The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
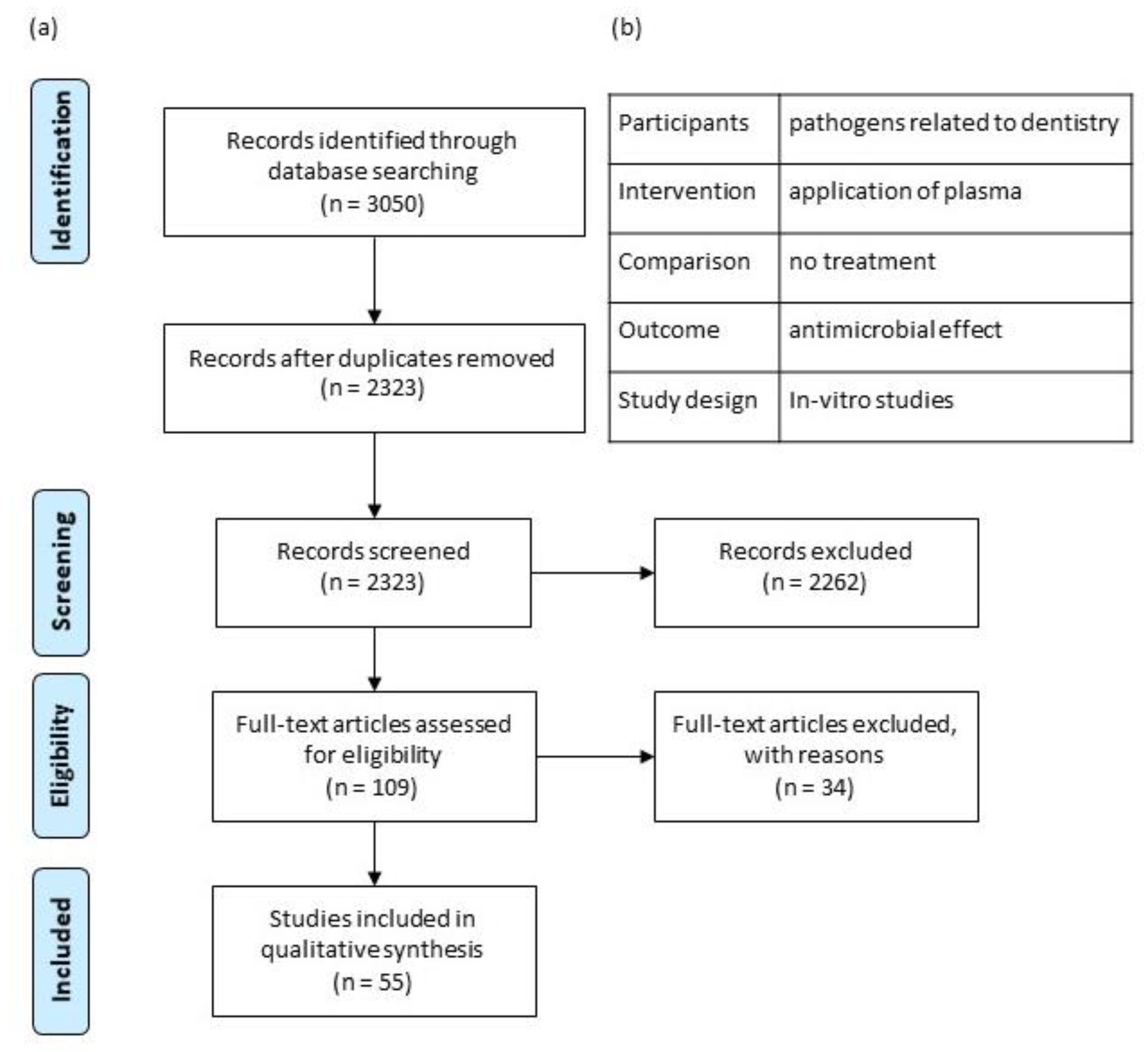
2.2. Eligibility Criteria
2.3. Data Extraction and Assessment of Quality
2.4. Statistical Analysis
3. Results
3.1. Oral Streptococci
3.2. Lactobacilli
3.3. Enterococcus faecalis
3.4. Periopathogens
3.5. Candida
3.6. Multi-Species Biofilm
3.7. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plasmamedizin: Kaltplasma in der Medizinischen Anwendung; Metelmann, H.-R., von Woedtke, T., Weltmann, K.-D., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-52644-6. [Google Scholar]
- Gherardi, M.; Tonini, R.; Colombo, V. Plasma in Dentistry: Brief History and Current Status. Trends Biotechnol. 2018, 36, 583–585. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Bandow, J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef]
- Müller, L.K.; Jungbauer, G.; Jungbauer, R.; Wolf, M.; Deschner, J. Biofilm and Orthodontic Therapy. In Oral Biofilms; Eick, S., Ed.; S. Karger AG: Berlin, Germany, 2020; pp. 201–213. ISBN 978-3-318-06851-1. [Google Scholar]
- Takahashi, N. Microbial ecosystem in the oral cavity: Metabolic diversity in an ecological niche and its relationship with oral diseases. Int. Congr. Ser. 2005, 1284, 103–112. [Google Scholar] [CrossRef]
- Caufield, P.W.; Schön, C.N.; Saraithong, P.; Li, Y.; Argimón, S. Oral Lactobacilli and Dental Caries: A Model for Niche Adaptation in Humans. J. Dent. Res. 2015, 94, 110S–118S. [Google Scholar] [CrossRef]
- Haapasalo, M.; Orstavik, D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 1987, 66, 1375–1379. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 717–725. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency Office of Pesticide Programs. Protocol for the Evaluation of Bactericidal Activity of Hard, Non-Porous Copper/Copper-Alloy Surfaces; Environmental Science Center: Fort Meade, MD, USA, 2015. [Google Scholar]
- European Committee for Standardization. Chemical Disinfectants and Antiseptics—Quantitative Carrier Test for the Evaluation of Bactericidal Activity for Instruments Used in the Medical Area—Test Method and Requirements (Phase 2, Step 2); EN 14561:2006; Beuth: Berlin, Germany, 2006. [Google Scholar]
- European Committee for Standardization. Chemical Disinfectants and Antiseptics—Quantitative Carrier Test for the Evaluation of Fungicidal or Yeasticidal Activity for Instruments Used in the Medical Area—Test Method and Requirements (Phase 2, Step 2); EN 14562:2006; Beuth: Berlin, Germany, 2006. [Google Scholar]
- International Organization for Standardization. Sterilization of Medical Devices—Microbiological Methods—Part 1: Determination of a Population of Microorganisms on Products; ISO 11737-1:2006; AAMI: Arlington, VA, USA, 2006. [Google Scholar]
- Chyderiotis, S.; Legeay, C.; Verjat-Trannoy, D.; Le Gallou, F.; Astagneau, P.; Lepelletier, D. New insights on antimicrobial efficacy of copper surfaces in the healthcare environment: A systematic review. Clin. Microbiol. Infect. 2018, 1130–1138. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Abonti, T.R.; Kaku, M.; Kojima, S.; Sumi, H.; Yamamoto, T.; Yashima, Y.; Miyahara, H.; Okino, A.; Kawata, T.; Tanne, K.; et al. Irradiation effects of low temperature multi gas plasma jet on oral bacteria. Dent. Mater. J. 2016, 35, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sirhan, S.; Hertel, M.; Preissner, S.; Wirtz, H.C.; Herbst, S.R.; Pierdzioch, P.; Raguse, J.D.; Hartwig, S. Bactericidal efficacy of cold plasma in processed bone. A new approach for adjuvant therapy of medication-related osteonecrosis of the jaw? Clin. Plasma Med. 2016, 4, 9–13. [Google Scholar] [CrossRef]
- Blumhagen, A.; Singh, P.; Mustapha, A.; Chen, M.; Wang, Y.; Yu, Q. Plasma Deactivation of Oral Bacteria Seeded on Hydroxyapatite Disks as Tooth Enamel Analogue. Am. J. Dent. 2014, 27, 84–90. [Google Scholar]
- Gorynia, S.; Koban, I.; Matthes, R.; Welk, A.; Gorynia, S.; Hübner, N.-O.; Kocher, T.; Kramer, A. In vitro efficacy of cold atmospheric pressure plasma on S. sanguinis biofilms in comparison of two test models. GMS Hyg. Infect. Control 2013, 8. [Google Scholar] [CrossRef]
- Hertel, M.; Schwill-Engelhardt, J.; Gerling, T.; Weltmann, K.-D.; Imiolczyk, S.M.; Hartwig, S.; Preissner, S. Antibacterial efficacy of plasma jet, dielectric barrier discharge, chlorhexidine, and silver diamine fluoride varnishes in caries lesions. Plasma Med. 2018, 8, 73–82. [Google Scholar] [CrossRef]
- Huang, W.-K.; Weng, C.-C.; Liao, J.-D.; Wang, Y.-C.; Chuang, S.-F. Capillary-tube-based micro-plasma system for disinfecting dental biofilm. Int. J. Radiat. Biol. 2013, 89, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Koban, I.; Holtfreter, B.; Hübner, N.-O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef]
- Park, S.R.; Lee, H.W.; Hong, J.W.; Lee, H.J.; Kim, J.Y.; Choi, B.; Kim, G.C.; Jeon, Y.C. Enhancement of the killing effect of low-temperature plasma on Streptococcus mutans by combined treatment with gold nanoparticles. J. Nanobiotechnol. 2014, 12, 29. [Google Scholar] [CrossRef]
- Preissner, S.; Wirtz, H.C.; Tietz, A.-K.; Abu-Sirhan, S.; Herbst, S.R.; Hartwig, S.; Pierdzioch, P.; Schmidt-Westhausen, A.M.; Dommisch, H.; Hertel, M. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: An in-vitro-study. J. Biophotonics 2016, 9, 637–644. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Yu, Q.; Li, H.; Lin, M.; Mustapha, A.; Hong, L.; Wang, Y. Oral Bacterial Deactivation Using a Low-Temperature Atmospheric Argon Plasma Brush. J. Dent. 2011, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Dong, X.; Chen, M.; Sun, H.; Hong, L.; Wang, Y.; Li, H.; Yu, Q. An in vitro and in vivo study of plasma treatment effects on oral biofilms. J. Oral Microbiol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Kovalová, Z.; Zahoran, M.; Zahoranová, A.; Machala, Z. Streptococci biofilm decontamination on teeth by low-temperature air plasma of dc corona discharges. J. Phys. D Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Liguori, A.; Cochis, A.; Stancampiano, A.; Laurita, R.; Azzimonti, B.; Sorrentino, R.; Varoni, E.M.; Petri, M.; Colombo, V.; Gherardi, M.; et al. Cold atmospheric plasma treatment affects early bacterial adhesion and decontamination of soft reline palatal obturators. Clin. Plasma Med. 2017, 7–8, 36–45. [Google Scholar] [CrossRef]
- Molnar, I.; Papp, J.; Simon, A.; Anghel, S.D. Deactivation of Streptococcus mutans biofilms on a tooth surface using He dielectric barrier discharge at atmospheric pressure. Plasma Sci. Technol. 2013, 15, 535–541. [Google Scholar] [CrossRef][Green Version]
- Rupf, S.; Lehmann, A.; Hannig, M.; Schäfer, B.; Schubert, A.; Feldmann, U.; Schindler, A. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J. Med. Microbiol. 2010, 59, 206–212. [Google Scholar] [CrossRef]
- Yoo, E.-M.; Choi, Y.-R.; Kang, M.-K. Antimicrobial Efficacy of Nitrogen-Based Non-thermal Atmospheric Pressure Plasma Jet on Dental Biofilm. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 1541–1547. [Google Scholar] [CrossRef]
- Ballout, H.; Hertel, M.; Doehring, J.; Kostka, E.; Hartwig, S.; Paris, S.; Preissner, S. Effects of plasma jet, dielectric barrier discharge, photodynamic therapy and sodium hypochlorite on infected curved root canals. J. Biophotonics 2018, 11. [Google Scholar] [CrossRef]
- Hüfner, A.; Steffen, H.; Holtfreter, B.; Schlüter, R.; Duske, K.; Matthes, R.; von Woedtke, T.; Weltmann, K.-D.; Kocher, T.; Jablonowski, L. Effects of Non-Thermal Atmospheric Pressure Plasma and Sodium Hypochlorite Solution on Enterococcus faecalis Biofilm: An Investigation in Extracted Teeth. Plasma Process. Polym. 2017, 14. [Google Scholar] [CrossRef]
- Herbst, S.R.; Hertel, M.; Ballout, H.; Pierdzioch, P.; Weltmann, K.-D.; Wirtz, H.C.; Abu-Sirhan, S.; Kostka, E.; Paris, S.; Preissner, S. Bactericidal Efficacy of Cold Plasma at Different Depths of Infected Root Canals In Vitro. Open Dent. J. 2015, 9, 486–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habib, M.; Hottel, T.L.; Hong, L. Antimicrobial effects of non-thermal atmospheric plasma as a novel root canal disinfectant. Clin. Plasma Med. 2014, 2, 17–21. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Ye, G.; Liang, Y.; Pan, H.; Wang, G.; Zhao, Y.; Pan, J.; Zhang, J.; Fang, J. Evaluation of Cold Plasma Treatment and Safety in Disinfecting 3-week Root Canal Enterococcus faecalis Biofilm in Vitro. J. Endod. 2015, 41, 1325–1330. [Google Scholar] [CrossRef]
- Pan, J.; Sun, K.; Liang, Y.; Sun, P.; Yang, X.; Wang, J.; Zhang, J.; Zhu, W.; Fang, J.; Becker, K.H. Cold plasma therapy of a tooth root canal infected with enterococcus faecalis biofilms in vitro. J. Endod. 2013, 39, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, H.; Sun, P.; Wu, H.; Pan, J.; Zhu, W.; Zhang, J.; Fang, J. The effect of an atmospheric pressure, DC nonthermal plasma microjet on tooth Root Canal, dentinal tubules infection and reinfection prevention. Plasma Med. 2011, 1, 143–155. [Google Scholar] [CrossRef]
- Chen, W.; Huang, J.; Du, N.; Liu, X.-D.; Lv, G.-H.; Wang, X.-Q.; Zhang, G.-P.; Guo, L.-H.; Yang, S.-Z. Deactivation of enterococcus faecalis bacteria by an atmospheric cold plasma brush. Chin. Phys. Lett. 2012, 29. [Google Scholar] [CrossRef]
- Chen, W.; Huang, J.; Du, N.; Liu, X.-D.; Wang, X.-Q.; Lv, G.-H.; Zhang, G.-P.; Guo, L.-H.; Yang, S.-Z. Treatment of enterococcus faecalis bacteria by a helium atmospheric cold plasma brush with oxygen addition. J. Appl. Phys. 2012, 112. [Google Scholar] [CrossRef]
- Armand, A.; Khani, M.; Asnaashari, M.; AliAhmadi, A.; Shokri, B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagn. Photodyn. 2019, 26, 327–333. [Google Scholar] [CrossRef]
- Simoncelli, E.; Barbieri, D.; Laurita, R.; Liguori, A.; Stancampiano, A.; Viola, L.; Tonini, R.; Gherardi, M.; Colombo, V. Preliminary investigation of the antibacterial efficacy of a handheld Plasma Gun source for endodontic procedures. Clin. Plasma Med. 2015, 3, 77–86. [Google Scholar] [CrossRef]
- Jiang, C.; Schaudinn, C.; Jaramillo, D.E.; Webster, P.; Costerton, J.W. In Vitro Antimicrobial Effect of a Cold Plasma Jet against Enterococcus faecalis Biofilms. ISRN Dent. 2012, 2012. [Google Scholar] [CrossRef]
- Du, T.; Ma, J.; Yang, P.; Xiong, Z.; Lu, X.; Cao, Y. Evaluation of antibacterial effects by atmospheric pressure nonequilibrium plasmas against enterococcus faecalis biofilms in vitro. J. Endod. 2012, 38, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Schaudinn, C.; Jaramillo, D.; Freire, M.O.; Sedghizadeh, P.P.; Nguyen, A.; Webster, P.; Costerton, J.W.; Jiang, C. Evaluation of a non-thermal plasma needle to eliminate ex vivo biofilms in root canals of extracted human teeth. Int. Endod. J. 2013, 46, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cao, Y.; Yang, P.; Xiong, Q.; Xiong, Z.; Xian, Y.; Pan, Y. An RC plasma device for sterilization of root canal of teeth. IEEE Trans. Plasma Sci. 2009, 37, 668–673. [Google Scholar] [CrossRef]
- Üreyen Kaya, B.; Kececi, A.D.; Güldas, H.E.; Cetin, E.S.; Öztürk, T.; Öksuz, L.; Bozduman, F. Efficacy of endodontic applications of ozone and low-temperature atmospheric pressure plasma on root canals infected with Enterococcus faecalis. Lett. Appl. Microbiol. 2014, 58, 8–15. [Google Scholar] [CrossRef]
- Zhou, X.; Xiong, Z.; Cao, Y.; Lu, X.; Liu, D. The antimicrobial activity of an atmospheric-pressure room-temperature plasma in a simulated root-canal model infected with enterococcus faecalis. IEEE Trans. Plasma Sci. 2010, 38, 3370–3374. [Google Scholar] [CrossRef]
- Zhou, X.-C.; Li, Y.-L.; Liu, D.-X.; Cao, Y.-G.; Lu, X.-P. Bactericidal effect of plasma jet with helium flowing through 3% hydrogen peroxide against Enterococcus faecalis. Exp. Med. 2016, 12, 3073–3077. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, P.; Lu, X.; Xiong, Z.; Ye, T.; Xiong, Q.; Sun, Z. Efficacy of atmospheric pressure plasma as an antibacterial agent against enterococcus faecalis in vitro. Plasma Sci. Technol. 2011, 13. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Chen, G. Oral bacterial inactivation using a novel low-temperature atmospheric-pressure plasma device. J. Dent. Sci. 2016, 11, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Theinkom, F.; Singer, L.; Cieplik, F.; Cantzler, S.; Weilemann, H.; Cantzler, M.; Hiller, K.-A.; Maisch, T.; Zimmermann, J.L. Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. PLoS ONE 2019, 14, e0223925. [Google Scholar] [CrossRef]
- Annunziata, M.; Canullo, L.; Donnarumma, G.; Caputo, P.; Nastri, L.; Guida, L. Bacterial inactivation/sterilization by argon plasma treatment on contaminated titanium implant surfaces:In vitro study. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e118-21. [Google Scholar] [CrossRef]
- Carreiro, A.F.P.; Delben, J.A.; Guedes, S.; Silveira, E.J.D.; Janal, M.N.; Vergani, C.E.; Pushalkar, S.; Duarte, S. Low-temperature plasma on peri-implantÔÇôrelated biofilm and gingival tissue. J. Periodontol. 2019, 90, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.; Zhou, X.; Liu, Z.; Wang, C.; Wang, K.; Zhang, J.; Wang, Z. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: An in vitro study. Dent. Mater. J. 2018, 37, 157–166. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, K.-H.; Park, S.-Y.; Yoon, S.-Y.; Kim, G.-H.; Lee, Y.-M.; Rhyu, I.-C.; Seol, Y.-J. The bactericidal effect of an atmospheric-pressure plasma jet on Porphyromonas gingivalis biofilms on sandblasted and acid-etched titanium discs. J. Periodontal Implant Sci. 2019, 49, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Handorf, O.; Weihe, T.; Bekeschus, S.; Graf, A.C.; Schnabel, U.; Riedel, K.; Ehlbeck, J. Nonthermal Plasma Jet Treatment Negatively Affects the Viability and Structure of Candida albicans SC5314 Biofilms. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Matthes, R.; Jablonowski, L.; Koban, I.; Quade, A.; Hübner, N.-O.; Schlueter, R.; Weltmann, K.-D.; von Woedtke, T.; Kramer, A.; Kocher, T. In vitro treatment of Candida albicans biofilms on denture base material with volume dielectric barrier discharge plasma (VDBD) compared with common chemical antiseptics. Clin. Oral Investig. 2015, 19, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Delben, J.A.; Zago, C.E.; Tyhovych, N.; Duarte, S.; Vergani, C.E. Effect of Atmospheric-Pressure Cold Plasma on Pathogenic Oral Biofilms and In Vitro Reconstituted Oral Epithelium. PLoS ONE 2016, 11, e0155427. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.C.O.C.; Figueira, F.R.; de Lima, J.S.B.; Figueira, J.A.N.; Castro, A.H.R.; Sismanoglu, B.N.; Petraconi, G.; Maciel, H.S.; Khouri, S.; Pessoa, R.S. Inactivation of Candida albicans biofilms by atmospheric gliding arc plasma jet: Effect of gas chemistry/flow and plasma pulsing. Plasma Res. Express 2019, 1. [Google Scholar] [CrossRef]
- Wanachantararak, P.; Suanpoot, P.; Nisoa, M. Inhibitory activity of cold atmospheric plasma on Candida albicans. Walailak J. Sci. Technol. 2019, 16, 401–408. [Google Scholar] [CrossRef]
- Kerlikowski, A.; Matthes, R.; Pink, C.; Steffen, H.; Schlüter, R.; Holtfreter, B.; Weltmann, K.-D.; von Woedtke, T.; Kocher, T.; Jablonowski, L. Effects of cold atmospheric pressure plasma and disinfecting agents on Candida albicans in root canals of extracted human teeth. J. Biophotonics 2020. [Google Scholar] [CrossRef]
- Wang, G.M.; Sun, P.P.; Pan, H.; Ye, G.P.; Sun, K.; Zhang, J.; Pan, J.; Fang, J. Inactivation of Candida albicans Biofilms on Polymethyl Methacrylate and Enhancement of the Drug Susceptibility by Cold Ar/O2 Plasma Jet. Plasma Chem. Plasma Process. 2016, 36, 383–396. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Isbary, G.; Heinlin, J.; Karrer, S.; Klämpfl, T.G.; Li, Y.-F.; Morfill, G.; Zimmermann, J.L. Contact-Free Inactivation of Candida albicans Biofilms by Cold Atmospheric Air Plasma. Appl. Environ. Microbiol. 2012, 78, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-M.; Seo, H.-Y.; Choi, Y.-R.; Kang, M.-K. Inhibition of C. albicans formation by non-thermal atmospheric pressure plasma jet (NTAPPJ) on acrylic resin surface. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 231–238. [Google Scholar] [CrossRef]
- Chiodi Borges, A.; Castaldelli Nishime, T.M.; Kostov, K.G.; de Morais Gouvêa Lima, G.; Lacerda Gontijo, A.V.; de Carvalho, J.N.M.M.; Yzumi Honda, R.; Koga-Ito, C.Y. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Clin. Plasma Med. 2017, 7–8, 9–15. [Google Scholar] [CrossRef]
- Song, Y.; Liu, D.; Ji, L.; Wang, W.; Niu, J.; Zhang, X. Plasma inactivation of Candida albicans by an atmospheric cold plasma brush composed of hollow fibers. IEEE Trans. Plasma Sci. 2012, 40, 1098–1102. [Google Scholar] [CrossRef]
- He, M.; Duan, J.; Xu, J.; Ma, M.; Chai, B.; He, G.; Gan, L.; Zhang, S.; Duan, X.; Lu, X.; et al. Candida albicans biofilm inactivated by cold plasma treatment in vitro and in vivo. Plasma Process. Polym. 2020, 17. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, S.; Sun, P.; Wu, H.; Zhu, W.; Liu, W.; Zhang, J.; Fang, J.; Li, R. Inactivation of Candida Biofilms by Non-Thermal Plasma and Its Enhancement for Fungistatic Effect of Antifungal Drugs. PLoS ONE 2012, 7, e40629. [Google Scholar] [CrossRef] [PubMed]
- Koban, I.; Matthes, R.; Hübner, N.-O.; Welk, A.; Meisel, P.; Holtfreter, B.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Kramer, A.; et al. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J. Phys. 2010, 12. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 864–870. [Google Scholar] [CrossRef]
- Al-rawaf, A.F.; Fuliful, F.K.; Khalaf, M.K.; Oudah, H.K. Studying the non-thermal plasma jet characteristics and application on bacterial decontamination. J. Appl. Phys. 2018, 12, 45–51. [Google Scholar] [CrossRef]
- Deng, X.; Shi, J.; Kong, M.G. Physical Mechanisms of Inactivation of Bacillus subtilis Spores Using Cold Atmospheric Plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]
- Bisag, A.; Manzini, M.; Simoncelli, E.; Stancampiano, A.; Tonini, R.; Gherardi, M.; Colombo, V. Cold atmospheric pressure plasma treatment to assist the restoration of the apical region of a root canal in endodontic procedures. Clin. Plasma Med. 2020, 19–20, 100100. [Google Scholar] [CrossRef]
- Lemos, J.A.; Quivey, R.G.; Koo, H.; Abranches, J. Streptococcus mutans: A new Gram-positive paradigm? Microbiology 2013, 159, 436–445. [Google Scholar] [CrossRef]
- Colombo, V.; Forgione, D.; Gherardi, M.; Laurita, R.; Simoncelli, E.; Nassar, M.; Stancampiano, A.; Tonini, R. Cold atmospheric pressure plasma treatment to improve the bonding strength of dentin-adhesive system interface in dental composite restoration. J. Appl. Biomater. Funct. Mater. 2017, 15, e280. [Google Scholar] [CrossRef][Green Version]
- Dong, X.; Chen, M.; Wang, Y.; Yu, Q. A Mechanistic study of Plasma Treatment Effects on Demineralized Dentin Surfaces for Improved Adhesive/Dentin Interface Bonding. Clin. Plasma Med. 2014, 2, 11–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.-M.; Zhou, J.-F.; Guo, H.; Zhang, X.-F.; Liu, X.-Q.; Li, H.-P.; Tan, J.-G. Effects of a modified cold atmospheric plasma jet treatment on resin-dentin bonding. Dent. Mater. J. 2018, 37, 798–804. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.Y.; Lee, H.J.; Kim, J.B.; Kim, S.; Joo, J.Y.; Kim, G.C. Retention Improvement in Fluoride Application with Cold Atmospheric Plasma. J. Dent. Res. 2018, 97, 179–183. [Google Scholar] [CrossRef] [PubMed]
- El-Wassefy, N.A. Remineralizing effect of cold plasma and/or bioglass on demineralized enamel. Dent. Mater. J. 2017, 36, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Das, J.; Foley, I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997, 11, 160–167. [Google Scholar] [CrossRef]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms With Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, S.A.; Varfolomeev, A.F.; Chernukha, M.Y.; Yurov, D.S.; Vasiliev, M.M.; Kaminskaya, A.A.; Moisenovich, M.M.; Romanova, J.M.; Murashev, A.N.; Selezneva, I.I.; et al. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med. Microbiol. 2011, 60, 75–83. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Henry, L.G.; McKenzie, R.M.E.; Robles, A.; Fletcher, H.M. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol. 2012, 7, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Barbara Nebe, J.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Matthes, R.; Duske, K.; Kebede, T.G.; Pink, C.; Schlüter, R.; von Woedtke, T.; Weltmann, K.-D.; Kocher, T.; Jablonowski, L. Osteoblast growth, after cleaning of biofilm-covered titanium discs with air-polishing and cold plasma. J. Clin. Periodontol. 2017, 44, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Keim, D.; Nickles, K.; Dannewitz, B.; Ratka, C.; Eickholz, P.; Petsos, H. In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin. Oral Implant. Res. 2019. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant Dent. 2015, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Song, K.; Zhou, X.; Xiong, Z.; Du, T.; Lu, X.; Cao, Y. Effects of non-equilibrium plasma in the treatment of ligature-induced peri-implantitis. J. Clin. Periodontol. 2015, 42, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Küçük, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Sağlam, M.; Köseoğlu, S. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef] [PubMed]
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.-F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.-U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.; Viola, K.S.; Coaguila-Llerena, H.; Oliveira, L.R.A.; Leonardo, R.T.; Aranda-García, A.J.; Guerreiro-Tanomaru, J.M. Penetration of sodium hypochlorite into root canal dentine: Effect of surfactants, gel form and passive ultrasonic irrigation. Int. Endod. J. 2019, 52, 385–392. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.; Aminsobhani, M.; Sohrabi, K.; Chiniforush, N.; Ghafari, S.; Shamshiri, A.R.; Noroozi, N. Penetration Depth of Sodium Hypochlorite in Dentinal Tubules after Conventional Irrigation, Passive Ultrasonic Agitation and Nd:YAG Laser Activated Irrigation. J. Lasers Med. Sci. 2016, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.S.; Cheung, G.S.P. Extension of bactericidal effect of sodium hypochlorite into dentinal tubules. J. Endod. 2014, 40, 825–829. [Google Scholar] [CrossRef]
- Zou, L.; Shen, Y.; Li, W.; Haapasalo, M. Penetration of sodium hypochlorite into dentin. J. Endod. 2010, 36, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Sky Driver, M.; Caruso, A.N.; Yu, Q.; Wang, Y. Surface modification of several dental substrates by non-thermal, atmospheric plasma brush. Dent. Mater. 2013, 29, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Koban, I.; Geisel, M.H.; Holtfreter, B.; Jablonowski, L.; Hübner, N.-O.; Matthes, R.; Masur, K.; Weltmann, K.-D.; Kramer, A.; Kocher, T. Synergistic Effects of Nonthermal Plasma and Disinfecting Agents against Dental Biofilms In Vitro. ISRN Dent. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Abreu, J.L.B.D.; Prado, M.; Simão, R.A.; Silva, E.M.D.; Dias, K.R.H.C. Effect of Non-Thermal Argon Plasma on Bond Strength of a Self-Etch Adhesive System to NaOCl-Treated Dentin. Braz. Dent. J. 2016, 27, 446–451. [Google Scholar] [CrossRef]
| Critical Steps of the in Vitro Protocol | Justification | Items | Points Attributed According to the Response for Each Critical Step |
|---|---|---|---|
| Preparation of micro-organisms and plasma device | Scientific robustness | 1. Preparation of microorganisms | 1 if described 0 if not described |
| 2. Technical data of plasma generator | 2 if at least 3 parameters described or commercially device 1 if at least 1 parameter described 0 if not described | ||
| Inoculation (Inoculum size) | Real inoculum size essential for the calculation of logarithmic reduction | 3. Experimental size presented | 2 for theoretical + true inoculum sizes 1 for theoretical inoculum size 0 if not described |
| Test conditions | Scientific robustness | 4. Experimental temperature | 1 if described 0 if not described or over 47 °C |
| 5. Protection of samples | 1 if described 0 if not described | ||
| Micro-organisms recovery | Impacts the results if not all micro-organisms are recovered, overestimated effect Scientific robustness | 6. Micro-organisms recovery | 1 if other method with mechanic action and validated with a test 0 if not clearly described or technic not validated |
| Microbial culture after treatment | Impacts the results if the growth duration is too short Scientific robustness | 7. Time, temperature and method indicated | 1 if described 0 if not or poorly described |
| 8. Culture media | 1 if described 0 if not described | ||
| Statistical analysis/tests repeatability | Scientific robustness | 9. Number of experiments | 1 if described with more than one experiment 0 if not described or described with onlyone experiment |
| 10. Statistical method (to compare differences) | 1 if described 0 if not described | ||
| Conflict of Interest | Bias | 11. Declaration | 1 if declared 0 if not declared |
| Gas | Author | Device | Plasma Mode | Distance (mm) | Surface | Biofilm | Species | Max Red t ≤ 60 s | Max Red t ≤ 120 s | Max Red t ≤ 300 s | Max Red t ≤ 600 s | Max Red t > 600 s | Additional |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | S. mutans | ns (1.4) | N/A | N/A | N/A | N/A | |
| self-constr. | jet | 20 | Agar | No | S. mutans | ns (1.6) | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 2 | Agar | No | L. fermentum | ns (1.1) | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 20 | Agar | No | L. fermentum | ns (0.7) | N/A | N/A | N/A | N/A | |||
| Abu-Sirhan et al. 2016 [20] | kINPen med | jet | 8 | bone (porcine) | Yes | S. mitis | ns (0.2) | N/A | N/A | N/A | N/A | ||
| Blumhagen et al. 2014 [21] | self-constr. | brush | 5 | HA discs | No | L. acidophilus | 1.7 | N/A | N/A | N/A | N/A | high conc. | |
| self-constr. | brush | 5 | HA discs | No | L. acidophilus | nd | N/A | N/A | N/A | N/A | med. conc., nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | No | L. acidophilus | nd | N/A | N/A | N/A | N/A | low conc., nd after 10 s | ||
| self-constr. | brush | 5 | HA discs | No | L. acidophilus | nd | N/A | N/A | N/A | N/A | nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | No | L. acidophilus | nd | N/A | N/A | N/A | N/A | nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | No | L. acidophilus | nd | N/A | N/A | N/A | N/A | nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | No | S. mutans | 2.6 | N/A | N/A | N/A | N/A | high conc. | ||
| self-constr. | brush | 5 | HA discs | No | S. mutans | nd | N/A | N/A | N/A | N/A | med. conc., nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | No | S. mutans | nd | N/A | N/A | N/A | N/A | low conc., nd after 6 s | ||
| self-constr. | brush | 5 | HA discs | No | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | No | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | No | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 17 s | ||
| Gorynia et al. 2013 [22] | kinpen 09 | jet | 10 | Ti discs | Yes | S. sanguinis | ns (0.1) | ns (0.3) | nd (0.5) | N/A | N/A | ||
| Hertel et al. 2018 [23] | kINPen med | jet | 8 | Dentin | Yes | L. rhamnosus | 1.1 | N/A | N/A | N/A | N/A | ||
| PlasmaDerm | VDBD | close contact | Dentin | Yes | L. rhamnosus | 0.6 | N/A | N/A | N/A | N/A | |||
| Huang et al. 2013 [24] | self-constr. | jet | 3 | Glass | No | S. mutans | 2.7 | 3.1 | nd | N/A | N/A | ||
| self-constr. | jet | 3 | Glass | No | S. mutans | 1.5 | 2.5 | 4.8 | N/A | N/A | |||
| Koban et al. 2011 [25] | kINPen 09 | jet | 7 | Ti discs | Yes | S. mutans | 3.1 | 2.8 | 3 | 3 | N/A | ||
| HDBD | 5 | Ti discs | Yes | S. mutans | 1 | 1.2 | 1.7 | 1.7 | N/A | ||||
| VDBD | 15 | Ti discs | Yes | S. mutans | 2 | 3.3 | 5.1 | 5.8 | N/A | ||||
| Park et al. 2014 [26] | self-constr. | jet | 8 | Glass | No | S. mutans | 3 | 3.8 | 5.3 | N/A | N/A | ||
| self-constr. | jet | 8 | Tooth | No | S. mutans | 3 | 3 | 3.4 | N/A | N/A | |||
| Preissner et al. 2016 [27] | kINPen MED | jet | 8 | Ti implants | Yes | S. mitis | 2.2 | 1.9 | N/A | N/A | N/A | ||
| Yang et al. 2011 [28] | self-constr. | brush | Filter paper | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 11 s | ||
| self-constr. | brush | Filter paper | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 9 s | |||
| self-constr. | brush | Filter paper | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 15 s | |||
| self-constr. | brush | Glass slide | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 15 s | |||
| self-constr. | brush | PTFE | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | nd after 13 s | |||
| self-constr. | brush | PTFE | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | 10% density, nd after 5 s | |||
| self-constr. | brush | PTFE | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | 1% density, nd after 3 s | |||
| self-constr. | brush | Filter paper | Yes | L. acidophilus | 2.2 | 3.9 | nd | N/A | N/A | ||||
| self-constr. | brush | Filter paper | Yes | L. acidophilus | 3.7 | 4.3 | nd | N/A | N/A | nd after 210 s | |||
| self-constr. | brush | Filter paper | Yes | L. acidophilus | 1.9 | 3.2 | nd | N/A | N/A | nd after 300 s | |||
| self-constr. | brush | Glass slide | Yes | L. acidophilus | 1.4 | 2.4 | nd | N/A | N/A | ||||
| self-constr. | brush | PTFE | Yes | L. acidophilus | 2 | 3 | nd | N/A | N/A | ||||
| self-constr. | brush | PTFE | Yes | L. acidophilus | 3.9 | nd | N/A | N/A | N/A | 10% density, nd after 90 s | |||
| self-constr. | brush | PTFE | Yes | L. acidophilus | nd | N/A | N/A | N/A | N/A | 1% density | |||
| Ar + O2 | Blumhagen et al. 2014 [21] | self-constr. | brush | 5 | HA discs | Yes | L. acidophilus | nd | N/A | N/A | N/A | N/A | Ar/O2: 60:1, nd after 13 s |
| self-constr. | brush | 5 | HA discs | Yes | L. acidophilus | nd | N/A | N/A | N/A | N/A | Ar/O2: 6:1, nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | Yes | L. acidophilus | nd | N/A | N/A | N/A | N/A | Ar/O2: 1:2, nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | Ar/O2: 300:5, nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | Ar/O2: 6:1, nd after 13 s | ||
| self-constr. | brush | 5 | HA discs | Yes | S. mutans | nd | N/A | N/A | N/A | N/A | Ar/O2: 1:2, nd after 17 s | ||
| Hong et al. 2019 [29] | self-constr. | brush | Steel wafers | Yes | S. mutans | 0.3 | 0.5 | N/A | N/A | N/A | Ar/O2: 100:1 | ||
| Koban et al. 2011 [25] | kINPen 09 | jet | 7 | Ti discs | Yes | Saliva | ns (0.3) | ns (0.5) | ns (0.4) | N/A | ns (0.7) | Ar/O2: 100:1 | |
| kINPen 09 | jet | 7 | Ti discs | Yes | S. mutans | 2 | 1.9 | 2.1 | N/A | 1.8 | Ar/O2: 100:1 | ||
| HDBD | 5 | Ti discs | Yes | Saliva | ns (0.5) | 1.4 | 3.1 | N/A | 2.2 | Ar/O2: 100:1 | |||
| HDBD | 5 | Ti discs | Yes | S. mutans | ns (0) | 0.9 | 3 | N/A | 3.7 | Ar/O2: 100:1 | |||
| Air | Hertel et al. 2018 [23] | PlasmaDerm | VDBD | 0 | Dentin | Yes | L. rhamnosus | 0.6 | N/A | N/A | N/A | N/A | |
| Kovalova et al. 2014 [30] | self-constr. | pos. CD | 5 | Teeth | Yes | Streptococci | N/A | 0.9 | 1 | 2.6 | N/A | ||
| self-constr. | neg. CD | 5 | Teeth | Yes | Streptococci | N/A | 0.8 | 1.3 | 2.4 | N/A | |||
| Liguori et al. 2017 [31] | self-constr. | DBD-Rod | 3 | Well Plate | No | S. mutans | 2.1 | 2.9 | N/A | N/A | N/A | ||
| self-constr. | DBD-Plate | 3 | Well Plate | No | S. mutans | 1.8 | 2.8 | N/A | N/A | N/A | |||
| self-constr. | DBD-Rod | 3 | Well Plate | Yes | S. mutans | 0.8 | 1.8 | N/A | N/A | N/A | |||
| self-constr. | DBD-Plate | 3 | Well Plate | Yes | S. mutans | 0.6 | 1.6 | N/A | N/A | N/A | |||
| He | Molnar et al. 2013 [32] | self-constr. | DBD | 2 | Tooth Slices | Yes | S. mutans | 1.3 | nd | N/A | N/A | N/A | high conc. |
| self-constr. | DBD | 2 | Tooth Slices | Yes | S. mutans | 2.4 | nd | N/A | N/A | N/A | med. conc. | ||
| self-constr. | DBD | 2 | Tooth Slices | Yes | S. mutans | 2.3 | nd | N/A | N/A | N/A | low conc. | ||
| He + O2 + N2 | Rupf et al. 2010 [33] | self-constr. | jet | 1.5 | Agar | Yes | S. mutans | 5.8 | 8.4 | N/A | N/A | N/A | He/O2/N2: 2:1.2:1.5 |
| self-constr. | jet | 1.5 | Dentin slice | No | S. mutans | 4 | N/A | N/A | N/A | N/A | He/O2/N2: 2:1.2:1.5 | ||
| self-constr. | jet | 1.5 | Agar | Yes | L. casei | nd | nd | N/A | N/A | N/A | He/O2/N2: 2:1.2:1.5 | ||
| self-constr. | jet | 1.5 | Dentin slice | No | L. casei | 4.5 | N/A | N/A | N/A | N/A | He/O2/N2: 2:1.2:1.5 | ||
| N2 | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | S. mutans | 6.2 | N/A | N/A | N/A | N/A | |
| self-constr. | jet | 20 | Agar | No | S. mutans | 7 | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 2 | Agar | No | L. fermentum | 1.8 | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 20 | Agar | No | L. fermentum | 2.3 | N/A | N/A | N/A | N/A | |||
| Yoo et al. 2020 [34] | self-constr. | jet | HA discs | Yes | S. mutans | 0.6 | 1 | N/A | N/A | N/A | |||
| O2 | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | S. mutans | nd | N/A | N/A | N/A | N/A | |
| self-constr. | jet | 20 | Agar | No | S. mutans | nd | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 2 | Agar | No | L. fermentum | 2.4 | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 20 | Agar | No | L. fermentum | nd | N/A | N/A | N/A | N/A | |||
| O2 + N2 | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | S. mutans | 5.2 | N/A | N/A | N/A | N/A | |
| self-constr. | jet | 20 | Agar | No | S. mutans | 6.8 | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 2 | Agar | No | L. fermentum | ns (1.7) | N/A | N/A | N/A | N/A | |||
| self-constr. | jet | 20 | Agar | No | L. fermentum | 2.3 | N/A | N/A | N/A | N/A |
| Gas | Author | Device | Plasma mode | Distance (mm) | Surface | Biofilm | Species | Max Red t ≤ 60 s | Max Red t ≤ 120 s | Max Red t ≤ 300 s | Max Red t ≤ 600 s | Max Red t > 600 s | Additional |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | Ballout et al. 2018 [35] | kINPen med | jet | 3 | Root canals | Yes | E. faecalis | 0.5 | N/A | N/A | N/A | N/A | |
| Hüfner et al. 2017 [36] | kINPen 08 | jet | 3 | Root canals | Yes | E. faecalis | N/A | N/A | N/A | 1.5 | 1.8 | ||
| Herbst et al. 2015 [37] | kINPen MED | jet | 3 | Root canals | Yes | E. faecalis | 3.2 | N/A | N/A | N/A | N/A | ||
| Ar + O2 | Hüfner et al. 2017 [36] | kINPen 08 | jet | 3 | Root canals | Yes | E. faecalis | N/A | N/A | N/A | 0.5 | 1.9 | Ar/O2: 100:1 |
| Habib et al. 2014 [38] | self-constr. | jet | Root canals | Yes | E. faecalis | N/A | 6.6 | N/A | N/A | N/A | Ar/O2: 1000:1 | ||
| Li et al. 2015 [39] | self-constr. | jet | 10 | Root canals | Yes | E. faecalis | N/A | N/A | 2.3 | 6.3 | nd | Ar/O2: 98:2 | |
| Pan et al. 2013 [40] | self-constr. | jet | 5 | Root canals | Yes | E. faecalis | N/A | 0.6 | 1.2 | nd | N/A | Ar/O2: 98:2 | |
| Wang et al. 2011 [41] | self-constr. | jet | 5 | Root canals | No | E. faecalis | N/A | 0.2 | 0.8 | 2 | N/A | Ar/O2 | |
| He | Chen et al. 2012 [42] | self-constr. | brush | 5 | Filter paper | Yes | E. faecalis | 2.7 | 3 | 4.5 | N/A | N/A | 16 W |
| self-constr. | brush | 5 | Filter paper | Yes | E. faecalis | 3.4 | 4 | nd | N/A | N/A | 20 W | ||
| self-constr. | brush | 5 | Filter paper | Yes | E. faecalis | 4 | nd | nd | N/A | N/A | 24 W | ||
| self-constr. | brush | 5 | Filter paper | Yes | E. faecalis | 5 | nd | nd | N/A | N/A | 28 W | ||
| self-constr. | brush | 5 | Filter paper | Yes | E. faecalis | nd | nd | nd | N/A | N/A | 32 W | ||
| Chen et al. 2012 [43] | self-constr. | brush | 5 | Filter paper | E. faecalis | 4 | nd | nd | N/A | N/A | |||
| Armand et al. 2019 [44] | self-constr. | jet | 2 | Root canals | Yes | E. faecalis | N/A | N/A | 1 | 2.1 | N/A | ||
| Simoncelli et al. 2015 [45] | self-constr. | jet | 2 | Root canals | No | E. faecalis | 0.2 | N/A | 4 | N/A | N/A | ||
| He + O2 | Jiang et al. 2012 [46] | self-constr. | jet | 10 | HA discs | Yes | E. faecalis | N/A | N/A | 1.2 | N/A | N/A | He/O2: 100:1 |
| Chen et al. 2012 [43] | self-constr. | brush | 5 | Filter paper | E. faecalis | 4.8 | nd | nd | N/A | N/A | He + 1% O2 | ||
| self-constr. | brush | 5 | Filter paper | E. faecalis | nd | nd | nd | N/A | N/A | He + 2.5% O2 | |||
| self-constr. | brush | 5 | Filter paper | E. faecalis | 3.7 | 5.2 | nd | N/A | N/A | He+ 5% O2 | |||
| self-constr. | brush | 5 | Filter paper | E. faecalis | 2.9 | 3 | 3.7 | N/A | N/A | He + 10% O2 | |||
| Armand et al. 2019 [44] | self-constr. | jet | 2 | Root canals | Yes | E. faecalis | N/A | N/A | 1.4 | 5.1 | N/A | He/O2: 200:1 | |
| Du et al. 2012 [47] | self-constr. | jet | 5 | Root canals | Yes | E. faecalis | N/A | N/A | 0.9 | 2.8 | 3.2 | He/O2: 100:1 | |
| Schaudinn et al. 2013 [48] | self-constr. | jet | Root canals | Yes | ex vivo | N/A | N/A | N/A | N/A | 1 | He/O2: 99:1 | ||
| Lu et al. 2009 [49] | self-constr. | jet | 2 | Root canals | Yes | E. faecalis | N/A | N/A | N/A | N/A | 2 | He/O2: 80:20 | |
| Üreyen et al. 2014 [50] | self-constr. | DBD | −1 | Root canals | Yes | E. faecalis | N/A | N/A | 3.1 | N/A | N/A | He/O2: 96:4 | |
| self-constr. | DBD | −1 | Root canals | Yes | E. faecalis | N/A | N/A | 3.2 | N/A | N/A | He/O2: 96:4 | ||
| self-constr. | DBD | −1 | Root canals | Yes | E. faecalis | N/A | N/A | nd | N/A | N/A | He/O2: 96:4 | ||
| self-constr. | DBD | −1 | Root canals | Yes | E. faecalis | N/A | N/A | 3.4 | N/A | N/A | He/O2: 96:4 | ||
| Zhou et al. 2010 [51] | self-constr. | jet | 0 | Root canals | Yes | E. faecalis | N/A | N/A | 1.7 | 2.2 | 4.5 | He/O2: 100:1 | |
| self-constr. | jet | 0 | Root canals | Yes | E. faecalis | N/A | N/A | 2.2 | 2.4 | 5.4 | He/O2: 100:1, He through NaOCl | ||
| Zhou et al. 2016 [52] | self-constr. | jet | 0 | Root canals | Yes | E. faecalis | 4.9 | 6.1 | 7 | N/A | N/A | He through H2O2 | |
| Air | Cao et al. 2011 [53] | self-constr. | jet | 10 | Cellulose | Yes | E. faecalis | N/A | N/A | N/A | 2 | N/A | |
| Chang et al. 2016 [54] | self-constr. | DBD | Glass | E. faecalis | N/A | 4 | 4.5 | 5.2 | 5.4 | ||||
| Theinkom et al. 2019 [55] | self-constr. | SMD | 10 | Agar | Yes | E. faecalis | 7.9 | N/A | 8.6 | 9.1 | N/A | ||
| self-constr. | SMD | 10 | Petri dish | Yes | E. faecalis | 0 | N/A | 3.2 | 5.4 | N/A | |||
| self-constr. | SMD | 10 | Petri dish | Yes | E. faecalis | 1.8 | N/A | 2.6 | 5.7 | N/A | |||
| self-constr. | SMD | 10 | Petri dish | Yes | E. faecalis | 1.7 | N/A | 2.4 | 4.9 | N/A | |||
| Ballout et al. 2018 [35] | Plasma Derm | DBD | 2 | Root canals | Yes | E. faecalis | 0.1 | N/A | N/A | N/A | N/A | ||
| Air + O2 | Zhou et al. 2016 [37] | self-constr. | jet | 0 | Root canals | Yes | E. faecalis | 2.9 | 3.1 | 3.6 | N/A | N/A | air through H2O2 |
| Gas | Author | Device | Plasma mode | Distance (mm) | Surface | Biofilm | Species | Max Red t ≤ 60 s | Max Red t ≤ 120 s | Max Red t ≤ 300 s | Max Red t ≤ 600 s | Max Red t > 600 s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | A. actinomycetemcomitans | ns (0.2) | N/A | N/A | N/A | N/A |
| self-constr. | jet | 20 | Agar | No | A. actinomycetemcomitans | ns (0.3) | N/A | N/A | N/A | N/A | ||
| Annunziata et al. 2016 [56] | Plasma R | DBD | Ti discs | Yes | A. actinomycetemcomitans | N/A | N/A | N/A | N/A | nd | ||
| Carreiro et al. 2019 [57] | kINPen med | jet | 7 | Ti discs | Yes | P. gingivalis | 1.2 | N/A | 1.2 | N/A | N/A | |
| Air | Liguori et al. 2017 [31] | self-constr. | DBD-Rod | 3 | Well Plate | No | A. actinomycetemcomitans | 2.2 | 2.8 | N/A | N/A | N/A |
| self-constr. | DBD-Plate | 3 | Well Plate | No | A. actinomycetemcomitans | 1.7 | 2.8 | N/A | N/A | N/A | ||
| self-constr. | DBD-Rod | 3 | Well Plate | Yes | A. actinomycetemcomitans | 1 | 1.8 | N/A | N/A | N/A | ||
| self-constr. | DBD-Plate | 3 | Well Plate | Yes | A. actinomycetemcomitans | 1.1 | 2 | N/A | N/A | N/A | ||
| Yang et al. 2018 [58] | self-constr. | jet | 15 | Agar | P. gingivalis | N/A | 4 | 4.5 | nd | N/A | ||
| He | Lee et al. 2019 [59] | self-constr. | jet | 30 | Ti discs | Yes | P. gingivalis | N/A | N/A | 1.2 | nd | N/A |
| O2 | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | A. actinomycetemcomitans | 1.9 | N/A | N/A | N/A | N/A |
| self-constr. | jet | 20 | Agar | No | A. actinomycetemcomitans | nd | N/A | N/A | N/A | N/A | ||
| N2 | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | A. actinomycetemcomitans | ns (0.4) | N/A | N/A | N/A | N/A |
| self-constr. | jet | 20 | Agar | No | A. actinomycetemcomitans | 1.6 | N/A | N/A | N/A | N/A | ||
| O2 + N2 | Abonti et al. 2016 [19] | self-constr. | jet | 2 | Agar | No | A. actinomycetemcomitans | 1.2 | N/A | N/A | N/A | N/A |
| self-constr. | jet | 20 | Agar | No | A. actinomycetemcomitans | 1.2 | N/A | N/A | N/A | N/A |
| Gas | Author | Device | Plasma Mode | Distance (mm) | Surface | Biofilm | Species | Max Red t ≤ 60 s | Max Red t ≤ 120 s | Max Red t ≤ 300 s | Max Red t ≤ 600 s | Max Red t > 600 s | Additional |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | Handorf et al. 2018 [60] | kINPen 09 | jet | 18 | Well-plate | Yes | C. albicans | 1 | 1.5 | 2 | N/A | N/A | |
| Koban et al. 2010 [40] | kINPen 09 | jet | 7 | Ti | Yes | C. albicans | 0.4 | 0.5 | 0.5 | 0.3 | N/A | ||
| self-constr. | HDBD | 7 | Ti | Yes | C. albicans | 1.7 | 1.4 | 2 | 2.9 | N/A | |||
| self-constr. | VDBD | 15 | Ti | Yes | C. albicans | 2.3 | 2.2 | 3.5 | 5.2 | N/A | |||
| Matthes et al. 2015 [61] | self-constr. | VDBD | PMMA | Yes | C. albicans | 1.2 | N/A | 2.8 | 4.1 | N/A | |||
| self-constr. | VDBD | PMMA | Yes | C. albicans | N/A | N/A | N/A | ns (0.1) | N/A | ||||
| self-constr. | VDBD | PMMA | Yes | C. albicans | N/A | N/A | N/A | ns (−0.26) | N/A | ||||
| Delben et al. 2016 [62] | kINPen | jet | 10 | Acrylic resin | Yes | C. albicans | 1.7 | N/A | N/A | N/A | N/A | ||
| Doria et al. 2019 [63] | self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.3 | pulsed | ||
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.5 | continous | |||
| Wanachantararak et al. 2019 [64] | self-constr. | jet | 10 | Agar | C. albicans | N/A | N/A | ns (0.1) | 0.4 | 0.5 | |||
| Ar + Air | Doria et al. 2019 [63] | self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.1 | Ar/Air: 1:9, continous | |
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 0.9 | Ar/Air: 1:9, pulsed | |||
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.9 | Ar/Air: 6:4, continous | |||
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.6 | Ar/Air: 6:4, pulsed | |||
| Ar + O2 | Kerlikowski et al. 2020 [65] | kINPen 08 | jet | 1–2 | Root canals | Yes | C. albicans | N/A | N/A | N/A | 2.1 | 2.7 | Ar/O2: 100:1 |
| Koban et al. 2010 [40] | kINPen 09 | jet | 7 | Ti | Yes | C. albicans | 0.6 | 1 | 0.8 | 0.5 | N/A | Ar/O2: 100:1 | |
| self-constr. | HDBD | Ti | Yes | C. albicans | 1.4 | 1.4 | 3 | 3.3 | N/A | Ar/O2: 100:1 | |||
| Matthes et al. 2015 [61] | self-constr. | VDBD | PMMA | Yes | C. albicans | ns (0.6) | N/A | 0.6 | 0.9 | N/A | Ar/O2: 100:1 | ||
| Wang et al. 2016 [66] | self-constr. | jet | 10 | PMMA | Yes | C. albicans | 1.9 | 2.6 | 3 | 5.9 | N/A | Ar/O2: 98:2 | |
| Air | Maisch et al. 2012 [67] | self-constr. | SMD | 6 | Well-plate | No | C. albicans | 5.5 | nd | nd | nd | N/A | |
| self-constr. | SMD | 6 | Well-plate | Yes | C. albicans | 0.1 | 0.7 | 0.6 | nd | N/A | |||
| Yoo et al. 2016 [68] | self-constr. | jet | 3 | PMMA | No | C. albicans | N/A | 1.3 | N/A | N/A | N/A | ||
| He | Chiodi et al. 2017 [69] | self-constr. | jet | 15 | Well-plate | Yes | C. albicans | ns (0.3) | N/A | 1.5 | 1.7 | N/A | |
| Song et al. 2012 [70] | self-constr. | brush | Glass | Yes | C. albicans | 0.5 | 0.9 | 1 | N/A | N/A | |||
| Doria et al. 2019 [63] | self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.1 | continous | ||
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.2 | pulsed | |||
| He + Air | Doria et al. 2019 [63] | self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 2.6 | He/Air: 6:4, continous | |
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1.7 | He/Air: 6:4, puseld | |||
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1 | He/Air: 1:9, continous | |||
| self-constr. | jet | polyurethane | Yes | C. albicans | N/A | N/A | N/A | N/A | 1 | He/Air: 1:9, pulsed | |||
| He + O2 | He et al. 2020 [71] | self-constr. | jet | 10 | Well-plate | Yes | C. albicans | N/A | 0.2 | 0.4 | 0.8 | N/A | He/O2: 99.5:0.5 |
| Song et al. 2012 [70] | self-constr. | brush | Glass | Yes | C. albicans | 0.4 | 0.8 | 1 | N/A | N/A | He + O2 1% | ||
| self-constr. | brush | Glass | Yes | C. albicans | 0.8 | 1.2 | 1.5 | N/A | N/A | He + O2 5% | |||
| self-constr. | brush | Glass | Yes | C. albicans | 0.5 | 1 | 1 | N/A | N/A | He + O2 7% | |||
| Sun et al. 2012 [72] | self-constr. | Well-plate | Yes | C. albicans | nd | N/A | N/A | N/A | N/A | He/O2: 98:2 | |||
| self-constr. | Well-plate | Yes | C. albicans | nd | N/A | N/A | N/A | N/A | He/O2: 98:2 | ||||
| self-constr. | Well-plate | Yes | C. krusei | nd | N/A | N/A | N/A | N/A | He/O2: 98:2 | ||||
| self-constr. | Well-plate | Yes | C. krusei | nd | N/A | N/A | N/A | N/A | He/O2: 98:2 | ||||
| He + O2 + N2 | Rupf et al. 2010 [33] | self-constr. | jet | 1.5 | Agar | Yes | C. albicans | 5 | N/A | N/A | N/A | N/A | He/O2/N2: 2:1.2:1.5 |
| N2 | Yoo et al. 2016 [68] | self-constr. | jet | 3 | PMMA | No | C. albicans | N/A | 1 | N/A | N/A | N/A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. https://doi.org/10.3390/antibiotics10020211
Jungbauer G, Moser D, Müller S, Pfister W, Sculean A, Eick S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics. 2021; 10(2):211. https://doi.org/10.3390/antibiotics10020211
Chicago/Turabian StyleJungbauer, Gert, Dominick Moser, Steffen Müller, Wolfgang Pfister, Anton Sculean, and Sigrun Eick. 2021. "The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies" Antibiotics 10, no. 2: 211. https://doi.org/10.3390/antibiotics10020211
APA StyleJungbauer, G., Moser, D., Müller, S., Pfister, W., Sculean, A., & Eick, S. (2021). The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics, 10(2), 211. https://doi.org/10.3390/antibiotics10020211









