Targeting Biofilm of MDR Providencia stuartii by Phages Using a Catheter Model
Abstract
1. Introduction
2. Results
2.1. Phage Isolation and Assessment of Host-Range Coverage
2.2. Genome Analysis
2.3. Characterization of Phage Lytic Activity
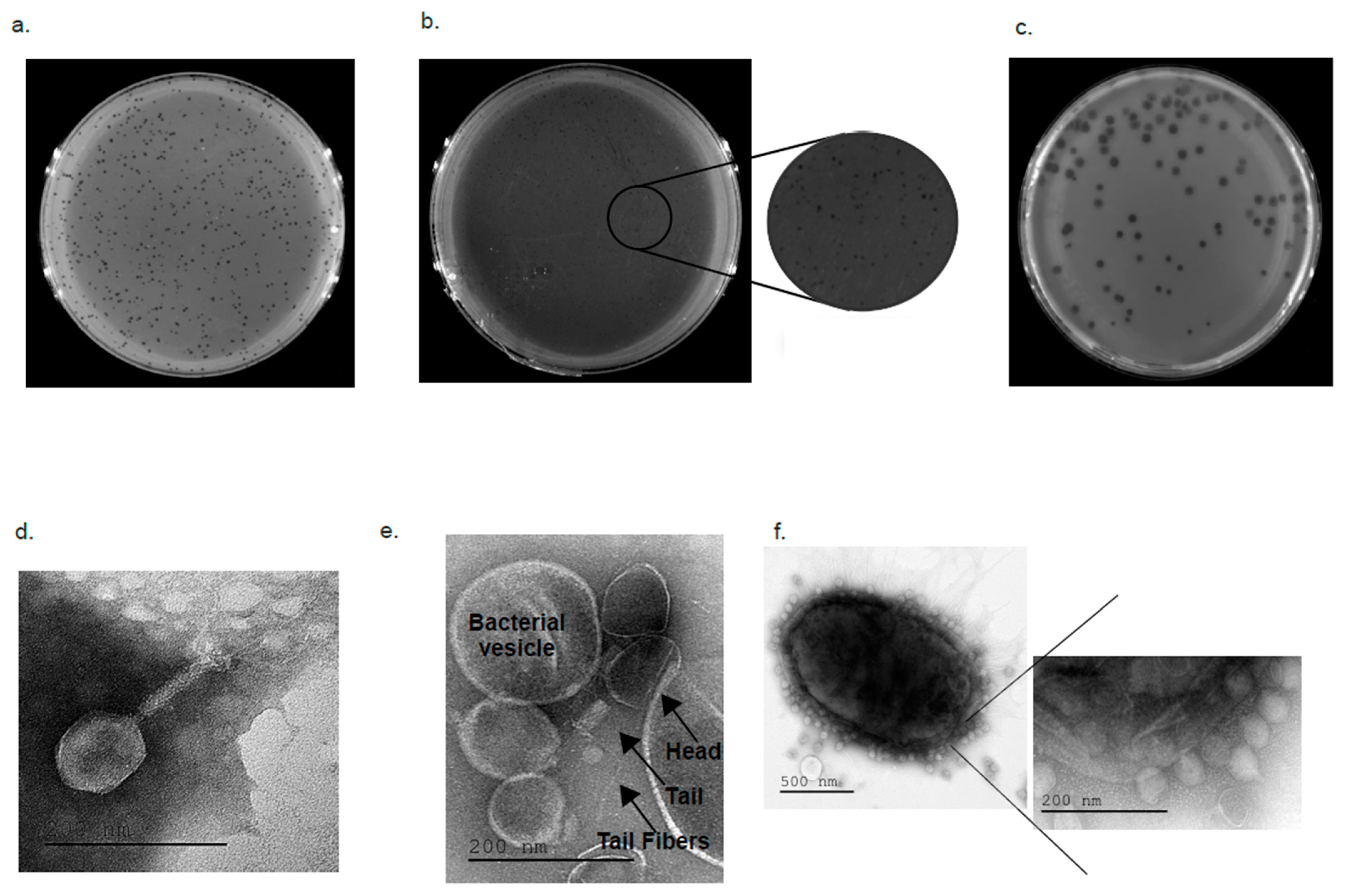
2.4. Electron Microscopy Visualizing
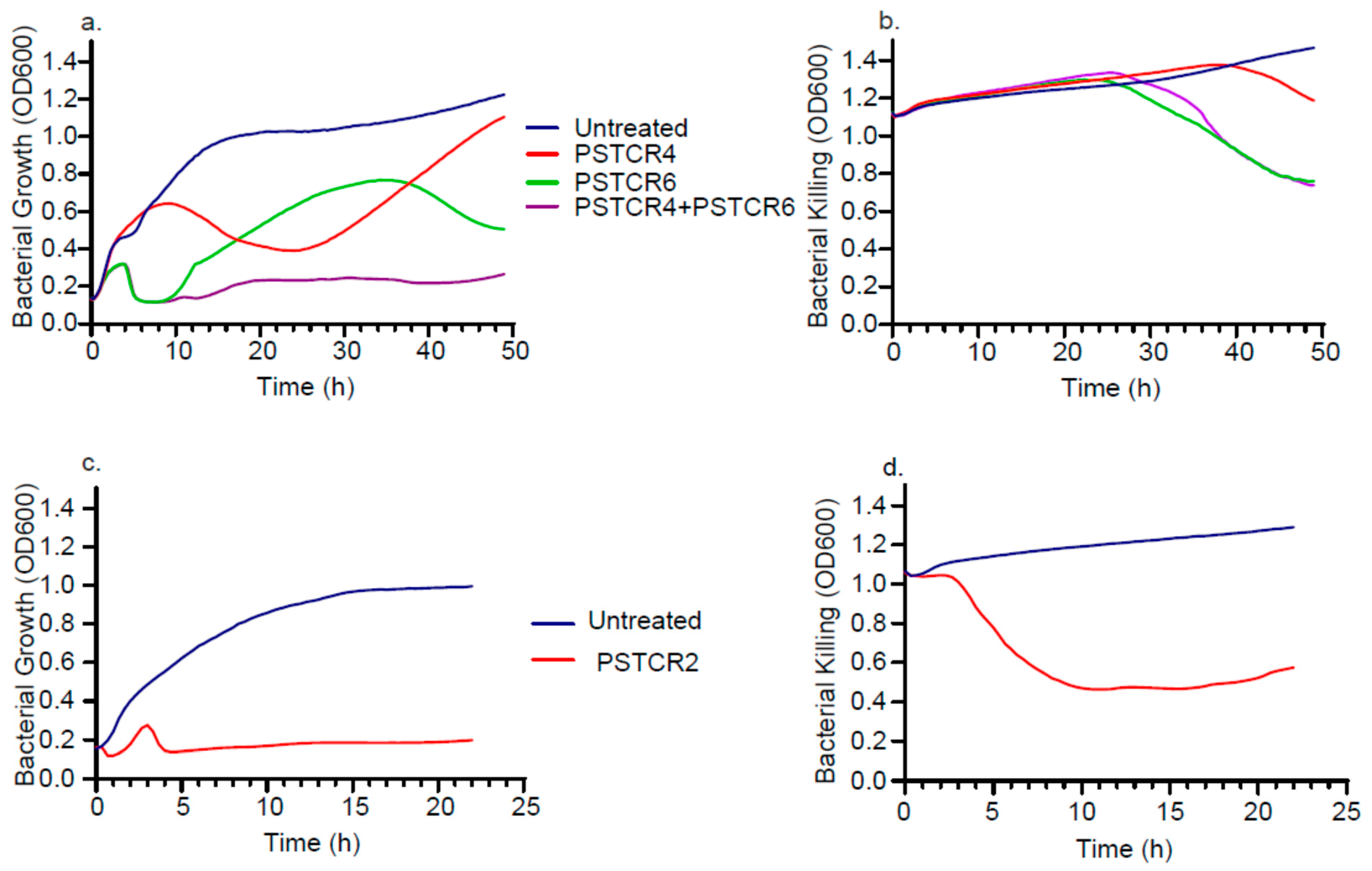
2.5. Kinetic of Lytic Activities in Liquid Culture
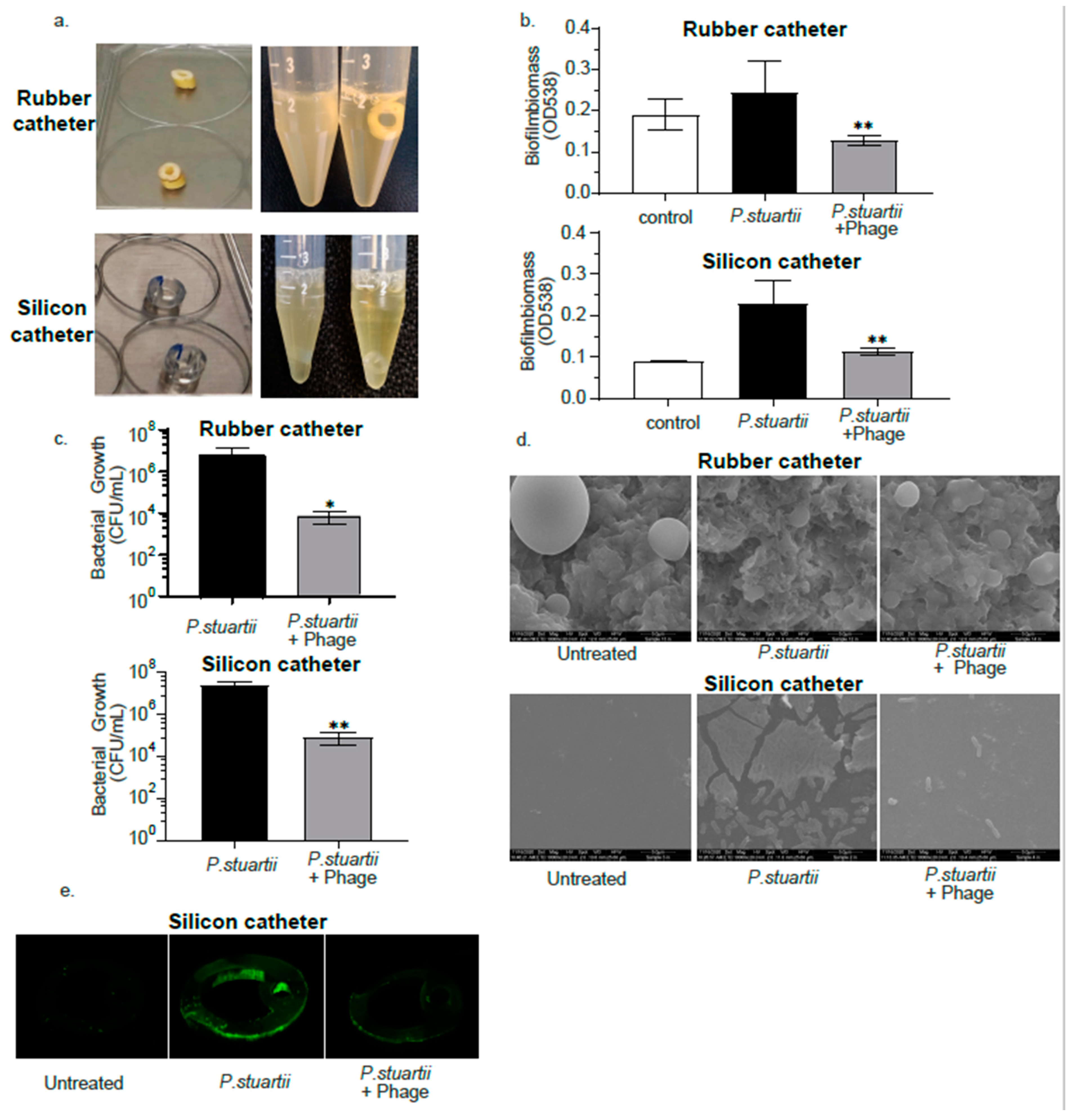
2.6. Biofilm Degradation Activity
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Phage Isolation and Propagation
4.3. Phgae Genome Sequencing
4.4. Bacteriophages for Phylogenetic Tree
4.5. TEM Visualization
4.6. Assessment of Phage Lytic Activity in Planktonic Cultures
4.7. Assessment of Phage Lytic Activity in Biofilm
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Hara, C.M.; Brenner, F.W.; Miller, J.M. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Washington, J.A.; Senjem, D.H.; Haldorson, A.; Schutt, A.H.; Martin, W.J. Nosocomially Acquired Bacteriuria Due to Proteus Rettgeri and Providencia Stuartii. Am. J. Clin. Pathol. 1973, 60, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.W. Providencia Stuartii: A Common Cause of Antibiotic-Resistant Bacteriuria in Patients with Long-Term Indwelling Catheters. Rev. Infect. Dis. 1986, 8, 61–67. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, Y.K.; Kim, H.Y.; Park, J.E.; Uh, Y. Clinical and microbiological features of providencia bacteremia: Experience at a tertiary care hospital. Korean J. Intern. Med. 2015, 30, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Molnár, S.; Flonta, M.M.M.; Almaş, A.; Buzea, M.; Licker, M.; Rus, M.; Földes, A.; Székely, E. Dissemination of NDM-1 carbapenemase-producer Providencia stuartii strains in Romanian hospitals: A multicentre study. J. Hosp. Infect. 2019, 103, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Marchaim, D.; Divine, G.W.; Pogue, J.M.; Kumar, S.; Lephart, P.; Risko, K.; Sobel, J.D.; Kaye, K.S. Growing prevalence of Providencia stuartii associated with the increased usage of colistin at a tertiary health care center. Int. J. Infect. Dis. 2012, 16, e646–e648. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Narasimhaswamy, N.; d’Souza, J. Providencia Rettgeri: An Emerging Nosocomial Uropathogen in an Indwelling Urinary Catheterised Patient. J. Clin. Diagn. Res. 2017, 11, DD01. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Carvalhaes, C.G.; da Silva, G.L.; Soares, S.P.T.; de Alcåntara, L.R.; Elias, L.S.; Sandri, A.M.; Gales, A.C. Outbreak of Carbapenem-Resistant Providencia stuartii in an Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2012, 33, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Rus, M.; Licker, M.; Musuroi, C.; Seclaman, E.; Muntean, D.; Cirlea, N.; Tamas, A.; Vulpie, S.; Horhat, F.G.; Baditoiu, L. Distribution of NDM1 carbapenemase-producing proteeae strains on high-risk hospital wards. Infect. Drug Resist. 2020, 13, 4751–4761. [Google Scholar] [CrossRef]
- El Khatib, M.; Tran, Q.T.; Nasrallah, C.; Lopes, J.; Bolla, J.M.; Vivaudou, M.; Pagès, J.M.; Colletier, J.P. Providencia stuartii form biofilms and floating communities of cells that display high resistance to environmental insults. PLoS ONE 2017, 12, e0174213. [Google Scholar] [CrossRef]
- Stickler, D.J. Bacterial biofilms in patients with indwelling urinary catheters. Nat. Clin. Pract. Urol. 2008, 5, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.; Oppenheimer, S.R.; Warren, J.W. Colonization of urinary catheters by Escherichia coli and providencia stuartii in a laboratory model system. J. Urol. 1994, 152, 232–236. [Google Scholar] [CrossRef]
- Fierer, J.; Ekstrom, M. An Outbreak of Providencia stuartii Urinary Tract Infections: Patients with Condom Catheters Are a Reservoir of the Bacteria. JAMA J. Am. Med. Assoc. 1981, 245, 1553–1555. [Google Scholar] [CrossRef]
- Tran, Q.T.; Mahendran, K.R.; Hajjar, E.; Ceccarelli, M.; Davin-Regli, A.; Winterhalter, M.; Weingart, H.; Pagès, J.M. Implication of porins in β-lactam resistance of Providencia stuartii. J. Biol. Chem. 2010, 285, 32273–32281. [Google Scholar] [CrossRef]
- Stock, I.; Wiedemann, B. Natural antibiotic susceptibility of providencia stuartii, P. rettgeri, P. alcalifaciens and P. rustigianii strains. J. Med. Microbiol. 1998, 47, 629–642. [Google Scholar] [CrossRef] [PubMed]
- McHale, P.J.; Keane, C.T.; Dougan, G. Antibiotic resistance in Providencia stuartii isolated in hospitals. J. Clin. Microbiol. 1981, 13, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Tshisevhe, V.S.; Lekalakala, M.R.; Tshuma, N.; Janse van Rensburg, S.; Mbelle, N. Outbreak of carbapenem-resistant Providencia rettgeri in a tertiary hospital. S. Afr. Med. J. 2016, 107, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Kurmasheva, N.; Vorobiev, V.; Sharipova, M.; Efremova, T.; Mardanova, A. The Potential Virulence Factors of Providencia stuartii: Motility, Adherence, and Invasion. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, G.W. Bacteriophages: An appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agents 2007, 30, 118–128. [Google Scholar] [CrossRef]
- Oliveira, H.; Pinto, G.; Hendrix, H.; Noben, J.P.; Gawor, J.; Kropinski, A.M.; Łobocka, M.; Lavigne, R.; Azeredo, J. A lytic Providencia rettgeri virus of potential therapeutic value is a deepbranching member of the T5virus genus. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Pinto, G.; Mendes, B.; Dias, O.; Hendrix, H.; Akturk, E.; Noben, J.P.; Gawor, J.; Łobocka, M.; Lavigne, R.; et al. A tailspike with exopolysaccharide depolymerase activity from a new Providencia stuartii phage makes multidrug-resistant bacteria susceptible to serum-mediated killing. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmy, O.; Khalifa, L.; Gold, N.; Rakov, C.; Alkalay-Oren, S.; Adler, K.; Ben-Porat, S.; Kraitman, R.; Gronovich, N.; Ginat, K.S.; et al. The israeli phage bank (IPB). Antibiotics 2020, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.J.; Kropinski, A.M. Bacteriophage: Methods and Protocols Volume 1: Isolation, Characterization, and Interactions; Springer: New York, NY, USA, 2009; ISBN 9781588296825. [Google Scholar]
- Clokie, M.R.J.; Kropinski, A.M. Bacteriophages Methods and Protocols Volume 2: Molecular and Applied Aspects; Springer: New York, NY, USA, 2009; ISBN 978-1-60327-564-4. [Google Scholar]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
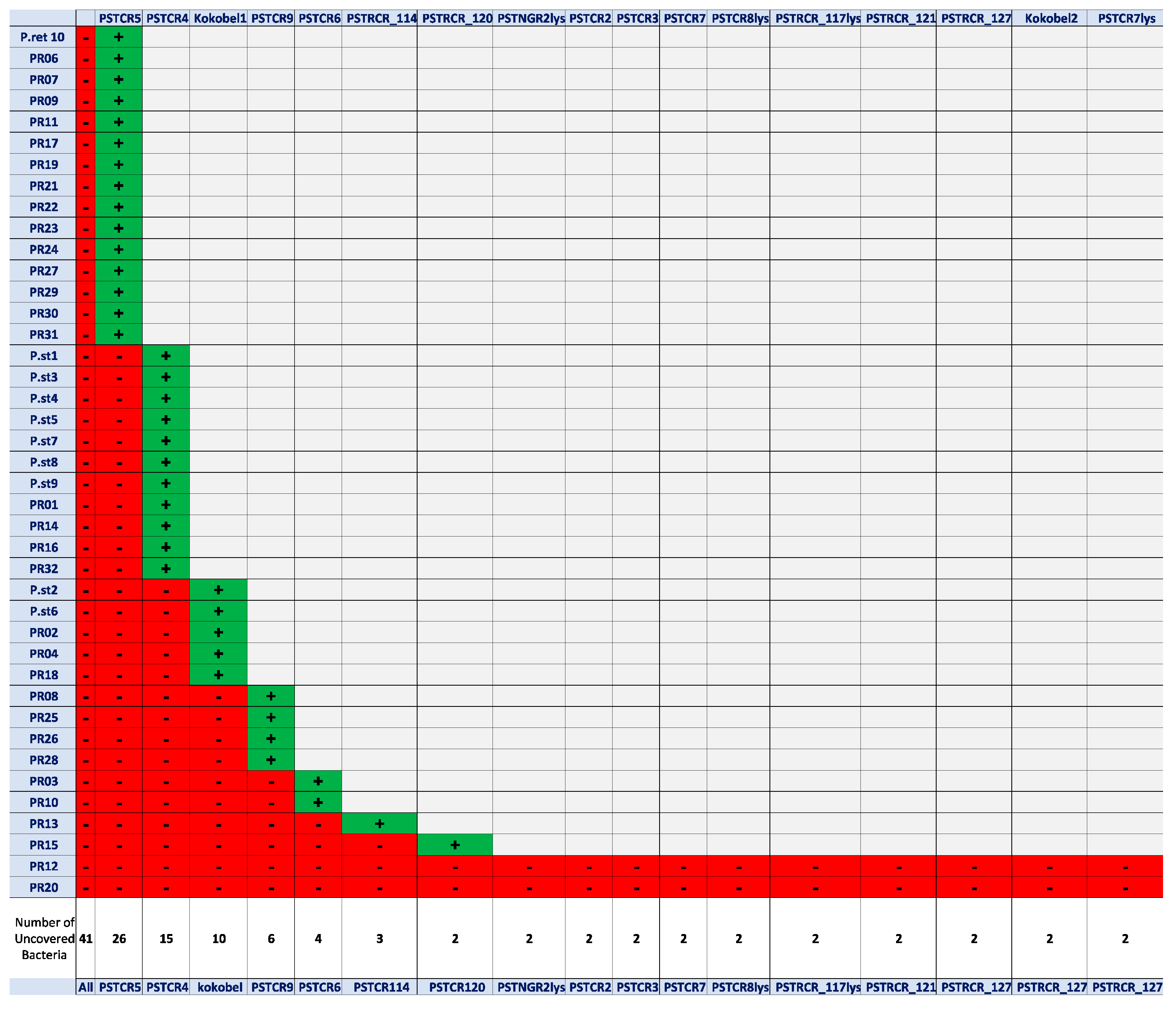

| Phage | Putative Life-Cycle Type 1 | Genome Organization 2 | Genome Size (bp) | Phylogeny | GenBank Accession |
|---|---|---|---|---|---|
| Kokobel1 | lytic | circular | 59,837 | Siphoridae | MW145139.1 |
| Kokobel2 | lytic | circular | 45,880 | Siphoridae | MW145138.1 |
| PSTNGR2lys | temperate | circular | 50,958 | Siphoridae | MW145137.1 |
| PSTCR2 | lytic | circular | 40,200 | Autographiviridae | MW057854.1 |
| PSTCR3 | temperate | circular | 39,447 | Myoviridae | MW057855.1 |
| PSTCR4 | lytic | circular | 57,214 | Siphoridae | MW057856.1 |
| PSTCR5 | lytic | circular | 109,434 | Siphoridae | MW057857.1 |
| PSTCR6 | lytic | circular | 155,737 | Myoviridae | MW057858.1 |
| PSTCR7 | lytic | circular | 57,986 | Siphoviridae | MW057861.1 |
| PSTCR7lys | temperate—partial genome | linear | 22,938 | Siphoridae | MW057862.1 |
| PSTCR8lys | temperate | linear | 40,280 | Siphoridae | MW057859.1 |
| PSTCR9 | lytic | circular | 58,873 | Siphoviridae | MW057860.1 |
| PSTRCR_114 | lytic | circular | 42,750 | Autographiviridae | MW358930.1 |
| PSTRCR_117lys | temperate | linear | 41,059 | Siphoviridae | MW358929.1 |
| PSTRCR_120 | lytic | circular | 39,396 | Autographiviridae; | MW358928.1 |
| PSTRCR_121 | lytic | circular | 165,776 | Myoviridae; | MW385300.1 |
| PSTRCR_127 | lytic | circular | 166,815 | Myoviridae; | MW358927.1 |
| Bacterial Strains | Source | Provided by 1 | Antibiotic Resistance 2 |
|---|---|---|---|
| Providencia stuartii | |||
| P.st 1 | blood | HMC | AMP, SAM, CRO, CXM, GEN, CFZ |
| P.st 2 | blood | HMC | AMP, SAM, CIP, CRO, CXM, GEN, CFZ |
| P.st 3 | blood | HMC | AMP, SAM, CIP, AMK, CRO, CXM, GEN, CFZ |
| P.st 4 | blood | HMC | AMP, SAM, CRO, CXM, GEN, CFZ |
| P.st 5 | blood | HMC | AMP, SAM, CIP, AMK, CRO, CXM, GEN, CFZ |
| P.st 6 | blood | HMC | AMP, SAM, CIP, AMK, CRO, CXM, GEN, CFZ |
| P.st 7 | blood | HMC | AMP, SAM, CIP, CXM, GEN, CFZ |
| P.st 8 | blood | HMC | AMP, SAM, CIP, AMK, CXM, CRO, GEN, CFZ |
| P.st 9 | blood | HMC | AMP, GEN |
| PR02 | urine | UCSD | AMP, SAM, CXM, GEN, CFZ |
| PR03 | urine | UCSD | AMP, SAM, CXM, GEN, CFZ |
| PR04 | urine | UCSD | AMP, SAM, AMK, CXM, GEN, CFZ |
| PR12 | leg lesion | UCSD | AMP, SAM, CIP, CFZ |
| PR13 | leg lesion | UCSD | AMP, SAM, CRO, CFZ |
| PR14 | urine | UCSD | AMP, SAM, CFZ |
| PR15 | blood | UCSD | AMP, SAM, CIP |
| PR16 | urine | UCSD | |
| PR25 | blood | UCSD | AMP, CXM, GEN |
| PR26 | blood | UCSD | AMP, CXM, GEN |
| PR32 | urine catheter | UCSD | AMP, GEN, CFZ |
| Providencia rettgeri | |||
| P. rett10 | blood | HMC | AMP, GEN |
| PR01 | breast | UCSD | AMP, SAM, GEN, CFZ |
| PR06 | blood | UCSD | SAM, CFZ |
| PR07 | urine | UCSD | AMP, SAM, CFZ |
| PR08 | sputum | UCSD | AMP, SAM, CFZ |
| PR09 | urine | UCSD | AMP, SAM, CRO, CFZ |
| PR10 | heel lesion | UCSD | AMP, SAM, CIP, CFZ |
| PR11 | lesion | UCSD | AMP, SAM, CFZ |
| PR17 | foot culture | UCSD | AMP, SAM |
| PR18 | urine | UCSD | AMP, SAM |
| PR19 | urine | UCSD | AMP, GEN |
| PR20 | body site, sacrum | UCSD | |
| PR21 | body tissue, foot | UCSD | AMP, GEN |
| PR22 | urine | UCSD | AMP, SAM, GEN |
| PR23 | urine | UCSD | AMP, GEN |
| PR24 | urine | UCSD | AMP, GEN |
| PR27 | body site, bladder stone | UCSD | AMP, CXM, GEN |
| PR28 | blood | UCSD | AMP, CXM, GEN |
| PR29 | urine | UCSD | AMP, CXM, GEN |
| PR30 | sputum | UCSD | AMP, GEN |
| PR31 | nasopharyngeal | UCSD | AMP, GEN |
| Type | Phage Name | Accession Number |
|---|---|---|
| Providencia phage | Redjac | NC_018832.1 |
| Providencia phage (P. stuartii) | vB PstP PS3 | NC_048148.1 |
| Providencia phage (P. stuartii) | vB_PstP_Stuart | MK387869.1 |
| Providencia phage (P. rettgeri) | vB_PreS_PR1 | NC_041913.1 |
| Providencia phage (P. rettgeri) | vB_PreS-PatoteraRojo | MT675126.1 |
| Providencia phage (P. rettgeri) | vB_PreS-Stilesk | MT675125.1 |
| Providencia phage (P. rettgeri) | vB_PreS-PibeRecoleta | MT675124.1 |
| Morganella phage | MmP1 | NC_011085.3 |
| Morganella phage | vB_MmoM_MP1 | NC_031020.1 |
| Proteus phage | pPM_01 | NC_028812.1 |
| Shigella phage | vB_SdyM_006 | MK295204.1 |
| Siphoviridae sp. isolate 404 | − | MN855780.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakov, C.; Ben Porat, S.; Alkalay-Oren, S.; Yerushalmy, O.; Abdalrhman, M.; Gronovich, N.; Huang, L.; Pride, D.; Coppenhagen-Glazer, S.; Nir-Paz, R.; et al. Targeting Biofilm of MDR Providencia stuartii by Phages Using a Catheter Model. Antibiotics 2021, 10, 375. https://doi.org/10.3390/antibiotics10040375
Rakov C, Ben Porat S, Alkalay-Oren S, Yerushalmy O, Abdalrhman M, Gronovich N, Huang L, Pride D, Coppenhagen-Glazer S, Nir-Paz R, et al. Targeting Biofilm of MDR Providencia stuartii by Phages Using a Catheter Model. Antibiotics. 2021; 10(4):375. https://doi.org/10.3390/antibiotics10040375
Chicago/Turabian StyleRakov, Chani, Shira Ben Porat, Sivan Alkalay-Oren, Ortal Yerushalmy, Mohanad Abdalrhman, Niv Gronovich, Lina Huang, David Pride, Shunit Coppenhagen-Glazer, Ran Nir-Paz, and et al. 2021. "Targeting Biofilm of MDR Providencia stuartii by Phages Using a Catheter Model" Antibiotics 10, no. 4: 375. https://doi.org/10.3390/antibiotics10040375
APA StyleRakov, C., Ben Porat, S., Alkalay-Oren, S., Yerushalmy, O., Abdalrhman, M., Gronovich, N., Huang, L., Pride, D., Coppenhagen-Glazer, S., Nir-Paz, R., & Hazan, R. (2021). Targeting Biofilm of MDR Providencia stuartii by Phages Using a Catheter Model. Antibiotics, 10(4), 375. https://doi.org/10.3390/antibiotics10040375






