Attacins: A Promising Class of Insect Antimicrobial Peptides
Abstract
1. Introduction
2. Insects Antimicrobial Peptides: A Brief Insight
3. Attacins
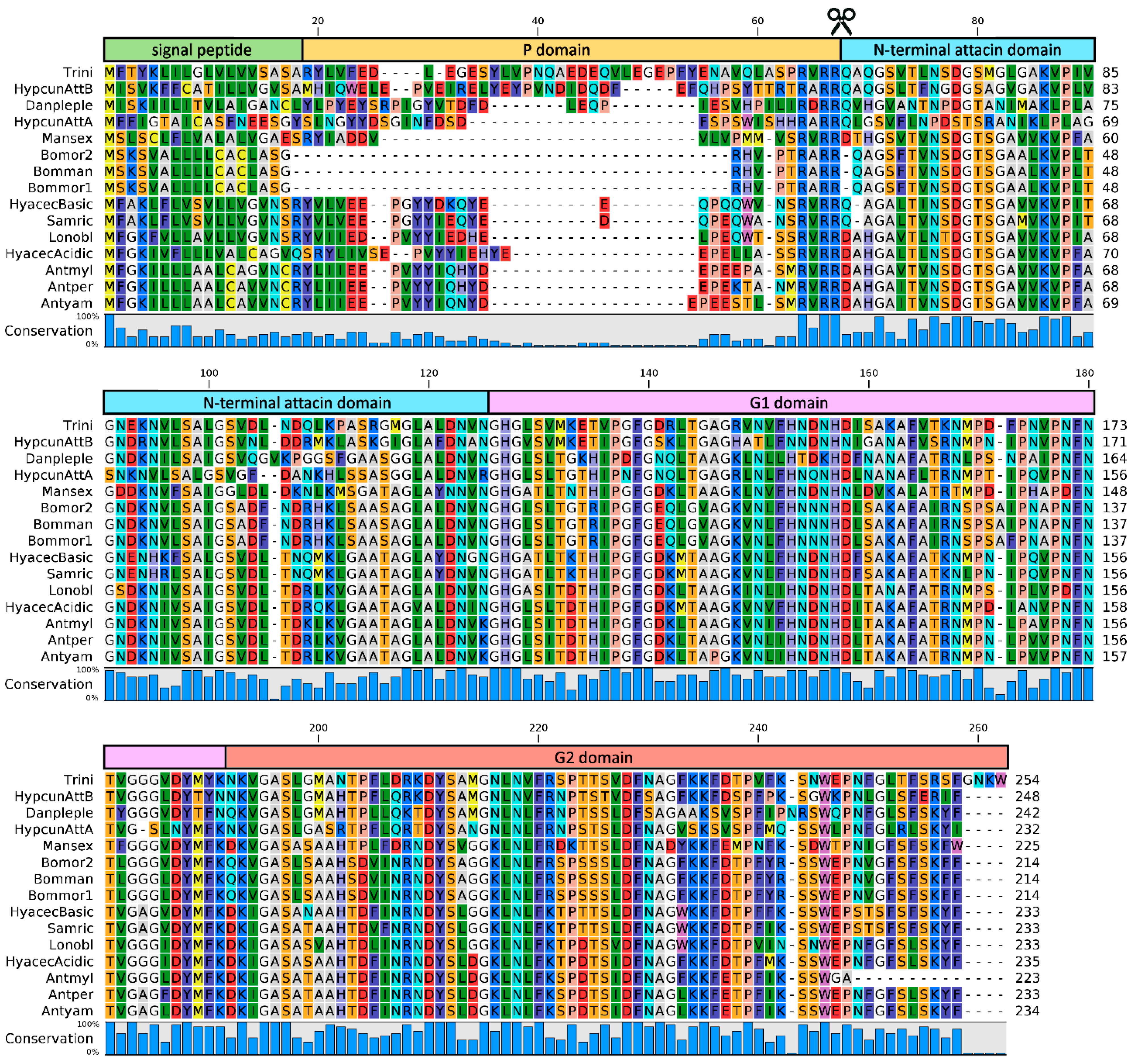
3.1. First Identification and Main Structural Features
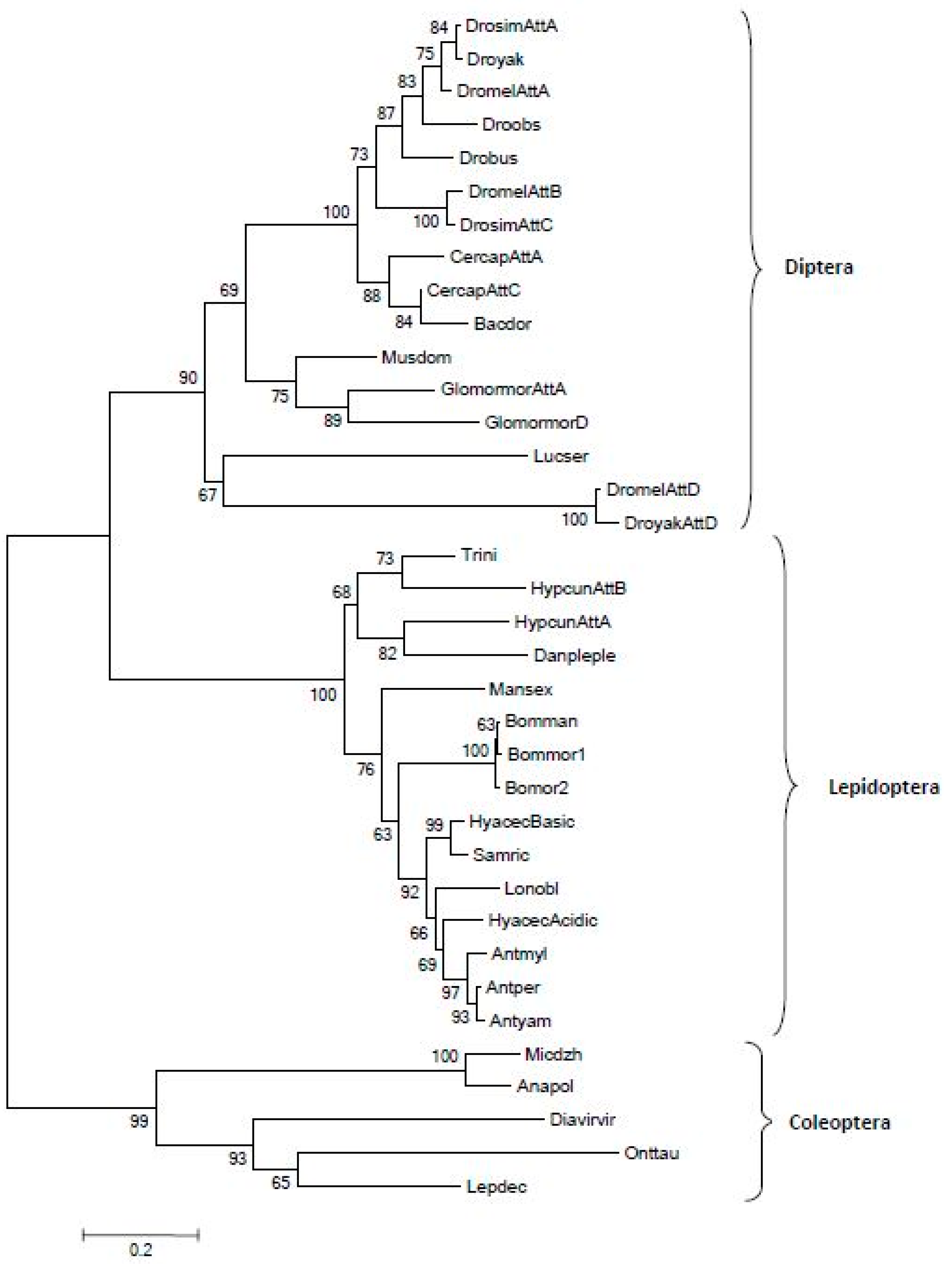
3.2. Phylogenetic Considerations
3.3. Antimicrobial Activity of Attacins and Therapeutical Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Intern. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Geitani, R.; Moubareck, C.A.; Xu, Z.; Karam Sarkis, D.; Touqui, L. Expression and Roles of Antimicrobial Peptides in Innate Defense of Airway Mucosa: Potential Implication in Cystic Fibrosis. Front. Immunol. 2020, 11, 1198. [Google Scholar] [CrossRef]
- Ganz, T.; Lehrer, R.I. Antimicrobial peptides of leukocytes. Curr. Opin. Hematol. 1997, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Q.; Yuan, J.; Ösapay, G.; Ösapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science (80-) 1999, 286, 498–502. [Google Scholar] [CrossRef]
- Bastos, P.; Trindade, F.; da Costa, J.; Ferreira, R.; Vitorino, R. Human Antimicrobial Peptides in Bodily Fluids: Current Knowledge and Therapeutic Perspectives in the Postantibiotic Era. Med. Res. Rev. 2018, 38, 101–146. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hooper, L.V. Antimicrobial defense of the intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K. The Roles of Antimicrobial Peptides in Innate Host Defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, 1087–1093. [Google Scholar] [CrossRef]
- Fields, F.R.; Freed, S.D.; Carothers, K.E.; Hamid, M.N.; Hammers, D.E.; Ross, J.N.; Kalwajtys, V.R.; Gonzalez, A.J.; Hildreth, A.D.; Friedberg, I.; et al. Novel antimicrobial peptide discovery using machine learning and biophysical selection of minimal bacteriocin domains. Drug Dev. Res. 2020, 81, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Porto, W.F.; Pires, A.S.; Franco, O.L. Computational tools for exploring sequence databases as a resource for antimicrobial peptides. Biotechnol. Adv. 2017, 35, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, J.E.; Van der Donk, W.A. Genome mining for ribosomally synthesized natural products. Curr. Opin. Chem. Biol. 2011, 15, 11–21. [Google Scholar] [CrossRef]
- Leikoski, N.; Liu, L.; Jokela, J.; Wahlsten, M.; Gugger, M.; Calteau, A.; Permi, P.; Kerfeld, C.A.; Sivonen, K.; Fewer, D.P. Genome mining expands the chemical diversity of the cyanobactin family to include highly modified linear peptides. Chem. Biol. 2013, 20, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, D.A.; Dennison, S.R.; Harris, F. Antimicrobial Peptides: Their History, Evolution, and Functional Promiscuity. Antimicrob. Pept. 2013, 1–37. [Google Scholar] [CrossRef]
- Hanson, M.A.; Lemaitre, B.; Unckless, R.L. Dynamic Evolution of Antimicrobial Peptides Underscores Trade-Offs between Immunity and Ecological Fitness. Front. Immunol. 2019, 10, 2620. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C.; White, S.H. Membrane Partitioning: Distinguishing Bilayer Effects from the Hydrophobic Effect. Biochemistry 1993, 32, 6307–6312. [Google Scholar] [CrossRef]
- Wimley, W.C.; White, S.H. Determining the membrane topology of peptides by fluorescence quenching. Biochemistry 2000, 39, 161–170. [Google Scholar] [CrossRef]
- Pan, C.Y.; Chen, J.Y.; Cheng, Y.S.E.; Chen, C.Y.; Ni, I.H.; Sheen, J.F.; Pan, Y.L.; Kuo, C.M. Gene expression and localization of the epinecidin-1 antimicrobial peptide in the grouper (Epinephelus coioides), and its role in protecting fish against pathogenic infection. DNA Cell Biol. 2007, 26, 403–413. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Nicoletto, S.F. Antimicrobial peptides: An overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Almeida, P.F.; Pokorny, A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: From kinetics to thermodynamics. Biochemistry 2009, 48, 8083–8093. [Google Scholar] [CrossRef]
- Rončević, T.; Puizina, J.; Tossi, A. Antimicrobial peptides as anti-infective agents in pre-post-antibiotic era? Int. J. Mol. Sci. 2019, 20, 5713. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Combination Effects of Antimicrobial Peptides. Antimicrob. Agents Chemother. 2016, 60, 1717–1724. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Mitani, Y.; Akada, K.Y.; Murase, O.; Yoneyama, S.; Zasloff, M.; Miyajima, K. Mechanism of synergism between antimicrobial peptides magainin 2 and PGLa. Biochemistry 1998, 37, 15144–15153. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W. Current Topics Action of Antimicrobial Peptides: Two-State Model. Biochemistry 2000. [Google Scholar] [CrossRef]
- Hara, T.; Mitani, Y.; Tanaka, K.; Uematsu, N.; Takakura, A.; Tachi, T.; Kodama, H.; Kondo, M.; Mori, H.; Otaka, A.; et al. Heterodimer formation between the antimicrobial peptides magainin 2 and PGLa in lipid bilayers: A cross-linking study. Biochemistry 2001, 40, 12395–12399. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Galanth, C.; Abbassi, F.; Lequin, O.; Ayala-Sanmartin, J.; Ladram, A.; Nicolas, P.; Amiche, M. Mechanism of antibacterial action of dermaseptin B2: Interplay between helix—Hinge—Helix structure and membrane curvature strain. Biochemistry 2009, 48, 313–327. [Google Scholar] [CrossRef]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial peptides: Interaction with model and biological membranes and synergism with chemical antibiotics. Front. Chem. 2018, 6, 204. [Google Scholar] [CrossRef]
- Olivieri, C.; Bugli, F.; Menchinelli, G.; Veglia, G.; Buonocore, F.; Scapigliati, G.; Stocchi, V.; Ceccacci, F.; Papi, M.; Sanguinetti, M.; et al. Design and characterization of chionodracine-derived antimicrobial peptides with enhanced activity against drug-resistant human pathogens. RSC Adv. 2018, 8, 41331–41346. [Google Scholar] [CrossRef]
- Oren, Z.; Shai, Y. Mode of action of linear amphipathic α-helical antimicrobial peptides. Biopolymers 1998, 47, 451–463. [Google Scholar] [CrossRef]
- Tennessen, J.A. Molecular evolution of animal antimicrobial peptides: Widespread moderate positive selection. J. Evol. Biol. 2005, 18, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Park, N.G.; Silphaduang, U.; Moon, H.S.; Seo, J.K.; Corrales, J.; Noga, E.J. Structure-Activity Relationships of Piscidin 4, a Piscine Antimicrobial Peptide. Biochemistry 2011, 50, 3288–3299. [Google Scholar] [CrossRef]
- Wheaten, S.A.; Lakshmanan, A.; Almeida, P.F. Statistical analysis of peptide-induced graded and all-or-none fluxes in giant vesicles. Biophys. J. 2013, 105, 432–443. [Google Scholar] [CrossRef]
- Niu, S.F.; Jin, Y.; Xu, X.; Qiao, Y.; Wu, Y.; Mao, Y.; Su, Y.Q.; Wang, J. Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish Shellfish. Immunol. 2013, 35, 513–524. [Google Scholar] [CrossRef]
- Ongey, E.L.; Pflugmacher, S.; Neubauer, P. Bioinspired designs, molecular premise and tools for evaluating the ecological importance of antimicrobial peptides. Pharmaceuticals 2018, 11, 68. [Google Scholar] [CrossRef]
- Armengol, E.; Domenech, O.; Fusté, E.; Pérez-Guillén, I.; Borrell, J.H.; Sierra, J.M.; Vinas, M. Efficacy of combinations of colistin with other antimicrobials involves membrane fluidity and efflux machinery. Infect. Drug Resist. 2019, 12, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Z.; Cao, Z.; Li, W.; Wu, Y. Defensins, a novel type of animal toxin-like potassium channel inhibitor. Toxicon 2019, 157, 101–105. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Zhang, K.; Ling, J.; Peng, Z.; Huang, X.; Liu, H.; Gu, L. Antimicrobial and DNA-binding activities of the peptide fragments of human lactoferrin and histatin 5 against Streptococcus mutans. Arch. Oral Biol. 2011, 56, 869–876. [Google Scholar] [CrossRef]
- Yan, J.; Wang, K.; Dang, W.; Chen, R.; Xie, J.; Zhang, B.; Song, J.; Wang, R. Two hits are better than one: Membrane-active and DNA binding-related double-action mechanism of NK-18, a novel antimicrobial peptide derived from mammalian NK-lysin. Antimicrob. Agents Chemother. 2013, 57, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, C.; Buonocore, F.; Picchietti, S.; Taddei, A.R.; Bernini, C.; Scapigliati, G.; Dicke, A.A.; Vostrikov, V.V.; Veglia, G.; Porcelli, F. Structure and membrane interactions of chionodracine, a piscidin-like antimicrobial peptide from the icefish Chionodraco hamatus. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L. Antibacterial peptides isolated from insects. J. Pept. Sci. 2000, 6, 497–511. [Google Scholar] [CrossRef]
- Feldhaar, H.; Gross, R. Insects as hosts for mutualistic bacteria. Int. J. Med. Microbiol. 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A. Evolutionary plasticity of insect immunity. J. Insect Physiol. 2013, 59, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Makarova, O.; Rodriguez-Rojas, A.; Eravci, M.; Weise, C.; Dobson, A.; Johnston, P.; Rolff, J. Antimicrobial defence and persistent infection in insects revisited. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef]
- Masson, F.; Zaidman-Rémy, A.; Heddi, A. Antimicrobial peptides and cell processes tracking endosymbiont dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150298. [Google Scholar] [CrossRef]
- Schaible, U.E.; Haas, A. Intracellular Niches of Microbes: A Pathogens Guide through the Host Cell; John Wiley and Sons: Hoboken, NJ, USA, 2009; ISBN 9783527322077. [Google Scholar]
- Doudoumis, V.; Alam, U.; Aksoy, E.; Abd-Alla, A.M.M.; Tsiamis, G.; Brelsfoard, C.; Aksoy, S.; Bourtzis, K. Tsetse-Wolbachia symbiosis: Comes of age and has great potential for pest and disease control. J. Invertebr. Pathol. 2013, 112, S94. [Google Scholar] [CrossRef][Green Version]
- Login, F.H.; Balmand, S.; Vallier, A.; Vincent-Monégat, C.; Vigneron, A.; Weiss-Gayet, M.; Rochat, D.; Heddi, A. Antimicrobial peptides keep insect endosymbionts under control. Science (80-) 2011, 334, 362–365. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Eleftherianos, I. Memory and specificity in the insect immune system: Current perspectives and future challenges. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Tanji, T.; Hu, X.; Weber, A.N.R.; Ip, Y.T. Toll and IMD Pathways Synergistically Activate an Innate Immune Response in Drosophila melanogaster. Mol. Cell. Biol. 2007, 27, 4578–4588. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamakawa, M. Regulation of the innate immune responses in the silkworm, Bombyx mori. Invertebr. Surviv. J. 2011, 8, 59–69. [Google Scholar]
- Khan, I.; Prakash, A.; Agashe, D. Experimental evolution of insect immune memory versus pathogen resistance. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171583. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef]
- Åsling, B.; Dushay, M.S.; Hultmark, D. Identification of early genes in the Drosophila immune response by PCR-based differential display: The Attacin A gene and the evolution of attacin-like proteins. Insect Biochem. Mol. Biol. 1995, 25, 511–518. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Orivel, J.; Redeker, V.; Le Caer, J.P.; Krier, F.; Revol-Junelles, A.M.; Longeon, A.; Chaffotte, A.; Dejean, A.; Rossier, J. Ponericins, New Antibacterial and Insecticidal Peptides from the Venom of the Ant Pachycondyla goeldii. J. Biol. Chem. 2001, 276, 17823–17829. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Kruse, F.; Vasudevarao, M.D.; Junker, J.P.; Zebrowski, D.C.; Fischer, K.; Noël, E.S.; Grün, D.; Berezikov, E.; Engel, F.B.; et al. Spatially Resolved Genome-wide Transcriptional Profiling Identifies BMP Signaling as Essential Regulator of Zebrafish Cardiomyocyte Regeneration. Dev. Cell 2016, 36, 36–49. [Google Scholar] [CrossRef]
- Mylonakis, E.; Podsiadlowski, L.; Muhammed, M.; Vilcinskas, A. Diversity, evolution and medical applications of insect antimicrobial peptides. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150290. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Levashina, E.A.; Ohresser, S.; Bulet, P.; Reichhart, J.-M.; Hetru, C.; Hoffmann, J.A. Metchnikowin, a Novel Immune-Inducible Proline-Rich Peptide from Drosophila with Antibacterial and Antifungal Properties. Eur. J. Biochem. 1995, 233, 694–700. [Google Scholar] [CrossRef]
- Axén, A.; Carlsson, A.; Engström, Å.; Bennich, H. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur. J. Biochem. 1997, 247, 614–619. [Google Scholar] [CrossRef]
- Hultmark, D.; Engström, A.; Andersson, K.; Steiner, H.; Bennich, H.; Boman, H.G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983, 2, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.; Engström, P.; Tao, Z.J.; Carlsson, A.; Bennich, H. Insect immunity. The primary structure of the antibacterial protein attacin F and its relation to two native attacins from Hyalophora cecropia. EMBO J. 1984, 3, 2065–2070. [Google Scholar]
- GUNNE, H.; HELLERS, M.; STEINER, H. Structure of preproattacin and its processing in insect cells infected with a recombinant baculovirus. Eur. J. Biochem. 1990, 187, 699–703. [Google Scholar] [CrossRef]
- Kockum, K.; Faye, I.; Hofsten, P.V.; Lee, J.Y.; Xanthopoulos, K.G.; Boman, H.G. Insect immunity. Isolation and sequence of two cDNA clones corresponding to acidic and basic attacins from Hyalophora cecropia. EMBO J. 1984, 3, 2071–2075. [Google Scholar] [CrossRef]
- Sun, S.-C.; Lindström, I.; Lee, J.-Y.; Faye, I. Structure and expression of the attacin genes in Hyalophora cecropia. Eur. J. Biochem. 1991, 196, 247–254. [Google Scholar] [CrossRef]
- Hedengren, M.; Borge, K.; Hultmark, D. Expression and evolution of the Drosophila Attacin/Diptericin gene family. Biochem. Biophys. Res. Commun. 2000, 279, 574–581. [Google Scholar] [CrossRef]
- Shelomi, M.; Jacobs, C.; Vilcinskas, A.; Vogel, H. The unique antimicrobial peptide repertoire of stick insects. Dev. Comp. Immunol. 2020, 103, 103471. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Nyström, T.; De Cock, H.; Bennich, H. Attacin—An insect immune protein—Binds LPS and triggers the specific inhibition of bacterial outer-membrane protein synthesis. Microbiology 1998, 144, 2179–2188. [Google Scholar] [CrossRef]
- Wenzler, E.; Bunnell, K.L.; Danziger, L.H. Clinical use of the polymyxins: The tale of the fox and the cat. Int. J. Antimicrob. Agents 2018, 51, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Yu, B.; Han, G.Q.; Chen, D.W. Molecular cloning, expression in Escherichia coli of Attacin A gene from Drosophila and detection of biological activity. Mol. Biol. Rep. 2010, 37, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.M.; Kim, H.J.; Kim, Y.I.; Kang, Y.J.; Lee, I.H.; Jin, B.R.; Han, Y.S.; Cheon, H.M.; Ha, N.G.; Seo, S.J. Comparative analysis of two attacin genes from Hyphantria cunea. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 213–220. [Google Scholar] [CrossRef]
- Bang, K.; Park, S.; Yoo, J.Y.; Cho, S. Characterization and expression of attacin, an antibacterial protein-encoding gene, from the beet armyworm, Spodoptera exigua (Hübner) (Insecta: Lepidoptera: Noctuidae). Mol. Biol. Rep. 2012, 39, 5151–5159. [Google Scholar] [CrossRef]
- Hu, Y.; Aksoy, S. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 2005, 35, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Park, S.I. Novel attacin from Hermetia illucens: cDNA cloning, characterization, and antibacterial properties. Prep. Biochem. Biotechnol. 2019, 49, 279–285. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Zuo, Y.H.; Tsai, C.L.; Hsu, C.M.; Chen, M.E. Cdna cloning and transcriptional regulation of the cecropin and attacin from the oriental fruit fly, Bactrocera dorsalis (DIPTERA: TEPHRITIDAE). Arch. Insect Biochem. Physiol. 2015, 89, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Liebold, K.; Schmidt, W.D.; Hartmann, M.; Fassler, D. Biosurgery supports granulation and debridement in chronic wounds—Clinical data and remittance spectroscopy measurement. Int. J. Dermatol. 2002, 41, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Kaihanfar, M.; Momeni-Moghaddam, M.; Moghaddam, M.J.M.; Hajar, T.; Pak, V.D.; Bidi, J.O. Investigation of antimicrobial effects of treated Lucilia sericata larvae extract on bacteria. Iran. J. Microbiol. 2018, 10, 409–416. [Google Scholar]
- Hirsch, R.; Wiesner, J.; Marker, A.; Pfeifer, Y.; Bauer, A.; Hammann, P.E.; Vilcinskas, A. Profiling antimicrobial peptides from the medical maggot Lucilia sericata as potential antibiotics for MDR Gram-negative bacteria. J. Antimicrob. Chemother. 2019, 74, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Asthana, N.; Yadav, S.P.; Ghosh, J.K. Dissection of antibacterial and toxic activity of melittin: A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J. Biol. Chem. 2004, 279, 55042–55050. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence | Charge | Pathogen | Antimicrobial Activity |
|---|---|---|---|---|
| Attacin B from Hyalophora cecropia | QAGALTINSDGTSGAV-VKVPITGNENHKFSALGSVDLT-NQMKL | +3 | E. coli D21 | 0.6 µM (inhibition zone assay) |
| GAATAGLAYDNGNGHGATLT KTHIPGFGDKMTAAGKVNLFHN | E. coli D22 | 0.06 µM | ||
| DNHDFSAKAFATKNMP-NIPQVPNFNTVGAGVDYMFKDKIG | P. maltophilia Pm1 | 4 µM | ||
| ASANAAHTDFINRNDYS-LGGKLNLFKTPTTSLDFNAGWKKF | A. calcoaceticus Ac11 | 0.4 µM | ||
| DTPFFKSSWEPSTSFSFSKYF | ||||
| Attacin E from Hyalophora cecropia | DAHGALTLNSDGTSGAVVKVPFAGNDKNIVSAIGSVDLT-DRQKL | −3 | E. coli D21 | 2 µM (inhibition zone assay) |
| GAATAGVALDNINGHGLSLTDT HIPGFGDKMTAAGKVNVFHNDNHD | E. coli D22 | 0.3 µM | ||
| ITAKAFATRNMPDIANVPN FNTVGGGIDYMFKDKIG | P. maltophilia Pm1 | 15 µM | ||
| ASASAAHTDFINRNDYSLDGKLNLFKTPDTSIDFNAGFKKFDTPFMKSS | A. calcoaceticus Ac11 | 1 µM | ||
| WEPNFGFSLSKYF | ||||
| Attacin A from Drosophila melanogaster | QVLGGSLTSNPAGGADARLDLT-KGIGNPNHNVVGQVFAAGNTQSG | +3 | E. coli DH5α | N.D. |
| PVTTGGTLAYNNAGHGASLTKT HTPGVKDVFQQEAHANLFNNGRH | S. maltophilia | |||
| NLDAKVFASQ NKLANGFEFQRN GAGLDYSHINGHGASLTHSNF | ||||
| PGIGQQLGLDGRANLWSSPNRAT-TLDLTGSASKWTSGPFANQKPNF | ||||
| GAGLGLSHHFG | ||||
| Attacin B from Hyphantria cunea | QAQGSLTFNGDGSAGVGAKVPLVGNDRNVLSAIGSVNLDDRMKLASKG | +5 | E. coli | 0.1 and 1.0 µg of protein (radial diffusion assay) |
| IGLAFDNANGHGVSVMKET IPGFGSKLTGAGHATLFNNDNHNIGANA | C. freundii | |||
| FVSRNMPNIPNVPNFNTVGGG LDYTYNNKVGASLGMAHTPFLQRKD | C. albicans | |||
| YSAMGNLNVFRNPTSTVDFSAGFKKFDSPFPKSGWKPNLGLSFERIF | ||||
| Attacin from Spodoptera exigua | QAQGSVTLNSDGGMGLGAKI-PLANNDRNVLSAVGSMDLNNN-MNPTSKG | +4 | E. coli DH5α | 1, 0.5, 0.1, and 0.05 µg of protein (radial diffusion assay) |
| FGLALDNVNGHGLTVMKES VPG-FGDRLSGAGKLNVFHNDNHNVAVTGSL | P. cichorii | |||
| TRNMPSIPNVPNFN-TIGGGVDYMYKNKVGASLGMASTPFLDRKDYSAMG | B. subtilis | |||
| NLNLFRSPTTSVDFSGGFKK-FESPFMSSGWKPNFGLTFGRSF | L. monocytogenes | |||
| Attacin A1 from Glossina morsitans morsitans | GQFGGTVSSNPNGGLDVNARLSKTIGDPNANVVGGVFAAGNTDGGPATRGA | +4 | E. coli K12 | 0.3 µM (minimum bactercidal concentration, MBC) |
| FLAANKDGHGLSLQHSKTDNFGSSLTSSAHAHLFNDKTHKLDANAFH | ||||
| SRTHLDNGFKFDRVGGGLRYDHVTGHGASLTASRIPQLDMNTLGLTGKANL | Trypanosoma brucei | 0.075 µM (50 % minimal inhibitory concentration, MIC50) | ||
| WSSPNRATTLDLTGGVSKHFGGPFDGQTNKQIGLGLNSRF | ||||
| Attacin from Hermetia illucens | KFLGNPNHNIGGGVFAAGN-TRSNTPSLGAF-GTLNLKDHSLGVSKTITPGVS | +10 | E. coli | N.D. |
| DTFSQNARLNILKTPDHR-VDANVFNSHTRLN-NGFAFDKRRGGSLDYTH | S. aureus KCCM 40881 | |||
| RAGHGLSLGA-SHIP-KFGTTAELTGKANLWRSPSGLSTFDLTGSASRTF | ||||
| GGPMAGRNNFGAGLGFSHRF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonocore, F.; Fausto, A.M.; Della Pelle, G.; Roncevic, T.; Gerdol, M.; Picchietti, S. Attacins: A Promising Class of Insect Antimicrobial Peptides. Antibiotics 2021, 10, 212. https://doi.org/10.3390/antibiotics10020212
Buonocore F, Fausto AM, Della Pelle G, Roncevic T, Gerdol M, Picchietti S. Attacins: A Promising Class of Insect Antimicrobial Peptides. Antibiotics. 2021; 10(2):212. https://doi.org/10.3390/antibiotics10020212
Chicago/Turabian StyleBuonocore, Francesco, Anna Maria Fausto, Giulia Della Pelle, Tomislav Roncevic, Marco Gerdol, and Simona Picchietti. 2021. "Attacins: A Promising Class of Insect Antimicrobial Peptides" Antibiotics 10, no. 2: 212. https://doi.org/10.3390/antibiotics10020212
APA StyleBuonocore, F., Fausto, A. M., Della Pelle, G., Roncevic, T., Gerdol, M., & Picchietti, S. (2021). Attacins: A Promising Class of Insect Antimicrobial Peptides. Antibiotics, 10(2), 212. https://doi.org/10.3390/antibiotics10020212








