Biosynthesis and Heterologous Expression of Cacaoidin, the First Member of the Lanthidin Family of RiPPs
Abstract
:1. Introduction
2. Results
2.1. Sequencing of Streptomyces Cacaoi CA-170360 Genome and Identification of Cacaoidin BGC
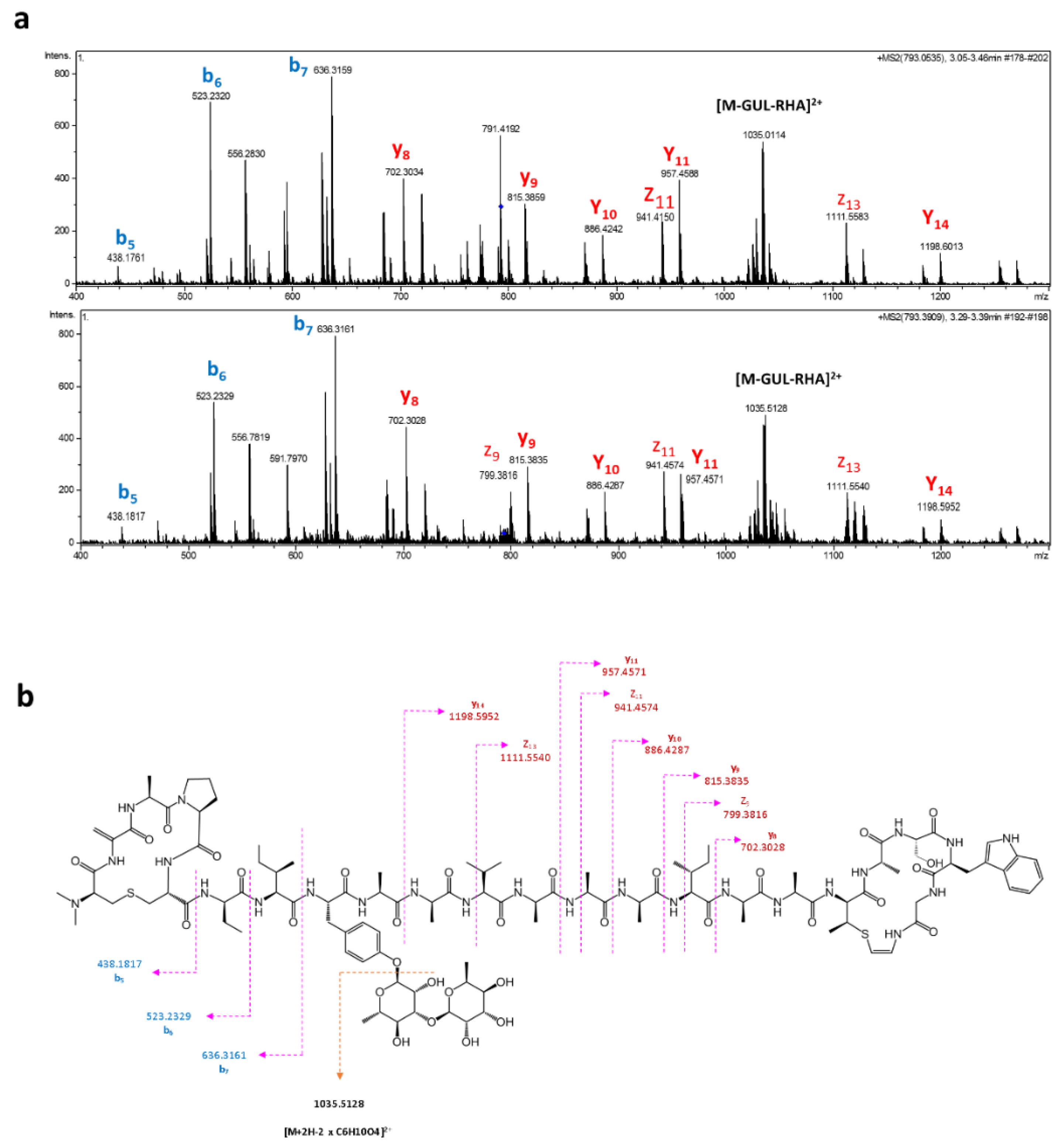
2.2. Cloning and Heterologous Expression of Cacaoidin BGC
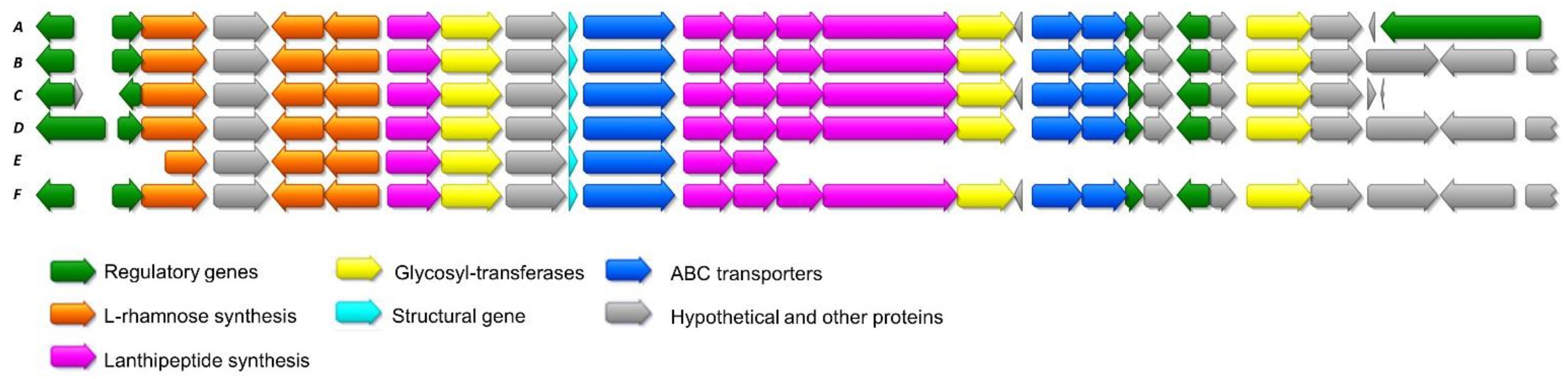
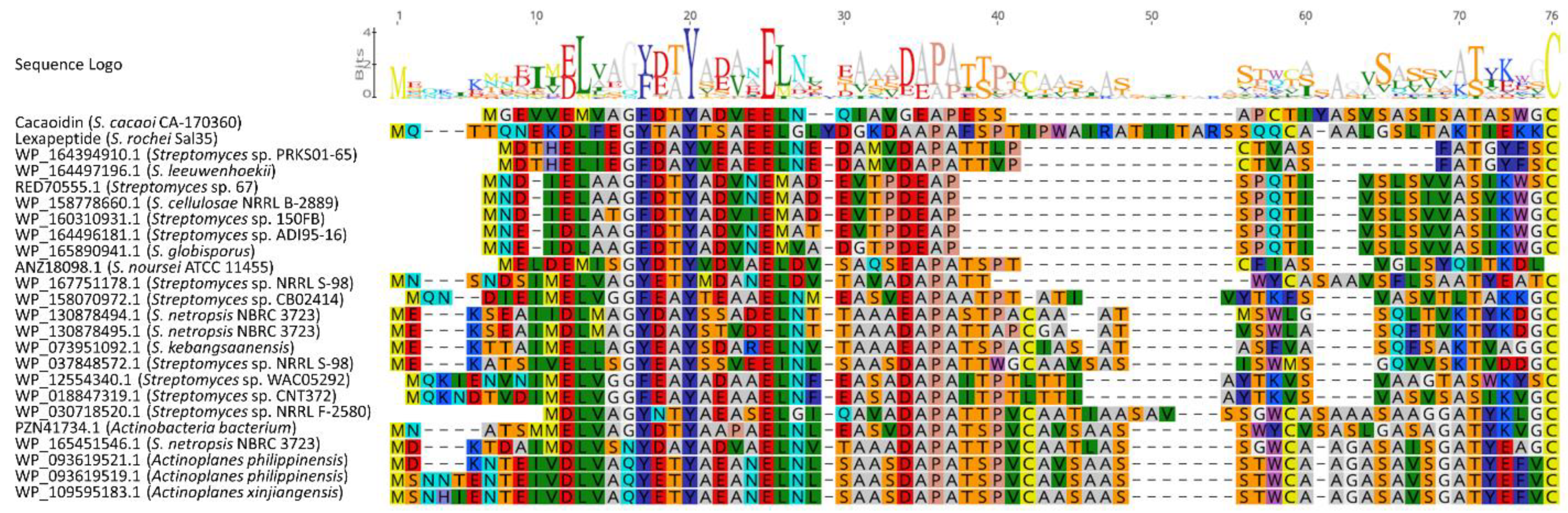
2.3. Additional Lanthidin Clusters in Public Databases
3. Materials and Methods
3.1. Strains and Plasmids
3.2. DNA Extraction and Genome Mining
3.3. Identification of Cacaoidin BGC
3.4. Cas-9 Assisted Targeting of CHromosome (CATCH) Cloning of Cacaoidin BGC
3.5. Heterologous Expression of Cacaoidin BGC
3.6. Extraction and Detection of Cacaoidin
3.7. Extraction and Detection of Cacaoidin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genilloud, O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Escribano, J.P.; Alt, S.; Bibb, M. Next generation sequencing of actinobacteria for the discovery of novel natural products. Mar. Drugs. 2016, 14, 78. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; van der Hooft, J.J.J.; van Santen, J.A.; Tracanna, V.; Suarez Duran, H.G.; Pascal Andreu, V.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, 454–458. [Google Scholar] [CrossRef] [Green Version]
- van Santen, J.A.; Kautsar, S.A.; Medema, M.H.; Linington, R.G. Microbial natural products databases: Moving forward in the multi-omics era. Nat. Prod. Rep. 2021, 38, 264–278. [Google Scholar] [CrossRef]
- Genilloud, O. Mining actinomycetes for novel antibiotics in the omics era: Are we ready to exploit this new paradigm? Antibiotics 2018, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Kloosterman, A.M.; Cimermancic, P.; Elsayed, S.S.; Du, C.; Hadjithomas, M.; Donia, M.S.; Fischbach, M.A.; van Wezel, G.P.; Medema, M.H. Expansion of RiPP biosynthetic space through integration of pan-genomics and machine learning uncovers a novel class of lanthipeptides. PLoS Biol. 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-López, F.J.; Carretero-Molina, D.; Sánchez-Hidalgo, M.; Martín, J.; González, I.; Román-Hurtado, F.; de la Cruz, M.; García-Fernández, S.; Reyes, F.; Deisinger, J.P.; et al. Cacaoidin, first member of the new lanthidin RiPP family. Angew. Chem. Int. Ed. 2020, 59, 12654–12658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.; Velásquez, J.E.; van der Donk, W.A. Evolution of lanthipeptide synthetases. Proc. Natl. Acad. Sci. USA 2012, 109, 18361–18366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Zhang, Q. Linaridin natural products. Nat. Prod. Rep. 2020, 37, 1152–1163. [Google Scholar] [CrossRef]
- Repka, L.M.; Chekan, J.R.; Nair, S.K.; van der Donk, W.A. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem. Rev. 2017, 117, 5457–5520. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Zhai, Y.; Ehrner, E.; Rath, C.M.; Wang, X.; Tabudravu, J.; Ebel, R.; Bibb, M.; Kyeremeh, K.; Dorrestein, P.C.; et al. Legonaridin, a new member of linaridin RiPP from a Ghanaian Streptomyces isolate. Org. Biomol. Chem. 2015, 13, 9585–9592. [Google Scholar] [CrossRef] [Green Version]
- Sit, C.S.; Yoganathan, S.; Vederas, J.C. Biosynthesis of aminovinyl-cysteine-containing peptides and its application in the production of potential drug candidates. Acc. Chem. Res. 2011, 44, 261–268. [Google Scholar] [CrossRef]
- Claesen, J.; Bibb, M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 16297–16302. [Google Scholar] [CrossRef] [Green Version]
- Mo, T.; Liu, W.-Q.; Ji, W.; Zhao, J.; Chen, T.; Ding, W.; Yu, S.; Zhang, Q. Biosynthetic insights into linaridin natural products from genome mining and precursor peptide mutagenesis. ACS Chem. Biol. 2017, 12, 1484–1488. [Google Scholar] [CrossRef]
- Xu, M.; Cheng, Z.; Bashiri, G.; Wang, J.; Hong, J.; Wang, Y.; Xu, L.; Chen, X.; Huang, S.-X.; Lin, S.; et al. Functional genome mining reveals a novel class V lanthipeptide containing a D-amino acid introduced by an F420H2-dependent reductase. Angew. Chem. Int. Ed. 2020, 59, 18029–18035. [Google Scholar] [CrossRef]
- Montalbán-López, M.; Scott, T.A.; Ramesh, S.; Rahman, I.R.; van Heel, A.J.; Viel, J.H.; Bandarian, V.; Dittmann, E.; Genilloud, O.; Goto, Y.; et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 2020, 38, 130–239. [Google Scholar] [CrossRef]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, 278–281. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Merwin, N.J.; Johnston, C.W.; Magarvey, N.A. PRISM 3: Expanded prediction of natural product chemical structures from microbial genomes. Nucleic Acids Res. 2017, 45, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.; Mo, T.; Mandalapu, D.; Zhang, Q. Substrate specificity of the cypemycin decarboxylase CypD. Synth. Syst. Biotechnol. 2018, 3, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Shin, I.; Rhee, S. Crystal structure of the effector protein HopA1 from Pseudomonas syringae. J. Struct. Biol. 2015, 189, 276–280. [Google Scholar] [CrossRef]
- Acedo, J.Z.; Bothwell, I.R.; An, L.; Trouth, A.; Frazier, C.; van der Donk, W.A. O-methyltransferase-mediated incorporation of a β-amino acid in lanthipeptides. J. Am. Chem. Soc. 2019, 141, 16790–16801. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Zhao, X.; Acedo, J.Z.; Estrada, P.; Nair, S.K.; van der Donk, W.A. Characterization of a dehydratase and methyltransferase in the biosynthesis of a ribosomally-synthesized and post-translationally modified peptide in Lachnospiraceae. ChemBioChem 2019, 21, 190–199. [Google Scholar] [CrossRef]
- Yang, X.; van der Donk, W.A. Post-translational introduction of D-alanine into ribosomally synthesized peptides by the dehydroalanine reductase NpnJ. J. Am. Chem. Soc. 2015, 137, 12426–12429. [Google Scholar] [CrossRef] [PubMed]
- Lohans, C.T.; Li, J.L.; Vederas, J.C. Structure and biosynthesis of carnolysin, a homologue of enterococcal cytolysin with D-amino acids. J. Am. Chem. Soc. 2014, 136, 13150–13153. [Google Scholar] [CrossRef]
- Huo, L.; van der Donk, W.A. Discovery and characterization of bicereucin, an unusual D-amino acid-containing mixed two-component lantibiotic. J. Am. Chem. Soc. 2016, 138, 5254–5257. [Google Scholar] [CrossRef]
- Girauld, M.F.; Naismith, J.H. The rhamnose pathway. Curr. Opin. Struct. Biol. 2000, 19, 687–696. [Google Scholar] [CrossRef]
- Galm, U.; Wendt-Pienkowski, E.; Wang, L.; Huang, S.-X.; Unsin, C.; Tao, M.; Coughlin, J.M.; Shen, B. Comparative analysis of the biosynthetic gene clusters and pathways for three structurally related antitumor antibiotics: Bleomycin, tallosomycin and zorbamycin. J. Nat. Prod. 2011, 74, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1999. [Google Scholar]
- He, H.; Williamson, R.T.; Shen, B.; Graziani, E.I.; Yang, H.Y.; Sakya, S.M.; Petersen, P.J.; Carter, G.T. Mannopeptimycins, novel antibacterial glycopeptides from Streptomyces hygroscopicus, LL-AC98. J. Am. Chem. Soc. 2002, 124, 9729–9736. [Google Scholar] [CrossRef]
- Brockhausen, I.; Ho, B.; Liu, B.; Lau, K.; Szarek, W.A.; Wang, L.; Feng, L. Characterization of two β-1,3-glucosyltransferases from Escherichia coli serotypes O56 and O152. J. Bacteriol. 2008, 190, 4922–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradel, E.; Parker, C.T.; Schnaitman, C.A. Structures of the rfaB, rfaI, rfaJ, and rfaS genes of Escherichia coli K-12 and their roles in assembly of the lipopolysaccharide core. J. Bacteriol. 1992, 174, 4736–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo, R.G.; Olano, C.; Fernández, R.; Braña, A.F.; Méndez, C.; de la Calle, F.; Salas, J.A. Elucidation of the glycosylation steps during biosynthesis of antitumor macrolides PM100117 and PM100118 and engineering for novel derivatives. Microb. Cell Fact. 2016, 15, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, S.M.; Weidenbach, S.; Rohr, J. Two cooperative glycosyltransferases are responsible for the sugar diversity of saquayamycins isolated from Streptomyces sp. KY 40-1. ACS Chem. Biol. 2017, 12, 2529–2534. [Google Scholar] [CrossRef] [Green Version]
- Malmierca, M.G.; Pérez-Victoria, I.; Martín, J.; Reyes, F.; Méndez, C.; Olano, C.; Salas, J.A. Cooperative involvement of glycosyltransferases in the transfer of amino sugars during the biosynthesis of the macrolactam sipanmycin by Streptomyces sp. strain CS149. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Kaunietis, A.; Buivydas, A.; Citavicius, D.J.; Kuipers, O.P. Heterologous biosynthesis and characterization of a glycocin from a thermophilic bacterium. Nat. Commun. 2019, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Xu, B.; Li, Y.; Liu, B.; Wang, H. Substrate tolerance of the biosynthetic enzymes of glycosylated lanthipeptide NAI-112. Org. Biomol. Chem. 2020, 18, 6095–6099. [Google Scholar] [CrossRef] [PubMed]
- Plat, A.; Kluskens, L.D.; Kuipers, A.; Rink, R.; Moll, G.N. Requirements of the engineered leader peptide of nisin for inducing modification, export, and cleavage. Appl. Environ. Microbiol. 2011, 77, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knerr, P.J.; van der Donk, W.A. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.; Sasso, O.; Maffioli, S.I.; Bertorelli, R.; Monciardini, P.; Sosio, M.; Bonezzi, F.; Summa, M.; Brunati, C.; Bordoni, R.; et al. A glycosylated, labionin-containing lanthipeptide with marked antinociceptive activity. ACS Chem. Biol. 2014, 9, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Xu, B.; Chen, E.; Wang, J.; Lu, J.; Donadio, S.; Ge, H.; Wang, H. Zn-dependent bifunctional proteases are responsible for leader peptide processing of class III lanthipeptides. Proc. Natl. Acad. Sci. USA 2019, 116, 2533–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk, J.M.; Völler, G.-H.; Krawczyk, B.; Kretz, J.; Brönstrup, M.; Süssmuth, R.D. Heterologous expression and engineering studies of labyrinthopeptins, class III lantibiotics from Actinomadura namibiensis. Chem. Biol. 2013, 20, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Geiger, C.; Korn, S.M.; Häsler, M.; Peetz, O.; Martin, J.; Kötter, P.; Morgner, N.; Entian, K.-D. LanI-mediated lantibiotic immunity in Bacillus subtilis: Functional analysis. Appl. Environ. Microbiol. 2019, 85, e00534-19. [Google Scholar] [CrossRef] [Green Version]
- Méndez, C.; Salas, J.A. The role of ABC transporters in antibiotics-producing organisms: Drug secretion and resistance mechanisms. Res. Microbiol. 2001, 152, 341–350. [Google Scholar] [CrossRef]
- Wood, H.E.; Devine, K.M.; McConnell, D.J. Characterisation of a repressor gene (xre) and a temperature-sensitive allele from the Bacillus subtilis prophage, PBSX. Gene 1990, 96, 83–88. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Nodwell, J.R. The TetR family of regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xie, J. Role and regulation of bacterial LuxR-like regulators. J. Cell Biochem. 2011, 112, 2694–2702. [Google Scholar] [CrossRef]
- Li, Y.; Kong, L.; Shen, J.; Wang, Q.; Liu, Q.; Yang, W.; Deng, Z.; You, D. Characterization of the positive SARP family regulator PieR for improving piericidin A1 production in Streptomyces piomogeues var. Hangzhouwanensis. Synth. Syst. Biotechnol. 2019, 4, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Lackner, P.; Breiteneder, H. The Bet v1 fold: An ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol. Biol. 2008, 8, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, L.M.; Koonin, E.V.; Aravind, L. Adaptations of the helix-grip fold for ligand binding and catalysis in the START domain superfamily. Proteins 2001, 43, 134–144. [Google Scholar] [CrossRef]
- Ames, B.D.; Korman, T.P.; Zhang, W.; Smith, P.; Vu, T.; Tang, Y.; Tsai, S.-C. Crystal structure and functional analysis of tetracenomycin ARO/CYC: Implications for cyclization specificity of aromatic polyketides. Proc. Natl. Acad. Sci. USA 2008, 105, 5349–5354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, J.; Brostromer, E.; Liu, X.Y.; Kristensen, O.; Su, X.D. Bioinformatics and structural characterization of a hypothetical protein from Streptococcus mutans: Implication of antibiotic resistance. PLoS ONE 2009, 4, e7245. [Google Scholar] [CrossRef]
- Burkhart, B.J.; Hudson, G.A.; Dunbar, K.L.; Mitchell, D.A. A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat. Chem. Biol. 2015, 11, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Klinman, J.P.; Bonnot, F. Intrigues and intricacies of the biosynthetic pathways for the enzymatic quinocofactors: PQQ, TTQ, CTQ, TPQ, and LTQ. Chem. Rev. 2014, 114, 4343–4365. [Google Scholar] [CrossRef] [Green Version]
- Latham, J.A.; Iavarone, A.T.; Barr, I.; Juthani, P.V.; Klinman, J.P. PqqD is a novel peptide chaperone that forms a ternary complex with the radical S-adenosylmethionine protein PqqE in the pyrroloquinoline quinone biosynthetic pathway. J. Biol. Chem. 2015, 290, 12908–12918. [Google Scholar] [CrossRef] [Green Version]
- Chater, K.F.; Wilde, L.C. Streptomyces albus G mutants defective in the SalGI restriction-modification system. J. Gen. Microbiol. 1980, 116, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Zhao, X.; Gabrieli, T.; Lou, G.; Ebenstein, Y.; Zhu, T.F. Cas9-assisted targeting of chromosome segments CATCH enables one-step targeted cloning of large gene clusters. Nat. Commun. 2015, 6, 8101. [Google Scholar] [CrossRef]
- Yamanaka, K.; Reynolds, K.A.; Kersten, R.D.; Ryan, K.S.; Gonzalez, D.J.; Nizet, V.; Dorrestein, P.C.; Moore, B.S. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. USA 2014, 111, 1957–1962. [Google Scholar] [CrossRef] [Green Version]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2002; Volume 291. [Google Scholar]
- Bilyk, B.; Luzhetskyy, A. Unusual site-specific DNA integration into the highly active pseudo-attB of the Streptomyces albus J1074 genome. Appl. Microbiol. Biotechnol. 2014, 98, 5095–5104. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [PubMed] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.M.; Thibessard, A.; Lorenzi, J.-N.; Benhadj, M.; Hôtel, L.; Gacemi-Kirane, D.; Lespinet, O.; Leblond, P.; Aigle, B. Comparative genomics among closely related Streptomyces strains revealed specialized metabolite biosynthetic gene cluster diversity. Antibiotics 2018, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Seipke, R.F. Strain-level diversity of secondary metabolism in Streptomyces albus. PLoS ONE 2015, 10, e0116457. [Google Scholar] [CrossRef] [Green Version]
- Choudoir, M.J.; Pepe-Ranney, C.; Buckley, D.H. Diversification of secondary metabolite biosynthetic gene clusters coincides with lineage divergence in Streptomyces. Antibiotics 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.J.; Yamanaka, K.; Tang, X.; Moore, B.S. Direct cloning and heterologous expression of natural product biosynthetic gene clusters by transformation-associated recombination. Methods Enzymol. 2019, 621, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Agrawal, P.; Khater, S.; Gupta, M.; Sain, N.; Mohanty, D. RiPPMiner: A bioinformatics resource for deciphering chemical structures of RiPPs based on prediction of cleavage and cross-links. Nucleic Acids Res. 2017, 45, W80–W88. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Stephens, A.; Nam, S.-Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at its Core. J. Mol. Biol. 2018, 2836, 30587–30589. [Google Scholar] [CrossRef]
- Jian, W.; Zhu, T.F. Targeted isolation and cloning of 100-kb microbial genomic sequences by Cas9-assisted targeting of chromosome segments. Nat. Protoc. 2016, 11, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Robertsen, H.L.; Blin, K.; Weber, T.; Lee, S.Y. CRISPR-Cas9 Toolkit for Actinomycete Genome Editing. Methods Mol. Biol. 2018, 1671, 163–184. [Google Scholar] [CrossRef] [PubMed]
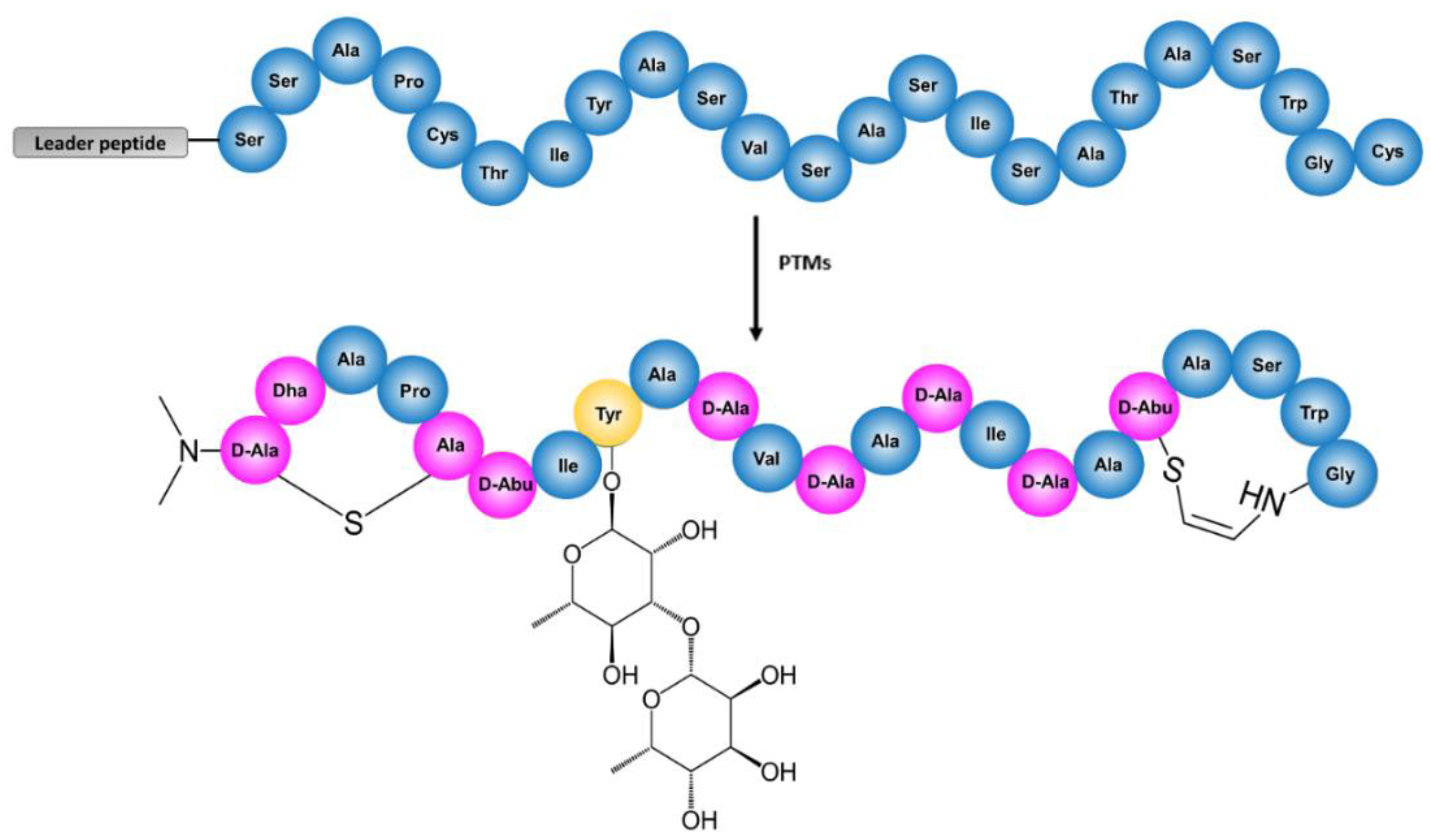
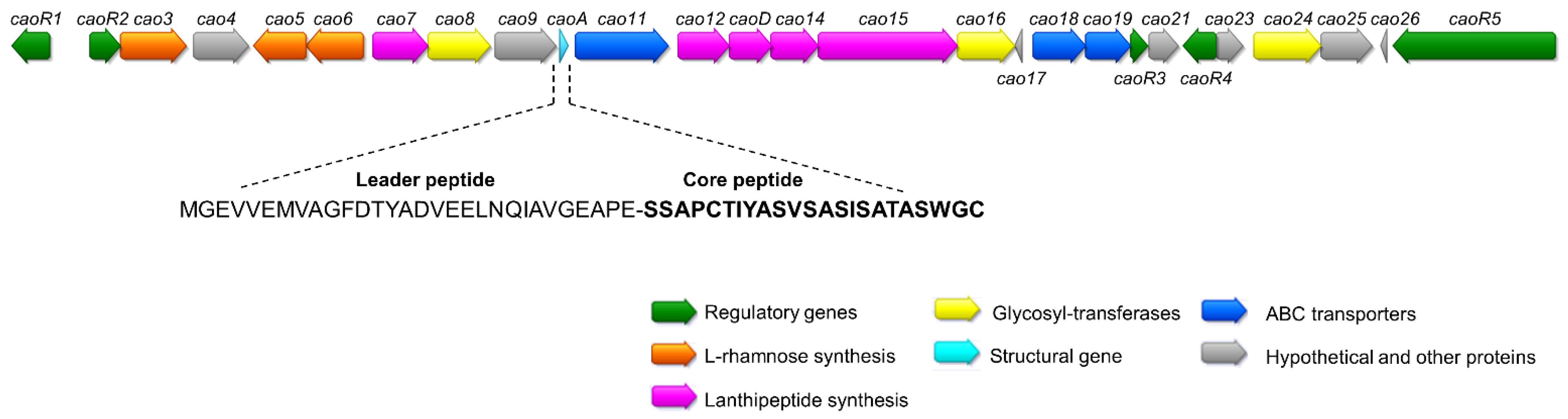


| ORF | Closest BLAST Homolog | %Identity/ Similarity | Conserved Domains | Possible Function |
|---|---|---|---|---|
| caoR1 | DNA-binding response regulator [Streptomyces cacaoi subsp. cacaoi] | 100/100 | CitB (NarL/FixJ family, contains REC and HTH domains); HTH_LUXR | Positive regulator |
| caoR2 | Helix-turn-helix domain-containing protein [Streptomyces cacaoi] | 98.44/100 | HTH_XRE superfamily | Negative regulator |
| cao3 | Hypothetical protein SCA03_05120 [Streptomyces cacaoi subsp. cacaoi] | 97.81/100 | RmlD_sub_bind | l-rhamnose synthesis |
| cao4 | Methyltransferase [Streptomyces cacaoi] | 100/100 | Methyltrans_2 (O-methyltransferase) | N-terminal dimethylation |
| cao5 | GDP-mannose 4,6-dehydratase [Streptomyces cacaoi] | 98.16/100 | dTDP_GD_SDR-e (dDTP-d-glucose 4,6-dehydratase) | l-rhamnose biosynthesis |
| cao6 | MULTISPECIES: glucose-1-phosphate thymidylyltransferase [Streptomyces] | 100/100 | RmlA_long (glucose-1-phosphate thymidylyltransferase) | l-rhamnose biosynthesis |
| cao7 | Hypothetical protein [Streptomyces cacaoi] | 100/100 | HopA1 superfamily | Unknown |
| cao8 | Glycosyltransferase family 2 protein [Streptomyces sp. NRRL S-1868] | 99.48/100 | Glycos_transf_2 | Glycosylation |
| cao9 | Phosphotransferase [Streptomyces sp. NRRL S-1868] | 100/100 | PKc_like (protein kinase catalytic domain) | Unknown |
| caoA | Hypothetical protein SCA03_05190 [Streptomyces cacaoi subsp. cacaoi] | 100/100 | No putative conserved domains detected | Structural gene |
| cao11 | ABC transporter ATP-binding protein [Streptomyces sp. NRRL F-5053] | 99.66/100 | MdlB (ABC-type multidrug transport system, ATPase and permease component) | Cacaoidin biosynthesis |
| cao12 | MULTISPECIES: LLM class flavin-dependent oxidoreductase [Streptomyces] | 100/100 | SsuD (Flavin-dependent oxidoreductase) | Cacaoidin biosynthesis |
| caoD | Hypothetical protein SCA03_05220 [Streptomyces cacaoi subsp. cacaoi] | 99.63/100 | PRK05579 (bifunctional phosphopantothenoylcysteine decarboxylase/phosphopantothenate synthase) | AviMeCys biosynthesis |
| cao14 | MULTISPECIES: hypothetical protein [unclassified Streptomyces] | 99.34/100 | No putative conserved domains detected | Unknown |
| cao15 | Hypothetical protein [Streptomyces sp. NHF165] | 99.42/100 | PqqL (predicted Zn-dependent peptidase) | Leader peptide cleavage |
| cao16 | Glycosyltransferase family 2 protein [Streptomyces cacaoi] | 100/100 | Glycos_transf_2 | Glycosylation |
| cao17 | Hypothetical protein [Streptomyces cacaoi] | 91.7/100 | No putative conserved domains detected | Unknown |
| cao18 | ABC transporter ATP-binding protein [Streptomyces sp. NRRL F-5053] | 100/100 | CcmA (ABC-type multidrug transport system) | Cacaoidin biosynthesis |
| cao19 | MULTISPECIES: ABC transporter permease [Streptomyces] | 99.65/100 | ABC2_membrane_3 (ABC-2 family transporter protein) | Cacaoidin biosynthesis |
| caoR3 | Hypothetical protein SCA03_05280 [Streptomyces cacaoi subsp. cacaoi] | 100/100 | HTH_XRE domain | Negative regulator |
| cao21 | Hypothetical protein [Streptomyces sp. NRRL S-1868] | 100/100 | No putative conserved domains detected | Unknown |
| caoR4 | TetR/AcrR family transcriptional regulator [Streptomyces cacaoi] | 100/100 | AcrR (DNA-binding transcriptional regulator) | Negative regulator |
| cao23 | Hypothetical protein SCA03_05310 [Streptomyces cacaoi subsp. cacaoi] | 99.39/100 | SRPBCC superfamily | Unknown |
| cao24 | MULTISPECIES: glycosyltransferase family 4 protein [Streptomyces] | 100/100 | GT4_AmsD-like | Glycosylation |
| cao25 | Hypothetical protein [Streptomyces cacaoi] | 99.06/100 | No putative conserved domains detected | Unknown |
| cao26 | No homologues found | - | - | Unknown |
| caoR5 | Tetratricopeptide repeat protein [Streptomyces sp. NRRL S-1868] | 99.31/100 | BTAD (Bacterial Transcriptional Activation) | Positive regulator |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román-Hurtado, F.; Sánchez-Hidalgo, M.; Martín, J.; Ortiz-López, F.J.; Genilloud, O. Biosynthesis and Heterologous Expression of Cacaoidin, the First Member of the Lanthidin Family of RiPPs. Antibiotics 2021, 10, 403. https://doi.org/10.3390/antibiotics10040403
Román-Hurtado F, Sánchez-Hidalgo M, Martín J, Ortiz-López FJ, Genilloud O. Biosynthesis and Heterologous Expression of Cacaoidin, the First Member of the Lanthidin Family of RiPPs. Antibiotics. 2021; 10(4):403. https://doi.org/10.3390/antibiotics10040403
Chicago/Turabian StyleRomán-Hurtado, Fernando, Marina Sánchez-Hidalgo, Jesús Martín, Francisco Javier Ortiz-López, and Olga Genilloud. 2021. "Biosynthesis and Heterologous Expression of Cacaoidin, the First Member of the Lanthidin Family of RiPPs" Antibiotics 10, no. 4: 403. https://doi.org/10.3390/antibiotics10040403
APA StyleRomán-Hurtado, F., Sánchez-Hidalgo, M., Martín, J., Ortiz-López, F. J., & Genilloud, O. (2021). Biosynthesis and Heterologous Expression of Cacaoidin, the First Member of the Lanthidin Family of RiPPs. Antibiotics, 10(4), 403. https://doi.org/10.3390/antibiotics10040403






