A Putative Amidase Endolysin Encoded by Clostridium perfringens St13 Exhibits Specific Lytic Activity and Synergizes with the Muramidase Endolysin Psm
Abstract
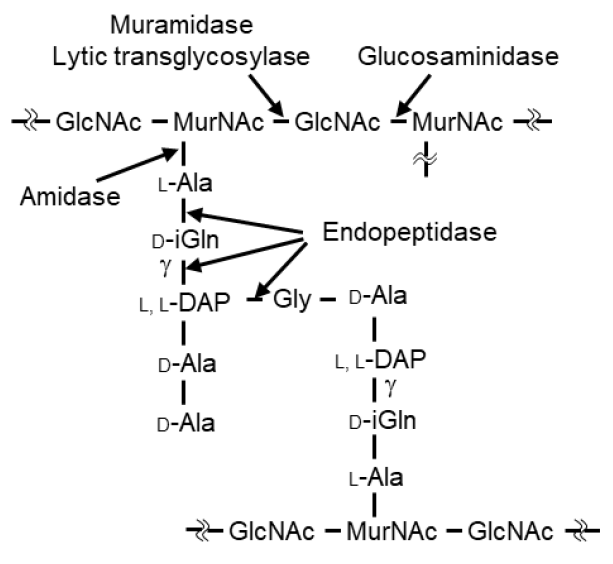
1. Introduction
2. Results
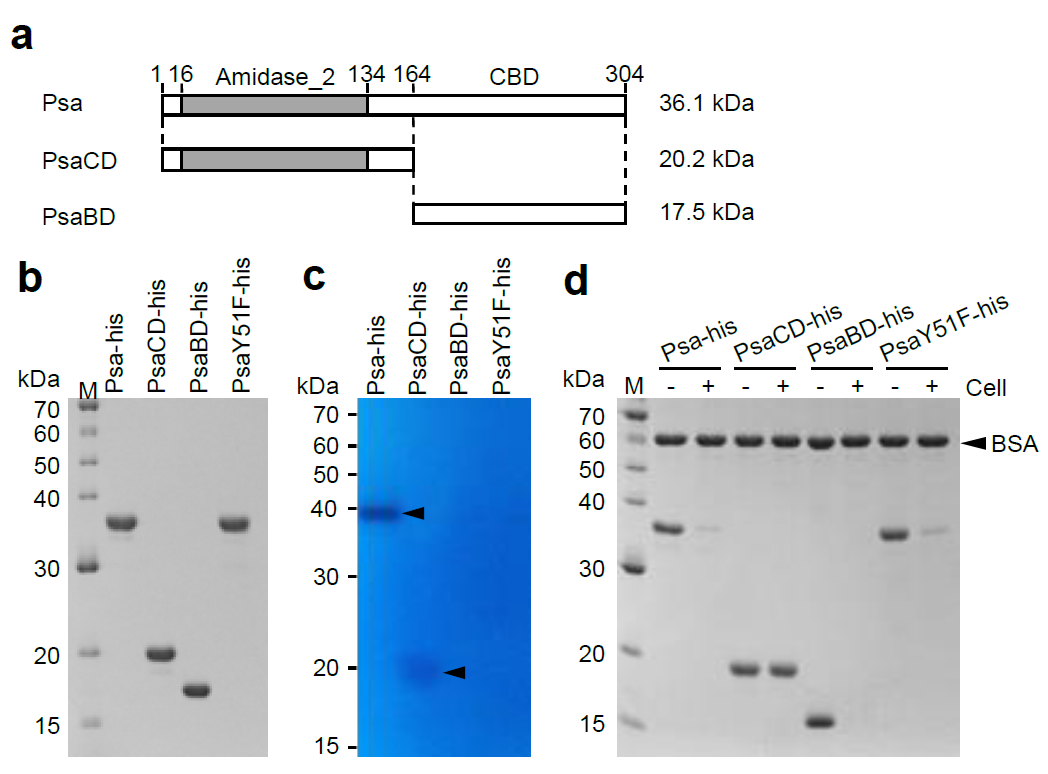
2.1. Identification and Cloning of Psa from the Genome of Clostridium perfringens st13, and Expression and Purification of the Psa Protein
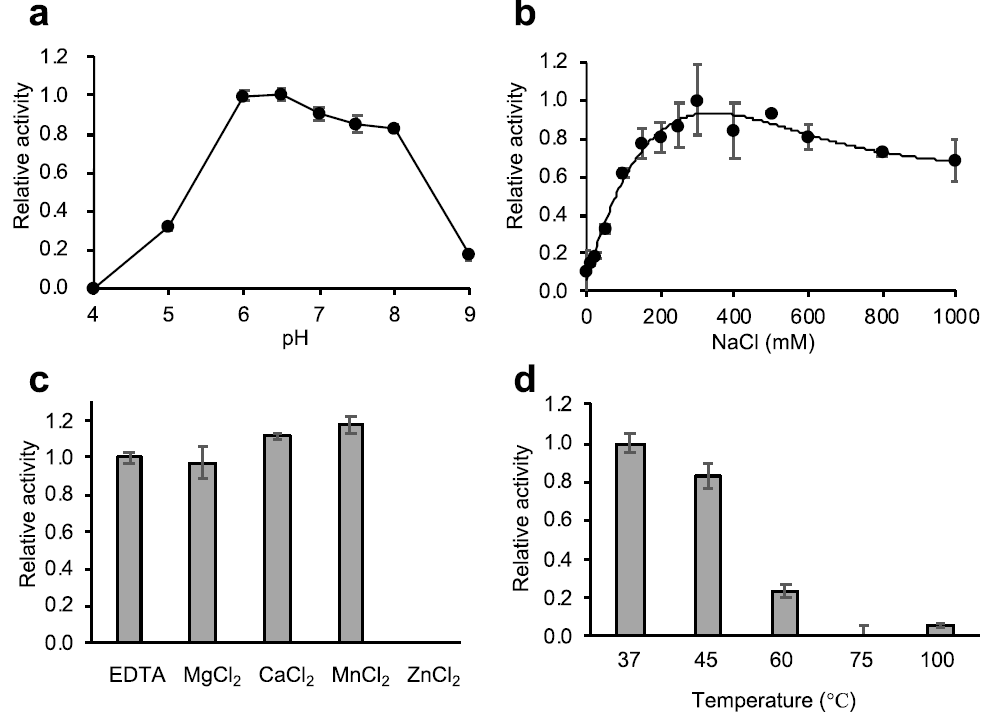
2.2. Characterization of the Enzyme Activity of Psa
2.3. Bacterial Specificity of Psa
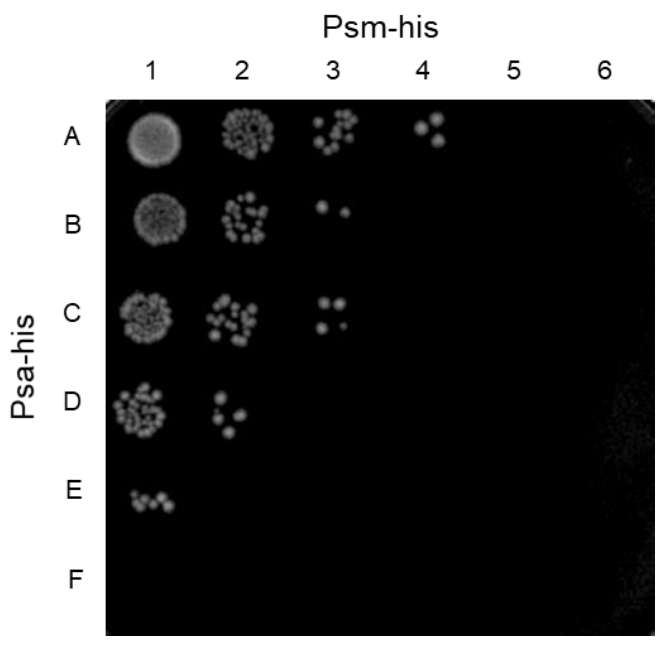
2.4. Synergistic Effect of Psa and the C. perfringens-Specific Endolysin Psm
3. Discussion
4. Materials and Methods
4.1. Construction of the Expression Vector
4.2. Expression and Purification of the Proteins
4.3. Turbidity Reduction Assay
4.4. Renaturing SDS-PAGE Analysis (Zymography)
4.5. Cell Binding Assay
4.6. Measurement of Synergistic Effect
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLAST | basic local alignment search tool |
| BSA | bovine serum albumin |
| CBD | cell wall binding domain |
| CD | catalytic domain |
| DAP | diaminopimelic acid |
| EDTA | ethylenediaminetetraacetic acid |
| FIC | fractional inhibitory concentration |
| OD600 | optical density at a wavelength of 600 nm |
| PBS (-) | phosphate-buffered saline without Ca and Mg |
| SDS | sodium dodecyl sulfate |
| SDS-PAGE | SDS polyacrylamide gel electrophoresis |
References
- Harbarth, S.; Samore, M.H. Antimicrobial resistance determinants and future control. Emerg. Infect. Dis. 2005, 11, 794–801. [Google Scholar] [CrossRef]
- Norrby, S.R.; Nord, C.E.; Finch, R.; European Society of Clinical Microbiology and Infectious Diseases. Lack of development of new antimicrobial drugs: A potential serious threat to public health. Lancet Infect. Dis. 2005, 5, 115–119. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Ross, R.P.; Coffey, A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 2009, 33, 801–881. [Google Scholar] [CrossRef]
- Courchesne, N.M.; Parisien, A.; Lan, C.Q. Production and application of bacteriophage and bacteriophage-encoded lysins. Recent Pat. Biotechnol. 2009, 3, 37–45. [Google Scholar] [CrossRef]
- Singh, P.K.; Donovan, D.M.; Kumar, A. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob. Agents Chemother. 2014, 58, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endo-lysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 2001, 294, 2170–2172. [Google Scholar] [CrossRef]
- Nelson, D.; Loomis, L.; Fischetti, V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 4107–4112. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef]
- Oechslin, F.; Daraspe, J.; Giddey, M.; Moreillon, P.; Resch, G. In vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia. Antimicrob. Agents Chemother. 2013, 57, 6276–6283. [Google Scholar] [CrossRef]
- Doehn, J.M.; Fischer, K.; Reppe, K.; Gutbier, B.; Tschernig, T.; Hocke, A.C.; Fischetti, V.A.; Loffler, J.; Suttorp, N.; Hippenstiel, S.; et al. Delivery of the endolysin Cpl-1 by inhalation rescues mice with fatal pneumococcal pneumonia. J. Antimicrob. Chemother. 2013, 68, 2111–2117. [Google Scholar] [CrossRef]
- Jun, S.Y.; Jung, G.M.; Yoon, S.J.; Choi, Y.-J.; Koh, W.S.; Moon, K.S.; Kang, S.H. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob. Agents Chemother. 2014, 58, 2084–2088. [Google Scholar] [CrossRef]
- Bae, J.Y.; Jun, K.I.; Kang, C.K.; Song, K.H.; Choe, P.G.; Bang, J.H.; Kim, E.S.; Park, S.W.; Kim, H.B.; Kim, N.J.; et al. Efficacy of intranasal administration of the recombinant endolysin SAL200 in a lethal murine Staphylococcus aureus pneumonia model. Antimicrob. Agents Chemother. 2019, 63, e02009–e02018. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Broendum, S.S.; Buckle, A.M.; McGowan, S. Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins. Mol. Microbiol. 2018, 110, 879–896. [Google Scholar] [CrossRef]
- Wang, I.N.; Smith, D.L.; Young, R. Holins: The protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 2000, 54, 799–825. [Google Scholar] [CrossRef]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef]
- Donovan, D.M. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat. Biotechnol. 2007, 1, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Blackman, S.A.; Foster, S.J. Autolysins of Bacillus subtilis: Multiple enzymes with multiple functions. Microbiology 2000, 146, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Joris, B.; Charlier, P.; Foster, S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008, 32, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, X.; Lu, P.; Zhu, Y.; Dong, W.; Roy, S.; Hejair, H.M.A.; Pan, Z.; Ma, J.; Yao, H. A novel autolysin AtlASS mediates bacterial cell separation during cell division and contributes to full virulence in Streptococcus suis. Vet. Microbiol. 2019, 234, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rainey, F.A.; Hollen, B.J.; Small, A.; Genus, I. Clostridium. In Bergey’s Manual of Systematic Bacteriology: Firmicutes, 2nd ed.; de Vos, P., Ed.; Springer: New York, USA, 2009; Volume 3, pp. 738–828. [Google Scholar]
- Ohtani, K.; Shimizu, T. Regulation of toxin production in Clostridium perfringens. Toxins Basel 2016, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996, 9, 216–234. [Google Scholar] [CrossRef]
- Bryant, A.E.; Bayer, C.R.; Aldape, M.J.; Wallace, R.J.; Titball, R.W.; Stevens, D.L. Clostridium perfringens phospholipase C-induced platelet/leukocyte interactions impede neutrophil diapedesis. J. Med. Microbiol. 2006, 55, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Present, D.A.; Meislin, R.; Shaffer, B. Gas gangrene. Rev. Orthop. Rev. 1990, 19, 333–341. [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Lindstrom, M.; Heikinheimo, A.; Lahti, P.; Korkeala, H. Novel insights into the epidemiology of Clostridium perfringens type A food poisoning. Food Microbiol. 2011, 28, 192–198. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S.; Jeong, W.; Jeoung, H.Y.; An, D.J. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011, 90, 1381–1390. [Google Scholar] [CrossRef]
- van Immerseel, F.; Rood, J.I.; Moore, R.J.; Titball, R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009, 17, 32–36. [Google Scholar] [CrossRef]
- Nariya, H.; Miyata, S.; Tamai, E.; Sekiya, H.; Maki, J.; Okabe, A. Identification and characterization of a putative endolysin encoded by episomal phage phiSM101 of Clostridium perfringens. Appl. Microbiol. Biotechnol. 2011, 90, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Tamai, E.; Yoshida, H.; Sekiya, H.; Nariya, H.; Miyata, S.; Okabe, A.; Kuwahara, T.; Maki, J.; Kamitori, S. X-ray structure of a novel endolysin encoded by episomal phage phiSM101 of Clostridium perfringens. Mol. Microbiol. 2014, 92, 326–337. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Fischetti, V.A. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 2003, 47, 375–377. [Google Scholar] [CrossRef]
- Inouye, M.; Arnheim, N.; Sternglanz, R. Bacteriophage T7 lysozyme is an N-acetylmuramyl-L-alanine amidase. J. Biol. Chem. 1973, 248, 7247–7252. [Google Scholar] [CrossRef]
- Pennartz, A.; Généreux, C.; Parquet, C.; Mengin-Lecreulx, D.; Joris, B. Substrate-induced inactivation of the Escherichia coli AmiD N-acetylmuramoyl-L-alanine amidase highlights a new strategy to inhibit this class of enzyme. Antimicrob. Agents Chemother. 2009, 53, 2991–2997. [Google Scholar] [CrossRef][Green Version]
- Cheng, X.; Zhang, X.; Pflugrath, J.W.; Studier, F.W. The structure of bacteriophage T7 lysozyme, a zinc amidase and an inhibitor of T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 1994, 91, 4034–4038. [Google Scholar] [CrossRef] [PubMed]
- Kerff, F.; Petrella, S.; Mercier, F.; Sauvage, E.; Herman, R.; Pennartz, A.; Zervosen, A.; Luxen, A.; Frère, J.M.; Joris, B.; et al. Specific structural features of the N-acetylmuramoyl-L-alanine amidase AmiD from Escherichia coli and mechanistic implications for enzymes of this family. J. Mol. Biol. 2010, 397, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Nariya, H.; Miyata, S.; Suzuki, M.; Tamai, E.; Okabe, A. Development and application of a method for counterselectable in-frame deletion in Clostridium perfringens. Appl. Environ. Microbiol. 2011, 77, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Tamai, E.; Sekiya, H.; Goda, E.; Makihata, N.; Maki, J.; Yoshida, H.; Kamitori, S. Structural and biochemical characterization of the Clostridium perfringens autolysin catalytic domain. FEBS Lett. 2017, 591, 231–239. [Google Scholar] [CrossRef]
- Leyh-Bouille, M.; Bonaly, R.; Ghuysen, J.M.; Tinelli, R.; Tipper, D. LL-diaminopimelic acid containing peptidoglycans in walls of Streptomyces sp. and of Clostridium perfringens (type A). Biochemistry 1970, 9, 2944–2952. [Google Scholar] [CrossRef]
- Royet, J.; Dziarski, R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 2007, 5, 264–277. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Sekiya, H.; Tamai, E.; Kawasaki, J.; Murakami, K.; Kamitori, S. Structural and biochemical characterizations of the novel autolysin Acd24020 from Clostridioides difficile and its full-function catalytic domain as a lytic enzyme. Mol. Microbiol. 2020, in press. [Google Scholar] [CrossRef]
- García, P.; Martínez, B.; Rodríguez, L.; Rodríguez, A. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. J. Food. Microbiol. 2010, 141, 151–155. [Google Scholar] [CrossRef]
- Becker, S.C.; Swift, S.; Korobova, O.; Schischkova, N.; Kopylov, P.; Donovan, D.M.; Abaev, I. Lytic activity of the staphylolytic Twort phage endolysin CHAP domain is enhanced by the SH3b cell wall binding domain. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Inhibitory zinc sites in enzymes. Biometals 2013, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Sitedirected mutagenesis by overlap extension using the polymerase chain reaction. Gene 2013, 77, 51–59. [Google Scholar] [CrossRef]
- Rashid, M.H.; Sato, N.; Sekiguchi, J. Analysis of the minor autolysins of Bacillus subtilis during vegetative growth by zymography. FEMS Microbiol. Lett. 1995, 132, 131–137. [Google Scholar] [CrossRef]
- Pendland, S.L.; Piscitelli, S.C.; Schreckenberger, P.C.; Danziger, L.H. In vitro activities of metronidazole and its hydroxy metabolite against Bacteroides spp. Antimicrob. Agents Chemother. 1994, 38, 2106–2110. [Google Scholar] [CrossRef]
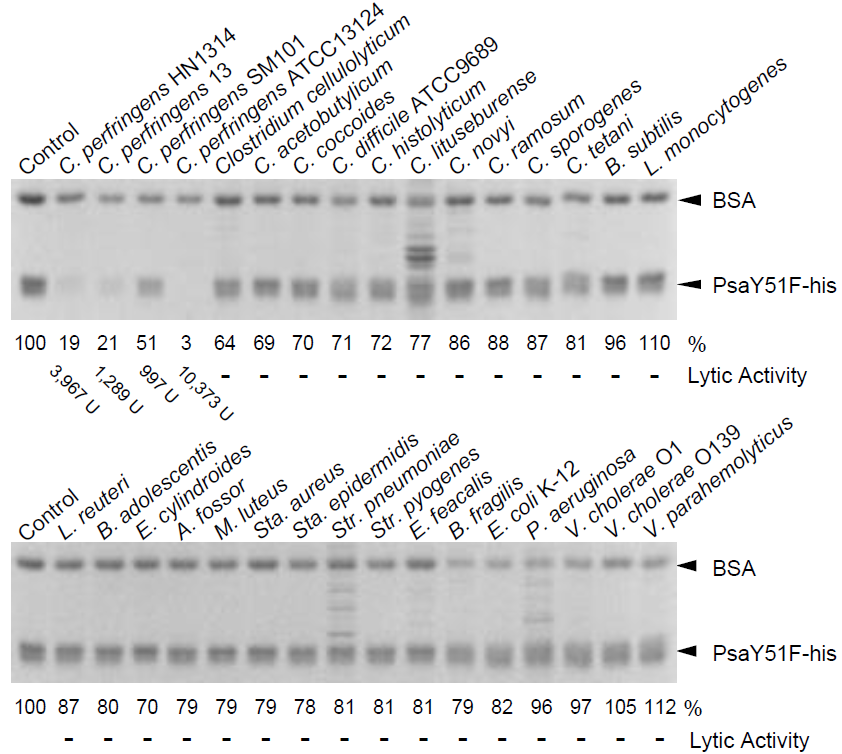
| Organism | Strain | Relative Activity (%) |
|---|---|---|
| Gram(+) | ||
| Clostridium perfringens | HN1314 | 100 (3967 U) |
| Clostridium perfringens | st13 | 32 (1289 U) |
| Clostridium perfringens | ATCC13124 | 261 (10,373 U) |
| Clostridium perfringens | SM101 | 25 (997 U) |
| Clostridium perfringens | SM101 (4 h) | 325 (12,911 U) |
| Clostridium perfringens | SM101 (spore) | - |
| Clostridium acetobutylicum | ATCC824 | - |
| Clostridium cellulolyticum | ATCC35319 | - |
| Clostridium coccoides | ATCC29236 | - |
| Clostridium histolyticum | ATCC19401 | - |
| Clostridium lituseburense | ATCC25759 | - |
| Clostridium novyi | ATCC17861 | - |
| Clostridium ramosum | ATCC25582 | - |
| Clostridium sporogenes | ATCC3584 | - |
| Clostridium tetani | KZ1113 | - |
| Clostridioidesdifficile | ATCC9689 | - |
| Atopobium fossor | ATCC43386 | - |
| Bacillus subtilis | 168 | - |
| Bifidobacterium adolescentis | ATCC15703 | - |
| Enterococcus faecalis | IID682 | - |
| Eubacterium cylindroides | ATCC27805 | - |
| Lactobacillus reuteri | ATCC23272 | - |
| Listeria monocytogenes | HCIPH A5-1 | - |
| Micrococcus luteus | ATCC4698 | - |
| Staphylococcus aureus | ATCC6538P | - |
| Staphylococcus epidermidis | ATCC35984 | - |
| Streptococcus pneumoniae | IID555 | - |
| Streptococcus pyogenes | 124/0207 | - |
| Gram(−) | ||
| Bacteroides fragilis | ATCC25285 | - |
| Escherichia coli | K-12 | - |
| Pseudomonas aeruginosa | PAO1 | - |
| Vibrio cholerae | O1/P1418 | - |
| Vibrio cholerae | O139/MDO-6 | - |
| Vibrio parahaemolyticus | RIMD2210115 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekiya, H.; Okada, M.; Tamai, E.; Shimamoto, T.; Shimamoto, T.; Nariya, H. A Putative Amidase Endolysin Encoded by Clostridium perfringens St13 Exhibits Specific Lytic Activity and Synergizes with the Muramidase Endolysin Psm. Antibiotics 2021, 10, 245. https://doi.org/10.3390/antibiotics10030245
Sekiya H, Okada M, Tamai E, Shimamoto T, Shimamoto T, Nariya H. A Putative Amidase Endolysin Encoded by Clostridium perfringens St13 Exhibits Specific Lytic Activity and Synergizes with the Muramidase Endolysin Psm. Antibiotics. 2021; 10(3):245. https://doi.org/10.3390/antibiotics10030245
Chicago/Turabian StyleSekiya, Hiroshi, Maho Okada, Eiji Tamai, Toshi Shimamoto, Tadashi Shimamoto, and Hirofumi Nariya. 2021. "A Putative Amidase Endolysin Encoded by Clostridium perfringens St13 Exhibits Specific Lytic Activity and Synergizes with the Muramidase Endolysin Psm" Antibiotics 10, no. 3: 245. https://doi.org/10.3390/antibiotics10030245
APA StyleSekiya, H., Okada, M., Tamai, E., Shimamoto, T., Shimamoto, T., & Nariya, H. (2021). A Putative Amidase Endolysin Encoded by Clostridium perfringens St13 Exhibits Specific Lytic Activity and Synergizes with the Muramidase Endolysin Psm. Antibiotics, 10(3), 245. https://doi.org/10.3390/antibiotics10030245






