycosylated Macrolactams from a Termite-Associated Amycolatopsis sp. M39. Org. Lett. 2017, 19, 1000–1003. [Google Scholar] [CrossRef]
- Guo, H.; Benndorf, R.; Leichnitz, D.; Klassen, J.L.; Vollmers, J.; Görls, H.; Steinacker, M.; Weigel, C.; Dahse, H.M.; Kaster, A.K.; et al. Isolation, Biosynthesis and Chemical Modifications of Rubterolones A–F: Rare Tropolone Alkaloids from Actinomadura sp. 5-2. Chem. A Eur. J. 2017, 23, 9338–9345. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, H.; Ball, R.G.; Zink, D.L.; Smith, J.L.; Goetz, M.A.; Jenkins, R.G.; Nallin-Omstead, M.; Silverman, K.C.; Bills, G.F.; Lingham, R.B.; et al. Barceloneic acid A, a new farnesyl-protein transferase inhibitor from a Phoma species. J. Nat. Prod. 1995, 58, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Supong, K.; Thawai, C.; Choowong, W.; Kittiwongwattana, C.; Thanaboripat, D.; Laosinwattana, C.; Koohakan, P.; Parinthawong, N.; Pittayakhajonwut, P. Antimicrobial compounds from endophytic Streptomyces sp. BCC72023 isolated from rice (Oryza sativa L.). Res. Microbiol. 2016, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Klassen, J.L.; Lee, S.R.; Poulsen, M.; Beemelmanns, C.; Kim, K.H. Efomycins K and L from a termite-associated Streptomyces sp. M56 and their putative biosynthetic origin. Front. Microbiol. 2019, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Von Bonin, A.; Buchmann, B.; Bader, B.; Rausch, A.; Venstrom, K.; Schäfer, M.; Gründemann, S.; Günther, J.; Zorn, L.; Nubbemeyer, R.; et al. Efomycine M: An inhibitor of selectins? Nat. Med. 2006, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Frobel, K.; Miller, H.; Bischoff, E.; Salcher, O.; de Jong, A.; Berschauer, F.; Scheer, M. Efomycin G and it’s use as yield Promoter in Animals. U.S. Patent 49,27,810, 22 May 1990. [Google Scholar]
- Wu, C.; Tan, Y.; Gan, M.; Wang, Y.; Guan, Y.; Hu, X.; Zhou, H.; Shang, X.; You, X.; Yang, Z.; et al. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using PCR-based screening. J. Nat. Prod. 2013, 76, 2153–2157. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Sørensen, D.; Ho, L.; Ziko, M.; Bueler, S.A.; Lu, S.; Tao, J.; Moser, A.; Lee, R.; Agard, D.; et al. Activity-Independent Discovery of Secondary Metabolites Using Chemical Elicitation and Cheminformatic Inference. ACS Chem. Biol. 2015, 10, 2616–2623. [Google Scholar] [CrossRef]
- Kretschmer, A.; Dorgerloh, M.; Deeg, M.; Hagenmaier, H. The structures of novel insecticidal macrolides: Bafilomycins D and E, and oxohygrolidin. Agric. Biol. Chem. 1985, 49, 2509–2511. [Google Scholar] [CrossRef]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.P.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 10666–10671. [Google Scholar] [CrossRef]
- Koba, M.; Konopa, J. Actinomycin D and its mechanisms of action. Postep. Hig. Med. Dosw. 2005, 59, 290–298. [Google Scholar]
- Guo, H.; Benndorf, R.; König, S.; Leichnitz, D.; Weigel, C.; Peschel, G.; Berthel, P.; Kaiser, M.; Steinbeck, C.; Werz, O.; et al. Expanding the Rubterolone Family: Intrinsic Reactivity and Directed Diversification of PKS-derived Pyrans. Chem. A Eur. J. 2018, 24, 11319–11324. [Google Scholar] [CrossRef] [PubMed]
- Carr, G.; Poulsen, M.; Klassen, J.L.; Hou, Y.; Wyche, T.P.; Bugni, T.S.; Currie, C.R.; Clardy, J. Microtermolides A and B from termite-associated Streptomyces sp. and structural revision of vinylamycin. Org. Lett. 2012, 14, 2822–2825. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kreuzenbeck, N.B.; Otani, S.; Garcia-Altares, M.; Dahse, H.M.; Weigel, C.; Aanen, D.K.; Hertweck, C.; Poulsen, M.; Beemelmanns, C. Pseudoxylallemycins A–F, Cyclic Tetrapeptides with Rare Allenyl Modifications Isolated from Pseudoxylaria sp. X802: A Competitor of Fungus-Growing Termite Cultivars. Org. Lett. 2016, 18, 3338–3341. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, D.; Yu, J.S.; Benndorf, R.; Lee, S.; Lee, D.-S.; Huh, J.; de Beer, Z.W.; Kim, Y.H.; Beemelmanns, C.; et al. Natalenamides A–C, Cyclic Tripeptides from the Termite-Associated Actinomadura sp. RB99. Molecules 2018, 23, 3003. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, J.A.V.; Oh, D.C.; Cao, S.; Currie, C.R.; Kolter, R.; Clardy, J. Common biosynthetic origins for polycyclic tetramate macrolactams from phylogenetically diverse bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.J.; Oh, D.C.; Yuceer, M.C.; Klepzig, K.D.; Clardy, J.; Currie, C.R. Bacterial protection of beetle-fungus mutualism. Science 2008, 322, 63. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Iizuka, T.; Ogura, K.; Maeda, M.; Tanaka, T. Isolation of some microorganisms associated with five species of Ambrosia beetles and two kinds of antibiotics produced by Xv-3 strain in these isolates. J. Fac. Agric. Hokkaido Univ. 1982, 61, 60–72. [Google Scholar]
- Kellner, R.L.L.; Dettner, K. Allocation of pederin during lifetime of Paederus rove beetles (Coleoptera: Staphylinidae): Evidence for polymorphism of hemolymph toxin. J. Chem. Ecol. 1995, 21, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ko, H.; Bang, H.S.; Park, S.H.; Kim, D.G.; Kwon, H.C.; Kim, S.Y.; Shin, J.; Oh, D.C. Coprismycins A and B, neuroprotective phenylpyridines from the dung beetle-associated bacterium, Streptomyces sp. Bioorganic Med. Chem. Lett. 2011, 21, 5715–5718. [Google Scholar] [CrossRef]
- Park, S.H.; Moon, K.; Bang, H.S.; Kim, S.H.; Kim, D.G.; Oh, K.B.; Shin, J.; Oh, D.C. Tripartilactam, a cyclobutane-bearing tricyclic lactam from a Streptomyces sp. in a dung beetle’s brood ball. Org. Lett. 2012, 14, 1258–1261. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwon, S.H.; Park, S.H.; Lee, J.K.; Bang, H.S.; Nam, S.J.; Kwon, H.C.; Shin, J.; Oh, D.C. Tripartin, a histone demethylase inhibitor from a bacterium associated with a dung beetle larva. Org. Lett. 2013, 15, 1834–1837. [Google Scholar] [CrossRef]
- Um, S.; Park, S.H.; Kim, J.; Park, H.J.; Ko, K.; Bang, H.S.; Lee, S.K.; Shin, J.; Oh, D.C. Coprisamides A and B, new branched cyclic peptides from a gut bacterium of the dung beetle Copris tripartitus. Org. Lett. 2015, 17, 1272–1275. [Google Scholar] [CrossRef]
- Um, S.; Bach, D.H.; Shin, B.; Ahn, C.H.; Kim, S.H.; Bang, H.S.; Oh, K.B.; Lee, S.K.; Shin, J.; Oh, D.C. Naphthoquinone-Oxindole Alkaloids, Coprisidins A and B, from a Gut-Associated Bacterium in the Dung Beetle, Copris tripartitus. Org. Lett. 2016, 18, 5792–5795. [Google Scholar] [CrossRef] [PubMed]
- An, J.S.; Hong, S.H.; Somers, E.; Lee, J.; Kim, B.Y.; Woo, D.; Kim, S.W.; Hong, H.J.; Jo, S.-I.; Shin, J.; et al. Lenzimycins A and B, Metabolites With Antibacterial Properties From Brevibacillus sp. Associated With the Dung Beetle Onthophagus lenzii. Front. Microbiol. 2020, 11, 599911. [Google Scholar] [CrossRef]
- Lu, J.; Sun, Q.; Tu, Z.C.; Lv, Q.; Shui, P.X.; Cheng, Y.X. Identification of N-acetyldopamine dimers from the dung beetle Catharsius molossus and their COX-1 and COX-2 inhibitory activities. Molecules 2015, 20, 15589–15596. [Google Scholar] [CrossRef]
- Mori, N.; Yoshinaga, N.; Sawada, Y.; Fukui, M.; Shimoda, M.; Fujisaki, K.; Nishida, R.; Kuwahara, Y. Identification of Volicitin-related Compounds from the Regurgitant of Lepidopteran Caterpillars. Biosci. Biotechnol. Biochem. 2003, 67, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Theodore, C.M.; King, J.B.; You, J.; Cichewicz, R.H. Production of cytotoxic glidobactins/luminmycins by Photorhabdus asymbiotica in liquid media and live crickets. J. Nat. Prod. 2012, 75, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Widdick, D.A.; Dodd, H.M.; Barraille, P.; White, J.; Stein, T.H.; Chater, K.F.; Gasson, M.J.; Bibb, M.J. Cloning and engineering of the cinnamycin biosynthetic gene cluster from Streptomyces cinnamoneus cinnamoneus DSM 40005. Proc. Natl. Acad. Sci. USA 2003, 100, 4316–4321. [Google Scholar] [CrossRef] [PubMed]
- Ökesli, A.; Cooper, L.E.; Fogle, E.J.; Van Der Donk, W.A. Nine post-translational modifications during the biosynthesis of cinnamycin. J. Am. Chem. Soc. 2011, 133, 13753–13760. [Google Scholar] [CrossRef]
- Repka, L.M.; Chekan, J.R.; Nair, S.K.; Van Der Donk, W.A. Mechanistic Understanding of Lanthipeptide Biosynthetic Enzymes. Chem. Rev. 2017, 117, 5457–5520. [Google Scholar] [CrossRef]
- Zhang, N.; Suh, S.O.; Blackwell, M. Microorganisms in the gut of beetles: Evidence from molecular cloning. J. Invertebr. Pathol. 2003, 84, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.E.; Wolfe, M.L. Response of understory vegetation to variable tree mortality following a mountain pine beetle epidemic in lodgepole pine stands in northern Utah. Vegetatio 1996, 122, 1–12. [Google Scholar] [CrossRef]
- Beemelmanns, C.; Guo, H.; Rischer, M.; Poulsen, M. Natural products from microbes associated with insects. Beilstein J. Org. Chem. 2016, 12, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Mohammed, W.S.; Shagimardanova, E.I.; Vankov, P.Y.; Gogoleva, N.E.; Ziganshin, A.M. Fungal, bacterial, and archaeal diversity in the digestive tract of several beetle larvae (coleoptera). Biomed Res. Int. 2018, 2018, 6765438. [Google Scholar] [CrossRef] [PubMed]
- Lavy, O.; Gophna, U.; Gefen, E.; Ayali, A. Locust Bacterial Symbionts: An Update. Insects 2020, 11, 655. [Google Scholar] [CrossRef]
- Dillon, R.; Charnley, K. Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Res. Microbiol. 2002, 153, 503–509. [Google Scholar] [CrossRef]
- Payne, D.W.; Davidson, L.M. Cellulose digestion in the locust, Schistocerra gregaria. J. Entomol. Ser. A Gen. Entomol. 2009, 48, 213–215. [Google Scholar] [CrossRef]
- Stevenson, J.P. The normal bacterial flora of the alimentary canal of laboratory stocks of the desert locust, Schistocerca gregaria. J. Invertebr. Pathol. 1966, 8, 205–211. [Google Scholar] [CrossRef]
- Cheseto, X.; Kuate, S.P.; Tchouassi, D.P.; Ndung’u, M.; Teal, P.E.A.; Torto, B. Potential of the Desert Locust Schistocerca gregaria (Orthoptera: Acrididae) as an Unconventional Source of Dietary and Therapeutic Sterols. PLoS ONE 2015, 10, e0127171. [Google Scholar] [CrossRef]
- Yen, A.L. Conservation of Lepidoptera used as human food and medicine. Curr. Opin. Insect Sci. 2015, 12, 102–108. [Google Scholar] [CrossRef]
- Ngute, A.S.K.; Dongmo, M.A.K.; Effa, J.A.M.; Ambombo Onguene, E.M.; Fomekong Lontchi, J.; Cuni-Sanchez, A. Edible caterpillars in central Cameroon: Host plants, value, harvesting, and availability. For. Trees Livelihoods 2020, 29, 16–33. [Google Scholar] [CrossRef]
- Hammer, T.J.; Janzen, D.H.; Hallwachs, W.; Jaffe, S.P.; Fierer, N. Caterpillars lack a resident gut microbiome. Proc. Natl. Acad. Sci. USA 2017, 114, 9641–9646. [Google Scholar] [CrossRef]
- Sawada, Y.; Yoshinaga, N.; Fujisaki, K.; Nishida, R.; Kuwahara, Y.; Mori, N. Absolute Configuration of Volicitin from the Regurgitant of Lepidopteran Caterpillars and Biological Activity of Volicitin-Related Compounds. Biosci. Biotechnol. Biochem. 2014, 70, 2185–2190. [Google Scholar] [CrossRef]
- Pemberton, R.W. Insects and other arthropods used as drugs in Korean traditional medicine. J. Ethnopharmacol. 1999, 65, 207–216. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Alves, H.N. The faunal drugstore: Animal-based remedies used in traditional medicines in Latin America. J. Ethnobiol. Ethnomed. 2011, 7, 1–43. [Google Scholar] [CrossRef]
- Ulrich, R.G.; Buthala, D.A.; Klug, M.J. Microbiota Associated with the Gastrointestinal Tract of the Common House Cricket, Acheta domestica. Appl. Environ. Microbiol. 1981, 41, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Goodrich-Blair, H.; Clarke, D.J. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: Two roads to the same destination. Mol. Microbiol. 2007, 64, 260–268. [Google Scholar] [CrossRef]
- Amatuni, A.; Renata, H. Identification of a lysine 4-hydroxylase from the glidobactin biosynthesis and evaluation of its biocatalytic potential. Org. Biomol. Chem. 2019, 17, 1736–1739. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).