Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice
Abstract
1. Introduction
2. Results
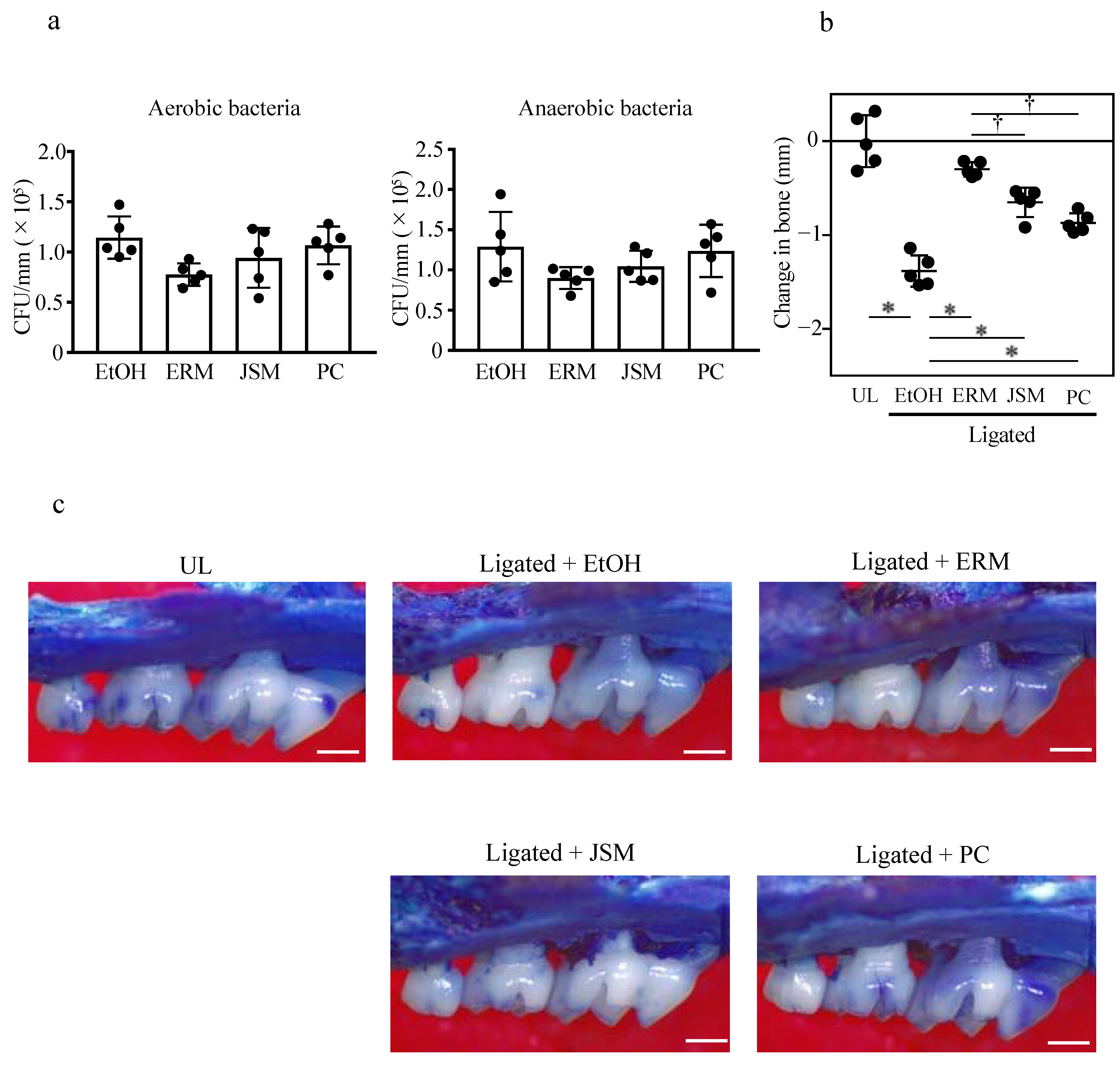
2.1. Oral Administration of Antibacterial Drugs Significantly Suppresses Periodontal Bone Loss Induced by Tooth Ligation
2.2. Intraperitoneal Injection of Erythromycin Significantly Suppresses Periodontal Bone Loss Induced by Tooth Ligation
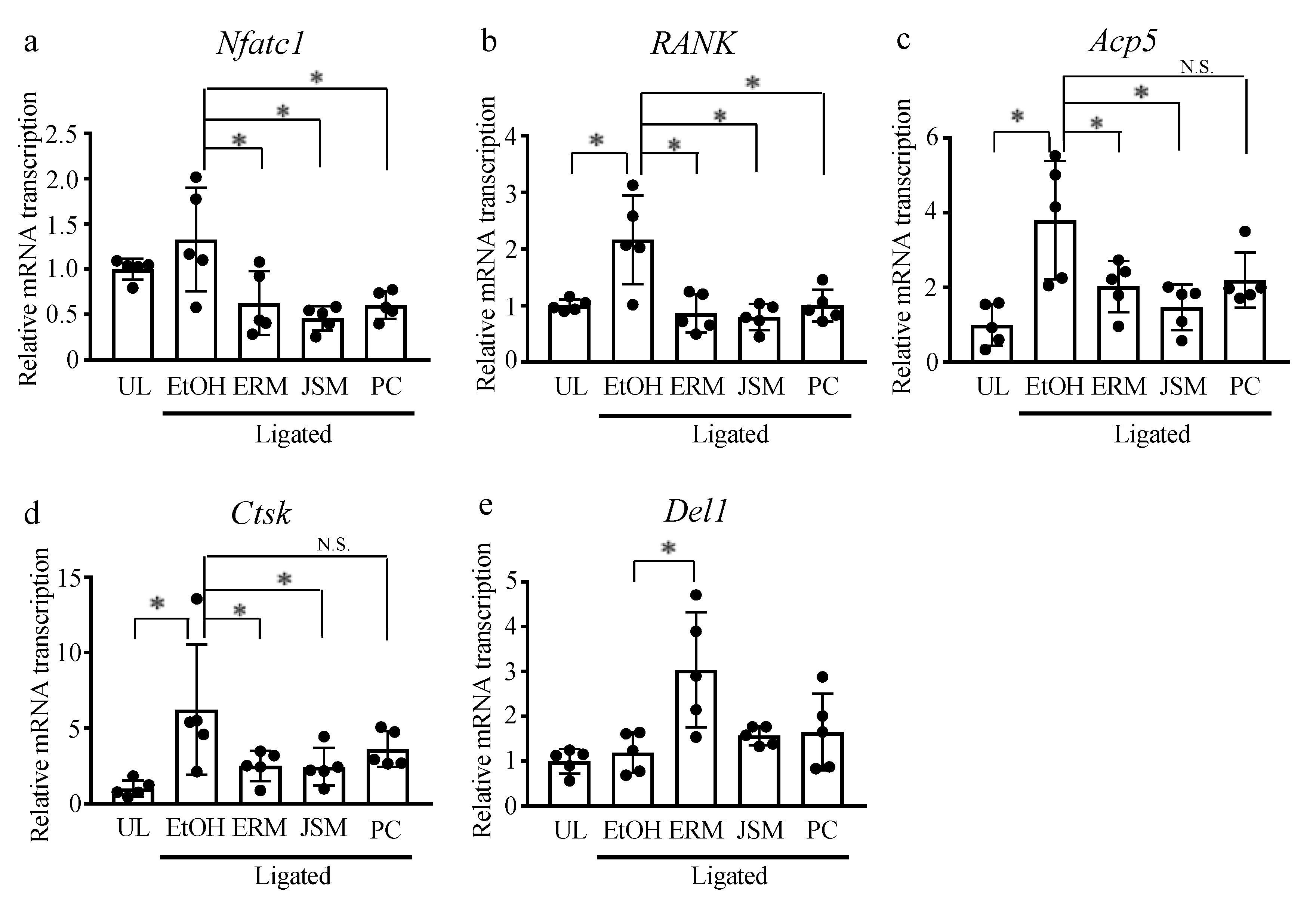
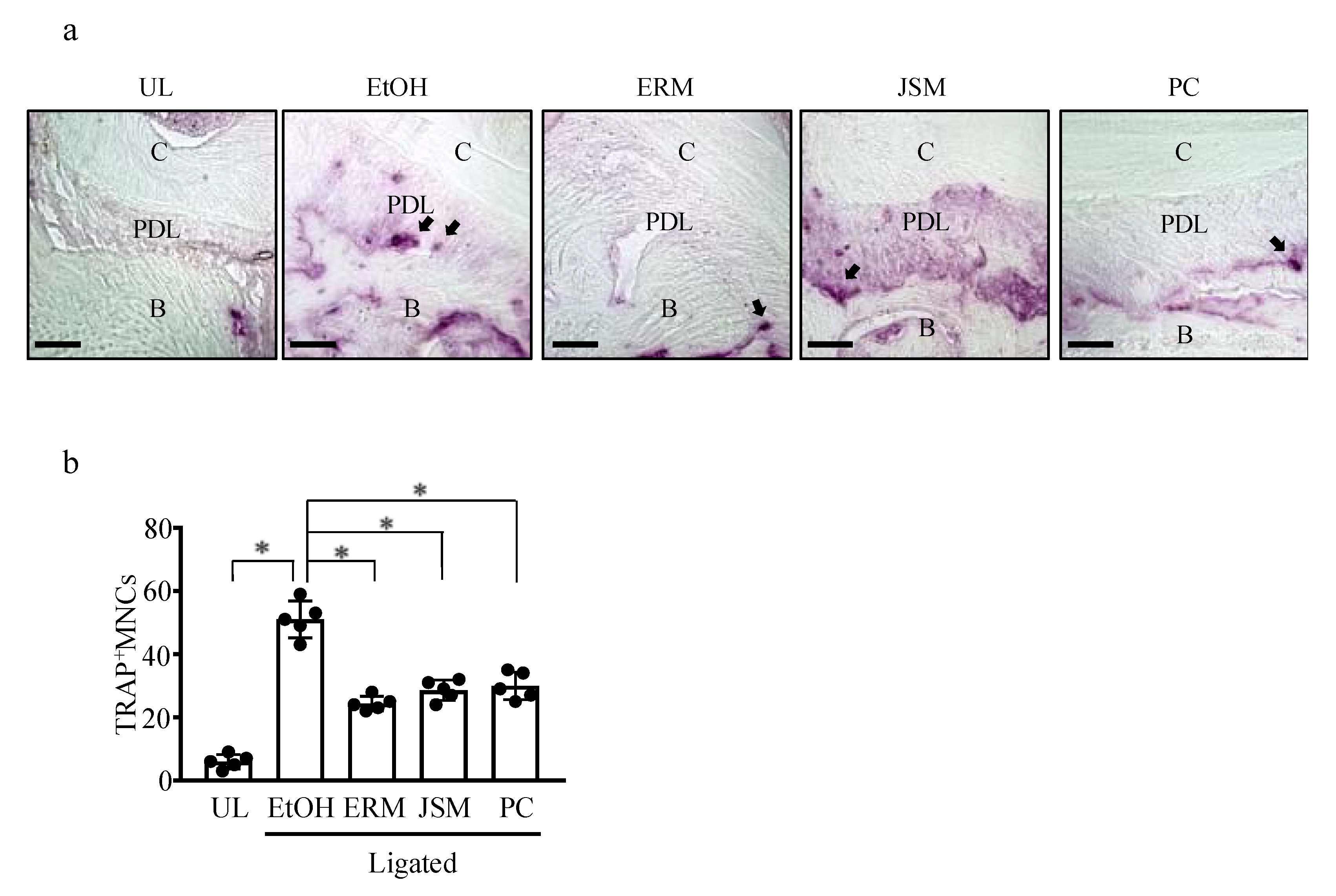
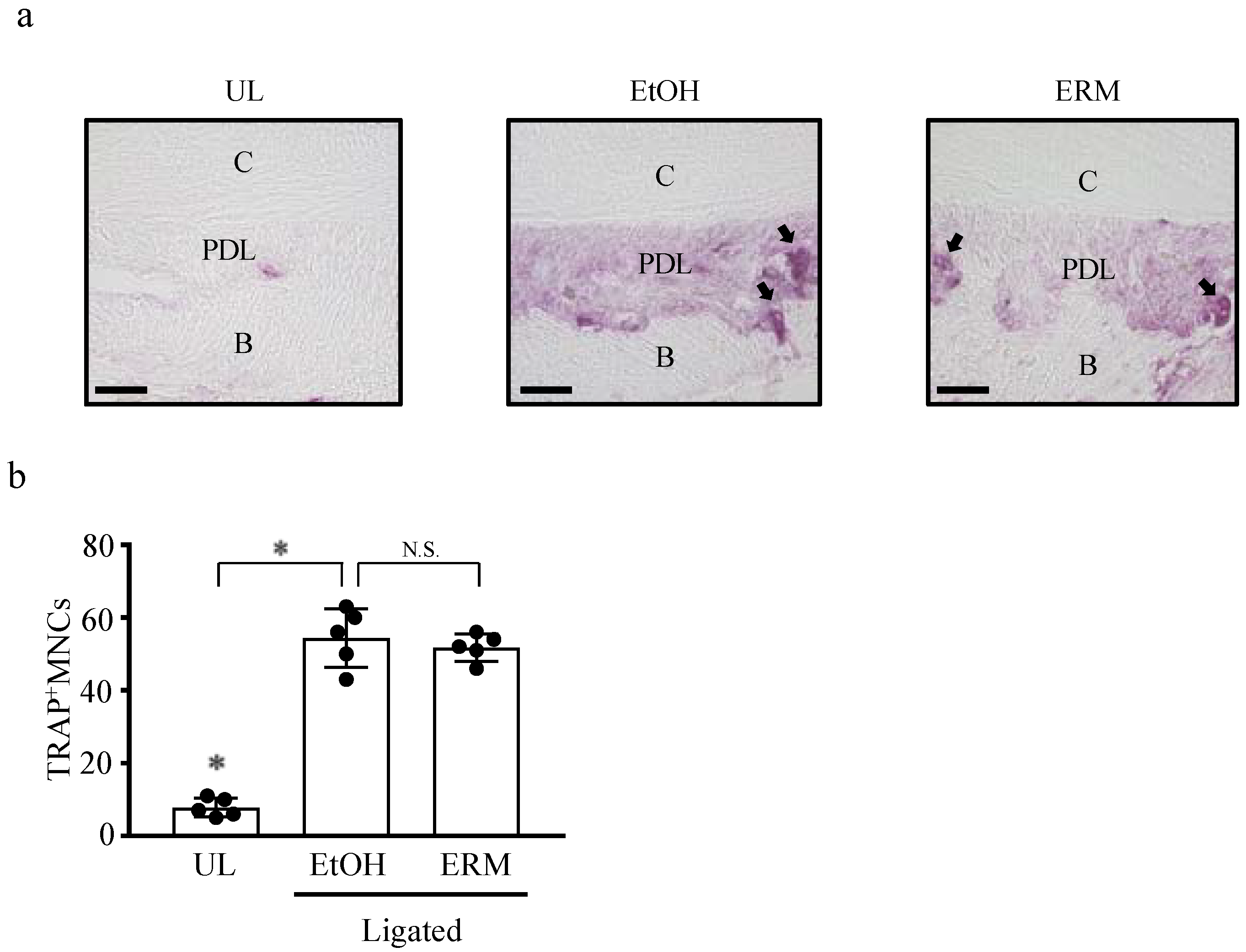
2.3. ERM Treatment Reduces the Number of Osteoclasts in Periodontal Ligament Tissue
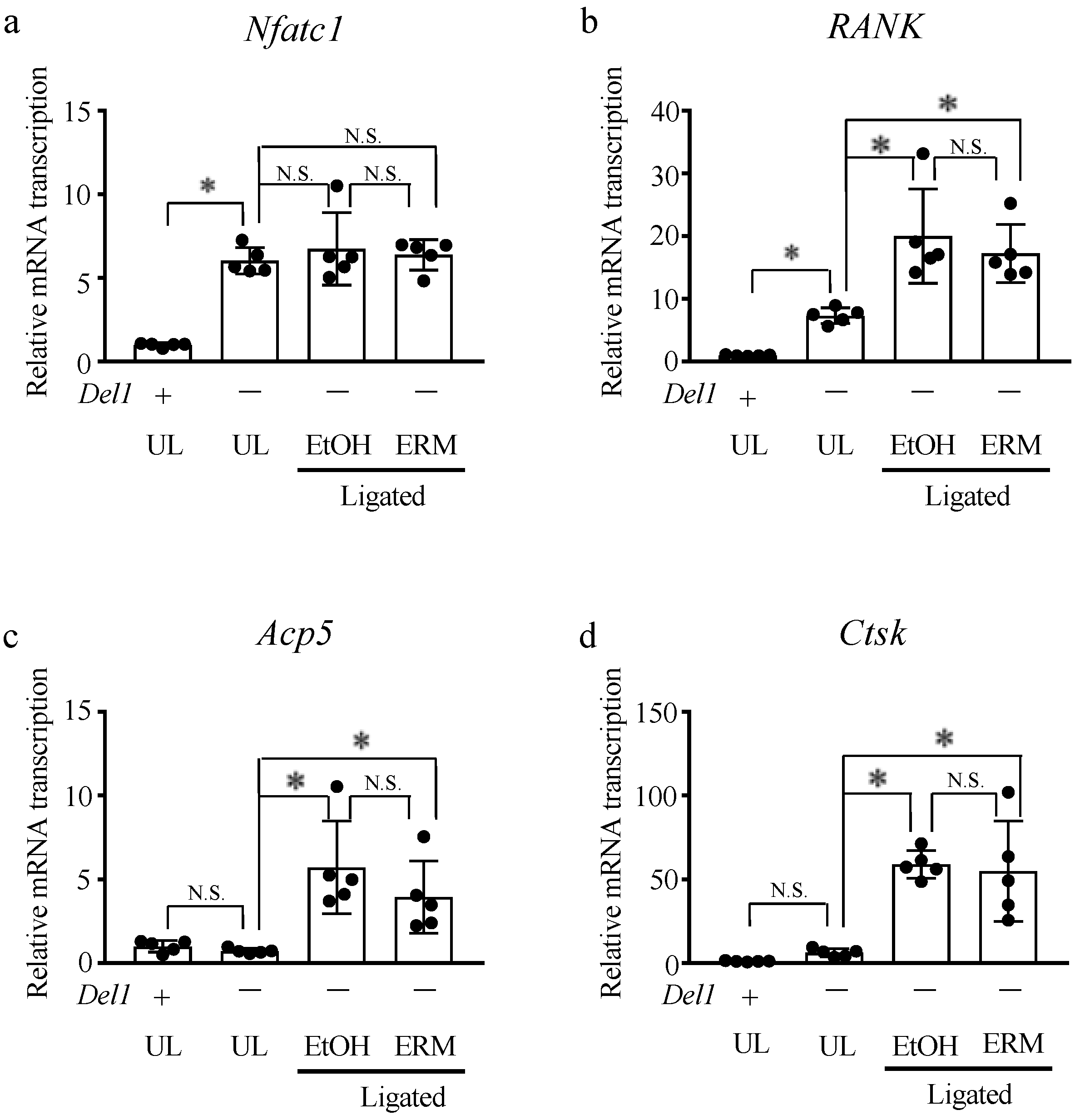
2.4. ERM Treatment Does Not Affect Alveolar Bone Resorption in Del1−/− Mice
2.5. ERM Treatment Suppresses Osteoclast Differentiation in Bone Marrow-Derived Macrophages
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Murine Tooth Ligating Model
4.3. Histologic Analysis
4.4. Cell Preparation and Culture
4.5. Quantitative Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Hajishengallis, E.; Lambris, J.D. Neutrophil homeostasis and inflammation: Novel paradigms from studying periodontitis. J. Leukoc. Biol. 2015, 98, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015, 21, 172–183. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Komatsu, N.; Nagashima, K.; Nitta, T.; Pluemsakunthai, W.; Shukunami, C.; Iwakura, Y.; Nakashima, T.; Okamoto, K.; Takayanagi, H. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Masek, E.; Ebersole, J.L. Dietary Polyphenols and Periodontitis-A Mini-Review of Literature. Molecules 2018, 23, 1786. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Domon, H.; Maekawa, T.; Yonezawa, D.; Kunitomo, E.; Tabeta, K.; Terao, Y. Protective effect of hinokitiol against periodontal bone loss in ligature-induced experimental periodontitis in mice. Arch. Oral. Biol. 2020, 112, 104679. [Google Scholar] [CrossRef]
- Ming, J.; Zhuoneng, L.; Guangxun, Z. Protective role of flavonoid baicalin from Scutellaria baicalensis in periodontal disease pathogenesis: A literature review. Complement. Ther. Med. 2018, 38, 11–18. [Google Scholar] [CrossRef]
- Tamura, H.; Maekawa, T.; Domon, H.; Hiyoshi, T.; Yonezawa, D.; Nagai, K.; Ochiai, A.; Taniguchi, M.; Tabeta, K.; Maeda, T.; et al. Peptides from rice endosperm protein restrain periodontal bone loss in mouse model of periodontitis. Arch. Oral. Biol. 2019, 98, 132–139. [Google Scholar] [CrossRef]
- Aoki-Nonaka, Y.; Tabeta, K.; Yokoji, M.; Matsugishi, A.; Matsuda, Y.; Takahashi, N.; Sulijaya, B.; Domon, H.; Terao, Y.; Taniguchi, M.; et al. A peptide derived from rice inhibits alveolar bone resorption via suppression of inflammatory cytokine production. J. Periodontol. 2019, 90, 1160–1169. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, V.J. Azithromycin as an adjunct to non-surgical periodontal therapy: A systematic review. Aust. Dent. J. 2017, 62, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Amsden, G.W. Anti-inflammatory effects of Macrolides—An underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J. Antimicrob. Chemother. 2005, 55, 10–21. [Google Scholar] [CrossRef][Green Version]
- Cervin, A.; Wallwork, B. Macrolide therapy of chronic rhinosinusitis. Rhinology 2007, 45, 259–267. [Google Scholar] [PubMed]
- Cui, Y.; Luo, L.; Li, C.; Chen, P.; Chen, Y. Long-term macrolide treatment for the prevention of acute exacerbations in COPD: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3813–3829. [Google Scholar] [CrossRef]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–698. [Google Scholar] [CrossRef]
- Lin, X.; Lu, J.; Yang, M.; Dong, B.R.; Wu, H.M. Macrolides for diffuse panbronchiolitis. Cochrane Database Syst Rev. 2015, 1, CD007716. [Google Scholar] [CrossRef]
- Rollins, D.R.; Good, J.T., Jr.; Martin, R.J. The role of atypical infections and macrolide therapy in patients with asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 511–517. [Google Scholar] [CrossRef]
- Gannon, S.C.; Cantley, M.D.; Haynes, D.R.; Hirsch, R.; Bartold, P.M. Azithromycin suppresses human osteoclast formation and activity in vitro. J. Cell Physiol. 2013, 228, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Tamura, H.; Domon, H.; Hiyoshi, T.; Isono, T.; Yonezawa, D.; Hayashi, N.; Takahashi, N.; Tabeta, K.; Maeda, T.; et al. Erythromycin inhibits neutrophilic inflammation and mucosal disease by upregulating DEL-1. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. DEL-1-Regulated Immune Plasticity and Inflammatory Disorders. Trends Mol. Med. 2019, 25, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Kourtzelis, I.; Li, X.; Mitroulis, I.; Grosser, D.; Kajikawa, T.; Wang, B.; Grzybek, M.; von Renesse, J.; Czogalla, A.; Troullinaki, M.; et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 2019, 20, 40–49. [Google Scholar] [CrossRef]
- Choi, E.Y. Inhibition of leukocyte adhesion by developmental endothelial locus-1 (del-1). Immune. Netw. 2009, 9, 153–157. [Google Scholar] [CrossRef]
- Shin, J.; Maekawa, T.; Abe, T.; Hajishengallis, E.; Hosur, K.; Pyaram, K.; Mitroulis, I.; Chavakis, T.; Hajishengallis, G. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci. Transl. Med. 2015, 7, 307ra155. [Google Scholar] [CrossRef]
- Lin, M.; Hu, Y.; Wang, Y.; Kawai, T.; Wang, Z.; Han, X. Different engagement of TLR2 and TLR4 in Porphyromonas gingivalis vs. ligature-induced periodontal bone loss. Braz. Oral. Res. 2017, 31, e63. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014, 15, 768–778. [Google Scholar] [CrossRef]
- Maekawa, T.; Abe, T.; Hajishengallis, E.; Hosur, K.B.; DeAngelis, R.A.; Ricklin, D.; Lambris, J.D.; Hajishengallis, G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J. Immunol. 2014, 192, 6020–6027. [Google Scholar] [CrossRef]
- Steel, H.C.; Theron, A.J.; Cockeran, R.; Anderson, R.; Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediat. Inflamm. 2012, 2012, 584262. [Google Scholar] [CrossRef] [PubMed]
- Arsic, B.; Barber, J.; Cikos, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [CrossRef]
- Ogrendik, M. Antibiotics for the treatment of rheumatoid arthritis. Int. J. Gen. Med. 2013, 7, 43–47. [Google Scholar] [CrossRef]
- Ogrendik, M.; Karagoz, N. Treatment of rheumatoid arthritis with roxithromycin: A randomized trial. Postgrad Med. 2011, 123, 220–227. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, L. Potential use of erythromycin to prevent and treat prosthetic failure. J. Clin. Pharmacol. 2010, 50, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Li, X.H.; Chen, B.D.; Wooley, P.H. Erythromycin inhibits wear debris-induced osteoclastogenesis by modulation of murine macrophage NF-kappaB activity. J. Orthop. Res. 2004, 22, 21–29. [Google Scholar] [CrossRef]
- Boyer, E.; Leroyer, P.; Malherbe, L.; Fong, S.B.; Loreal, O.; Bonnaure Mallet, M.; Meuric, V. Oral dysbiosis induced by Porphyromonas gingivalis is strain-dependent in mice. J. Oral. Microbiol. 2020, 12, 1832837. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, G.; Heyman, O.; Van Dyke, T.E.; Wilensky, A. Resolvin D2 Restrains Th1 Immunity and Prevents Alveolar Bone Loss in Murine Periodontitis. Front. Immunol. 2018, 9, 785. [Google Scholar] [CrossRef]
- Papathanasiou, E.; Kantarci, A.; Konstantinidis, A.; Gao, H.; Van Dyke, T.E. SOCS-3 Regulates Alveolar Bone Loss in Experimental Periodontitis. J. Dent. Res. 2016, 95, 1018–1025. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol. 2000 2016, 71, 82–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Y.; Bian, X. Clinical effect of azithromycin as an adjunct to non-surgical treatment of chronic periodontitis: A meta-analysis of randomized controlled clinical trials. J. Periodontol. Res. 2016, 51, 275–283. [Google Scholar] [CrossRef]
- Saleh, A.; Rincon, J.; Tan, A.; Firth, M. Comparison of adjunctive azithromycin and amoxicillin/metronidazole for patients with chronic periodontitis: Preliminary randomized control trial. Aust. Dent. J. 2016, 61, 469–481. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, Y.S.; Choi, E.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. Subantibiotic dose of azithromycin attenuates alveolar bone destruction and improves trabecular microarchitectures in a rat model of experimental periodontitis: A study using micro-computed tomography. Int Immunopharmacol. 2017, 47, 212–217. [Google Scholar] [CrossRef]
- Kobuchi, S.; Kabata, T.; Maeda, K.; Ito, Y.; Sakaeda, T. Pharmacokinetics of Macrolide Antibiotics and Transport into the Interstitial Fluid: Comparison among Erythromycin, Clarithromycin, and Azithromycin. Antibiotics 2020, 9, 199. [Google Scholar] [CrossRef]
- Amsden, G.W. Advanced-generation macrolides: Tissue-directed antibiotics. Int. J. Antimicrob. Agents 2001, 18 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef]
- Jelic, D.; Antolovic, R. From Erythromycin to Azithromycin and New Potential Ribosome-Binding Antimicrobials. Antibiotics 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Heta, S.; Robo, I. The Side Effects of the Most Commonly Used Group of Antibiotics in Periodontal Treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef]
- Konstantinidis, T.; Tsigalou, C.; Karvelas, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Effects of Antibiotics upon the Gut Microbiome: A Review of the Literature. Biomedicines 2020, 8, 502. [Google Scholar] [CrossRef]
- Villedieu, A.; Diaz-Torres, M.L.; Roberts, A.P.; Hunt, N.; McNab, R.; Spratt, D.A.; Wilson, M.; Mullany, P. Genetic basis of erythromycin resistance in oral bacteria. Antimicrob. Agents Chemother. 2004, 48, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, A.; Blanc, V.; Mor, C.; Nart, J.; Leon, R. Azithromycin and erythromycin susceptibility and macrolide resistance genes in Prevotella from patients with periodontal disease. Oral. Dis. 2019, 25, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Ishinaga, H.; Suzuki, S.; Sugawara, A.; Sunazuka, T.; Omura, S.; Jono, H.; Takeuchi, K. Effects of a novel nonantibiotic macrolide, EM900, on cytokine and mucin gene expression in a human airway epithelial cell line. Pharmacology 2011, 88, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Hidai, C.; Zupancic, T.; Penta, K.; Mikhail, A.; Kawana, M.; Quertermous, E.E.; Aoka, Y.; Fukagawa, M.; Matsui, Y.; Platika, D.; et al. Cloning and characterization of developmental endothelial locus-1: An embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998, 12, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, J.W.; Kim, Y.; Lee, M.N.; Kook, M.S.; Choi, E.Y.; Im, S.Y.; Koh, J.T. The extracellular matrix protein Edil3 stimulates osteoblast differentiation through the integrin alpha5beta1/ERK/Runx2 pathway. PLoS ONE 2017, 12, e0188749. [Google Scholar] [CrossRef]
- Chavakis, E.; Choi, E.Y.; Chavakis, T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb. Haemost. 2009, 102, 191–197. [Google Scholar] [CrossRef]
- Hidai, C.; Kawana, M.; Kitano, H.; Kokubun, S. Discoidin domain of Del1 protein contributes to its deposition in the extracellular matrix. Cell Tissue Res. 2007, 330, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Lim, J.H.; Neuwirth, A.; Economopoulou, M.; Chatzigeorgiou, A.; Chung, K.J.; Bittner, S.; Lee, S.H.; Langer, H.; Samus, M.; et al. Developmental endothelial locus-1 is a homeostatic factor in the central nervous system limiting neuroinflammation and demyelination. Mol. Psychiatry 2015, 20, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.K.; Le, A.; Chavakis, T.; Rumbaut, R.E.; Thiagarajan, P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation 2012, 125, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Nagata, S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J. Immunol. 2004, 172, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, L.; Zhao, Q.; Liu, Y.N.; Hou, R.; Yu, J.; Zhang, H. Developmental endothelial locus-1 (Del-1) antagonizes Interleukin-17-mediated allergic asthma. Immunol. Cell Biol. 2018, 96, 526–535. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Kim, D.Y.; Lee, S.H.; Choi, E.Y. Deficiency of developmental endothelial locus-1 (Del-1) aggravates bleomycin-induced pulmonary fibrosis in mice. Biochem. Biophys. Res. Commun. 2014, 445, 369–374. [Google Scholar] [CrossRef]
- Hyun, Y.M.; Seo, S.U.; Choi, W.S.; Kwon, H.J.; Kim, D.Y.; Jeong, S.; Kang, G.Y.; Yi, E.; Kim, M.; Ryu, H.J.; et al. Endogenous DEL-1 restrains melanoma lung metastasis by limiting myeloid cell-associated lung inflammation. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Yuh, D.Y.; Maekawa, T.; Li, X.; Kajikawa, T.; Bdeir, K.; Chavakis, T.; Hajishengallis, G. The secreted protein DEL-1 activates a beta3 integrin-FAK-ERK1/2-RUNX2 pathway and promotes osteogenic differentiation and bone regeneration. J. Biol. Chem. 2020, 295, 7261–7273. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Hosur, K.; Abe, T.; Kantarci, A.; Ziogas, A.; Wang, B.; Van Dyke, T.E.; Chavakis, T.; Hajishengallis, G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat. Commun. 2015, 6, 8272. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Takayanagi, H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 2012, 23, 582–590. [Google Scholar] [CrossRef]
- Nakamura, I.; Duong, L.T.; Rodan, S.B.; Rodan, G.A. Involvement of alpha(v)beta3 integrins in osteoclast function. J. Bone Miner. Metab. 2007, 25, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Yamashita, Y.; Takemoto, T. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 2016, 418, 1–9. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takemoto, T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci. Rep. 2015, 5, 11315. [Google Scholar] [CrossRef]
- Tamura, H.; Maekawa, T.; Hiyoshi, T.; Terao, Y. Analysis of Experimental Ligature-Induced Periodontitis Model in Mice. Methods Mol. Biol. 2021, 2210, 237–250. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyauchi, S.; Anada, T.; Imaizumi, H.; Suzuki, O. Evaluation of osteoclastic resorption activity using calcium phosphate coating combined with labeled polyanion. Anal. Biochem. 2011, 410, 7–12. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, H.; Maekawa, T.; Domon, H.; Hiyoshi, T.; Hirayama, S.; Isono, T.; Sasagawa, K.; Yonezawa, D.; Takahashi, N.; Oda, M.; et al. Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice. Antibiotics 2021, 10, 312. https://doi.org/10.3390/antibiotics10030312
Tamura H, Maekawa T, Domon H, Hiyoshi T, Hirayama S, Isono T, Sasagawa K, Yonezawa D, Takahashi N, Oda M, et al. Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice. Antibiotics. 2021; 10(3):312. https://doi.org/10.3390/antibiotics10030312
Chicago/Turabian StyleTamura, Hikaru, Tomoki Maekawa, Hisanori Domon, Takumi Hiyoshi, Satoru Hirayama, Toshihito Isono, Karin Sasagawa, Daisuke Yonezawa, Naoki Takahashi, Masataka Oda, and et al. 2021. "Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice" Antibiotics 10, no. 3: 312. https://doi.org/10.3390/antibiotics10030312
APA StyleTamura, H., Maekawa, T., Domon, H., Hiyoshi, T., Hirayama, S., Isono, T., Sasagawa, K., Yonezawa, D., Takahashi, N., Oda, M., Maeda, T., Tabeta, K., & Terao, Y. (2021). Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice. Antibiotics, 10(3), 312. https://doi.org/10.3390/antibiotics10030312






