Rapid Methods for Antimicrobial Resistance Diagnostics
Abstract
1. Introduction
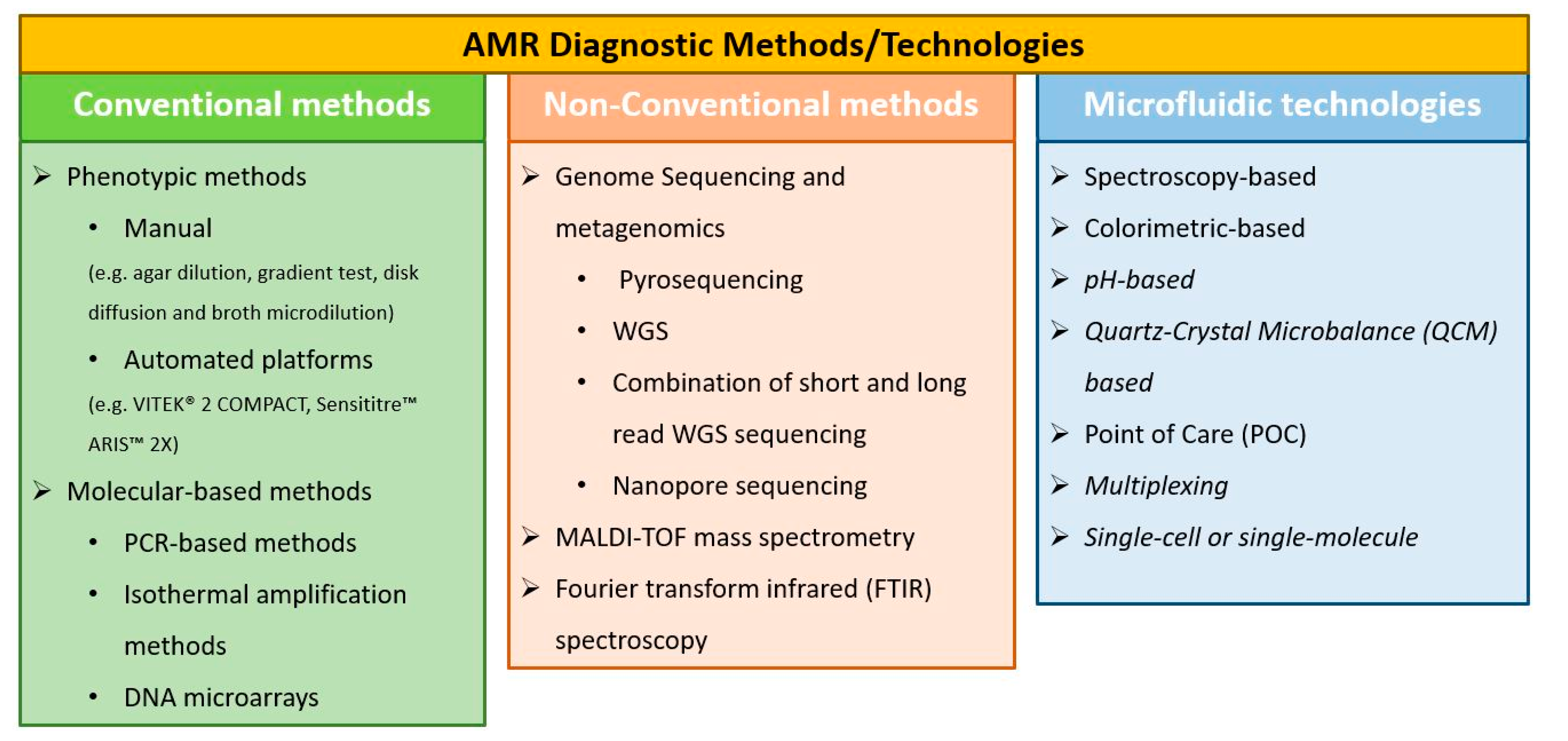
2. Conventional AMR Diagnostic Methods
2.1. Phenotypic Methods
2.2. Molecular-Based Methods
2.2.1. PCR-Based Methods
2.2.2. Isothermal Amplification Methods
2.2.3. DNA Microarrays
3. Non-Conventional AST Methods
3.1. Genome Sequencing and Metagenomics in AMR Diagnostics
3.1.1. Pyrosequencing
3.1.2. WGS
3.1.3. Combination of Short and Long Read WGS Sequencing
3.1.4. Nanopore Sequencing
3.2. MALDI-TOF Mass Spectrometry in AMR Diagnostics
3.3. Fourier Transform Infrared (FTIR) Spectroscopy in AMR Diagnostics
4. Microfluidics and Lab-on-a-Chip Technologies towards Rapid Diagnostics
4.1. Spectroscopy-Based Approaches
4.2. Colorimetric-Based Approaches
4.3. pH-Based Approaches
4.4. Quartz-Crystal Microbalance (QCM)-Based Approaches
4.5. POC Approaches
4.6. Multiplex Approaches
4.7. Single-Cell or Single-Molecule Approaches
5. Overview of Commercially Available AST Platforms
Comparison of Platforms
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM-IR | Atomic Force Microscopy-Infrared Spectroscopy |
| AMR | Antimicrobial Resistance |
| ANN | Artificial Neural Networks |
| ARG | Antimicrobial Resistance Gene |
| AST | Antimicrobial Susceptibility Testing |
| CLSI | Clinical and Laboratory Standards Institute |
| EFSA | European Food Safety Authority |
| ESBL | Extended-spectrum β-lactamase |
| EU | European Union |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| FDA | Food and Drug Administration |
| FTIR | Fourier Transform Infrared |
| FT-RIFS | Fourier Transform Reflectometric Interference Spectroscopy |
| GMO | Genetically Modified Organism |
| HAD | Helicase-Dependent Amplification |
| HT-qPCR | High Throughput Quantitative PCR |
| IR | Infrared |
| IVD | in vitro Diagnostic |
| KPC | Klebsiella pneumoniae Carbapenemase |
| LAMP | Loop-Mediated Isothermal Amplification |
| LoC | Lab-on-a-Chip |
| LoD | limit of detection |
| MALDI-TOF MS | Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry |
| MCA | Morphokinetic Cellular Analysis |
| MDR | Multidrug-Resistant |
| MIC | Minimum Inhibitory Concentration |
| MLST | multi-locus sequence typing |
| MRSA | methicillin resistant S. aureus |
| MSSA | methicillin susceptible S. aureus |
| MZO | Magnesium Zinc Oxide |
| NAAT | Nucleic Acid Amplification Technology |
| NASBA | Nucleic Acid Sequence-Based Amplification |
| NGS | Next-Generation Sequencing |
| PCR | Polymerase Chain Reaction |
| PMF | Peptide Mass Fingerprint |
| POC | Point-of-Care |
| QCM | Quartz-Crystal Microbalance |
| RAA | Residual Antimicrobial Activity |
| RCA | Rolling Circle Amplification |
| RDT | Rapid Diagnostic Test |
| RPA | Recombinase Polymerase Amplification |
| SDA | Strand Displacement Amplification |
| SERS | Surface Enhanced Raman Spectroscopy |
| TAT | Turnaround Time |
| TMA | Transcription Mediated Amplification |
| UTI | Urinary Tract Infection |
| WGS | Whole Genomic Sequencing |
| WHO | World Health Organization |
| WMS | Whole Metagenome Sequencing |
References
- McAdams, D.; Wollein Waldetoft, K.; Tedijanto, C.; Lipsitch, M.; Brown, S.P. Resistance diagnostics as a public health tool to combat antibiotic resistance: A model-based evaluation. PLoS Biol. 2019, 17, e3000250. [Google Scholar] [CrossRef]
- Collignon, P.C.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A.; for the World Health Organization Advisory Group, Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR); Agerso, Y.; Andremont, A.; Collignon, P.; Conly, J.; et al. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies to Control Antimicrobial Resistance From Food Animal Production. Clin. Infect. Dis. 2016, 63, 1087–1093. [Google Scholar] [CrossRef]
- Leonard, H.; Colodner, R.; Halachmi, S.; Segal, E. Recent Advances in the Race to Design a Rapid Diagnostic Test for Antimicrobial Resistance. Acs Sens. 2018, 3, 2202–2217. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Hosseini, H.; Shojaee-Aliabadi, S.; Torbati, M.; Alizadeh, A.M.; Alizadeh, M. Drug Resistance and the Prevention Strategies in Food Borne Bacteria: An Update Review. Adv. Pharm. Bull. 2019, 9, 335–347. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government and Welcome Trust: London, UK, 2016.
- Bell, G.; MacLean, C. The Search for ’Evolution-Proof’ Antibiotics. Trends Microbiol. 2018, 26, 471–483. [Google Scholar] [CrossRef]
- Anderson, M.; Clift, C.; Schulze, K.; Sagan, A.; Nahrgang, S.; Ouakrim, D.A.; Mossialos, E. Averting the AMR Crisis: What Are the Avenues for Policy Action for Countries in Europe? European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2019. [Google Scholar]
- Raoult, D.; Leone, M.; Roussel, Y.; Rolain, J.-M. Attributable deaths caused by infections with antibiotic-resistant bacteria in France. Lancet Infect. Dis. 2019, 19, 128–129. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Rafiqi, F. Antimicrobial Resistance Benchmark 2020; Access to Medicine Foundation: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Cansizoglu, M.F.; Tamer, Y.T.; Farid, M.; Koh, A.Y.; Toprak, E. Rapid ultrasensitive detection platform for antimicrobial susceptibility testing. PLoS Biol. 2019, 17, e3000291. [Google Scholar] [CrossRef]
- Dubourg, G.; Raoult, D. Emerging methodologies for pathogen identification in positive blood culture testing. Expert Rev. Mol. Diagn. 2016, 16, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Piccoli, E.; Brucculeri, V.; Barnini, S. A Prospective Evaluation of Two Rapid Phenotypical Antimicrobial Susceptibility Technologies for the Diagnostic Stewardship of Sepsis. Biomed Res. Int. 2018, 2018, 6976923. [Google Scholar] [CrossRef] [PubMed]
- Cals, J.W.L.; Ament, A.J.H.A.; Hood, K.; Butler, C.C.; Hopstaken, R.M.; Wassink, G.F.; Dinant, G.-J. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: Economic evaluation of a cluster randomized trial. J. Eval. Clin. Pract. 2011, 17, 1059–1069. [Google Scholar] [CrossRef]
- Holmes, E.A.F.; Harris, S.D.; Hughes, A.; Craine, N.; Hughes, D.A. Cost-Effectiveness Analysis of the Use of Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing in Primary Care. Antibiotics 2018, 7, 106. [Google Scholar] [CrossRef]
- Hunter, R. Cost-effectiveness of point-of-care C-reactive protein tests for respiratory tract infection in primary care in England. Adv. Ther. 2015, 32, 69–85. [Google Scholar] [CrossRef]
- Richter, S.S.; Karichu, J.; Otiso, J.; Van Heule, H.; Keller, G.; Cober, E.; Rojas, L.J.; Hujer, A.M.; Hujer, K.M.; Marshall, S.; et al. Evaluation of Sensititre Broth Microdilution Plate for determining the susceptibility of carbapenem-resistant Klebsiella pneumoniae to polymyxins. Diagn. Microbiol. Infect. Dis. 2018, 91, 89–92. [Google Scholar] [CrossRef]
- Álvarez-Molina, A.; de Toro, M.; Alexa, E.A.; Álvarez-Ordóñez, A. Applying Genomics to Track Antimicrobial Resistance in the Food Chain. Ref. Modul. Food Sci. 2020. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Kahlmeter, G.; Brown, D. CHAPTER 9—Laboratory control of antimicrobial therapy. In Antibiotic and Chemotherapy, 9th ed.; Finch, R.G., Greenwood, D., Norrby, S.R., Whitley, R.J., Eds.; W.B. Saunders: London, UK, 2010; pp. 115–122. [Google Scholar] [CrossRef]
- Tenover, F.C. Antibiotic Susceptibility Testing. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 67–77. [Google Scholar] [CrossRef]
- McGill, K.; Kelly, L.; Madden, R.H.; Moran, L.; Carroll, C.; O’Leary, A.; Moore, J.E.; McNamara, E.; O’Mahony, M.; Fanning, S.; et al. Comparison of disc diffusion and epsilometer (E-test) testing techniques to determine antimicrobial susceptibiliy of Campylobacter isolates of food and human clinical origin. J. Microbiol. Methods 2009, 79, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Fluit, A.C.; Visser, M.R.; Schmitz, F.-J. Molecular Detection of Antimicrobial Resistance. Clin. Microbiol. Rev. 2001, 14, 836. [Google Scholar] [CrossRef]
- Sundsfjord, A.; Simonsen, G.S.; Haldorsen, B.C.; Haaheim, H.; Hjelmevoll, S.-O.; Littauer, P.I.A.; Dahl, K.H. Genetic methods for detection of antimicrobial resistance. APMIS 2004, 112, 815–837. [Google Scholar] [CrossRef]
- Waseem, H.; Jameel, S.; Ali, J.; Saleem Ur Rehman, H.; Tauseef, I.; Farooq, U.; Jamal, A.; Ali, M.I. Contributions and Challenges of High Throughput qPCR for Determining Antimicrobial Resistance in the Environment: A Critical Review. Molecules 2019, 24, 163. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.A.; Owen, R.J.; Teare, E.L.; Saverymuttu, S. PCR-Based Diagnosis of Helicobacter pylori Infection and Real-Time Determination of Clarithromycin Resistance Directly from Human Gastric Biopsy Samples. J. Clin. Microbiol. 2001, 39, 1217. [Google Scholar] [CrossRef]
- Seedy, F.R.E.; Samy, A.A.; Salam, H.S.H.; Khairy, E.A.; Koraney, A.A. Polymerase chain reaction detection of genes responsible for multiple antibiotic resistance Staphylococcus aureus isolated from food of animal origin in Egypt. Vet. World 2017, 10, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Mackay, I.M. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 2004, 10, 190–212. [Google Scholar] [CrossRef]
- Böckelmann, U.; Dörries, H.-H.; Ayuso-Gabella, M.N.; de Salgot Marçay, M.; Tandoi, V.; Levantesi, C.; Masciopinto, C.; Van Houtte, E.; Szewzyk, U.; Wintgens, T.; et al. Quantitative PCR Monitoring of Antibiotic Resistance Genes and Bacterial Pathogens in Three European Artificial Groundwater Recharge Systems. Appl. Environ. Microbiol. 2009, 75, 154. [Google Scholar] [CrossRef]
- Whale, A.S.; Bushell, C.A.; Grant, P.R.; Cowen, S.; Gutierrez-Aguirre, I.; Sullivan, D.M.; Žel, J.; Milavec, M.; Foy, C.A.; Nastouli, E.; et al. Detection of Rare Drug Resistance Mutations by Digital PCR in a Human Influenza A Virus Model System and Clinical Samples. J. Clin. Microbiol. 2016, 54, 392. [Google Scholar] [CrossRef] [PubMed]
- Bogožalec Košir, A.; Cvelbar, T.; Kammel, M.; Grunert, H.-P.; Zeichhardt, H.; Milavec, M. Digital PCR method for detection and quantification of specific antimicrobial drug-resistance mutations in human cytomegalovirus. J. Virol. Methods 2020, 281, 113864. [Google Scholar] [CrossRef]
- Jousset, A.B.; Bernabeu, S.; Bonnin, R.A.; Creton, E.; Cotellon, G.; Sauvadet, A.; Naas, T.; Dortet, L. Development and validation of a multiplex polymerase chain reaction assay for detection of the five families of plasmid-encoded colistin resistance. Int. J. Antimicrob. Agents 2019, 53, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Waseem, H.; Saleem ur Rehman, H.; Ali, J.; Iqbal, M.J.; Ali, M.I. Chapter 14—Global trends in ARGs measured by HT-qPCR platforms. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Hashmi, M.Z., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 206–222. [Google Scholar]
- Wang, F.-H.; Qiao, M.; Su, J.-Q.; Chen, Z.; Zhou, X.; Zhu, Y.-G. High Throughput Profiling of Antibiotic Resistance Genes in Urban Park Soils with Reclaimed Water Irrigation. Environ. Sci. Technol. 2014, 48, 9079–9085. [Google Scholar] [CrossRef] [PubMed]
- Phuklia, W.; Panyanivong, P.; Sengdetka, D.; Sonthayanon, P.; Newton, P.N.; Paris, D.H.; Day, N.P.J.; Dittrich, S. Novel high-throughput screening method using quantitative PCR to determine the antimicrobial susceptibility of Orientia tsutsugamushi clinical isolates. J. Antimicrob. Chemother. 2018, 74, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, H.; Canales, M.; Ciric, L. Use of synthesized double-stranded gene fragments as qPCR standards for the quantification of antibiotic resistance genes. J. Microbiol. Methods 2019, 164, 105670. [Google Scholar] [CrossRef] [PubMed]
- Donà, V.; Kasraian, S.; Lupo, A.; Guilarte, Y.N.; Hauser, C.; Furrer, H.; Unemo, M.; Low, N.; Endimiani, A. Multiplex Real-Time PCR Assay with High-Resolution Melting Analysis for Characterization of Antimicrobial Resistance in Neisseria gonorrhoeae. J. Clin. Microbiol. 2016, 54, 2074–2081. [Google Scholar] [CrossRef]
- Wang, H.; Hecht, S.; Kline, D.; Leber, A.L. Staphylococcus aureus and methicillin resistance detection directly from pediatric samples using PCR assays with differential cycle threshold values for corroboration of methicillin resistance. J. Microbiol. Methods 2019, 159, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Masny, A.; Płucienniczak, A. Ligation mediated PCR performed at low denaturation temperatures—PCR melting profiles. Nucleic Acids Res. 2003, 31, e114. [Google Scholar] [CrossRef][Green Version]
- Woksepp, H.; Jernberg, C.; Tärnberg, M.; Ryberg, A.; Brolund, A.; Nordvall, M.; Olsson-Liljequist, B.; Wisell, K.T.; Monstein, H.-J.; Nilsson, L.E.; et al. High-resolution melting-curve analysis of ligation-mediated real-time PCR for rapid evaluation of an epidemiological outbreak of extended-spectrum-beta-lactamase-producing Escherichia coli. J. Clin. Microbiol. 2011, 49, 4032. [Google Scholar] [CrossRef][Green Version]
- Nijhuis, R.; van Zwet, A.; Stuart, J.C.; Weijers, T.; Savelkoul, P. Rapid molecular detection of extended-spectrum β-lactamase gene variants with a novel ligation-mediated real-time PCR. J. Med. Microbiol. 2012, 61, 1563–1567. [Google Scholar] [CrossRef]
- Woksepp, H.; Ryberg, A.; Billström, H.; Hällgren, A.; Nilsson, L.E.; Marklund, B.-I.; Olsson-Liljequist, B.; Schön, T. Evaluation of high-resolution melting curve analysis of ligation-mediated real-time PCR, a rapid method for epidemiological typing of ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens. J. Clin. Microbiol. 2014, 52, 4339. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Ghaemi, A. Nucleic acid isothermal amplification technologies: A review. Nucleosidesnucleotides Nucleic Acids 2008, 27, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.-M.; Kim, B.N.; Kwon, O.S.; Rho, W.-Y.; Jun, B.-H. Emerging ultrafast nucleic acid amplification technologies for next-generation molecular diagnostics. Biosens. Bioelectron. 2019, 141, 111448. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Mason, M.-G.; Botella, J.-R. Evaluation and improvement of isothermal amplification methods for point-of-need plant disease diagnostics. PLoS ONE 2020, 15, e0235216. [Google Scholar] [CrossRef]
- Zanoli, L.M.; Spoto, G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors 2012, 3, 18–43. [Google Scholar] [CrossRef]
- Karami, A.; Gill, P.; Motamedi, M.; Saghafinia, M. A review of the current isothermal amplification techniques: Applications, advantages and disadvantages. J. Glob. Infect. Dis. 2011, 3, 293–302. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Papadakis, G.; Papageorgiou, D.P.; Kokkoris, G.; Papadopoulos, V.; Kefala, I.; Gizeli, E.; Tserepi, A. Miniaturized devices for isothermal DNA amplification addressing DNA diagnostics. Microsyst. Technol. 2016, 22, 1529–1534. [Google Scholar] [CrossRef]
- Srimongkol, G.; Ditmangklo, B.; Choopara, I.; Thaniyavarn, J.; Dean, D.; Kokpol, S.; Vilaivan, T.; Somboonna, N. Rapid colorimetric loop-mediated isothermal amplification for hypersensitive point-of-care Staphylococcus aureus enterotoxin A gene detection in milk and pork products. Sci. Rep. 2020, 10, 7768. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Chakraborty, S.; Tiwari, R.; Kapoor, S.; Kumar, A.; Thomas, P. Loop-mediated isothermal amplification of DNA (LAMP): A new diagnostic tool lights the world of diagnosis of animal and human pathogens: A review. Pak. J. Biol. Sci. 2016, 17, 151–166. [Google Scholar] [CrossRef]
- Morin, S.; Bazarova, N.; Jacon, P.; Vella, S. The Manufacturers’ Perspective on World Health Organization Prequalification of In Vitro Diagnostics. Clin. Infect. Dis. 2017, 66, 301–305. [Google Scholar] [CrossRef]
- Cantera, J.L.; White, H.; Diaz, M.H.; Beall, S.G.; Winchell, J.M.; Lillis, L.; Kalnoky, M.; Gallarda, J.; Boyle, D.S. Assessment of eight nucleic acid amplification technologies for potential use to detect infectious agents in low-resource settings. PLoS ONE 2019, 14, e0215756. [Google Scholar] [CrossRef] [PubMed]
- Call, D.R.; Bakko, M.K.; Krug, M.J.; Roberts, M.C. Identifying antimicrobial resistance genes with DNA microarrays. Antimicrob. Agents Chemother. 2003. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.F.; Zankari, E.; Hasman, H. Molecular Methods for Detection of Antimicrobial Resistance. Microbiol. Spectr. 2017, 33–50. [Google Scholar] [CrossRef]
- Strauss, C.; Endimiani, A.; Perreten, V. A novel universal DNA labeling and amplification system for rapid microarray-based detection of 117 antibiotic resistance genes in Gram-positive bacteria. J. Microbiol. Methods 2015, 108, 25–30. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Zhang, Z.-W.; Wang, C.; Yang, H.-W.; Jiang, D.; Zhang, Q.; Mitchelson, K.; Cheng, J. Use of a DNA Microarray for Simultaneous Detection of Antibiotic Resistance Genes among Staphylococcal Clinical Isolates. J. Clin. Microbiol. 2007, 45, 3514. [Google Scholar] [CrossRef]
- Havlicek, J.; Dachsel, B.; Slickers, P.; Andres, S.; Beckert, P.; Feuerriegel, S.; Niemann, S.; Merker, M.; Labugger, I. Rapid microarray-based assay for detection of pyrazinamide resistant Mycobacterium tuberculosis. Diagn. Microbiol. Infect. Dis. 2019, 94, 147–154. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Maxam, A.M.; Gilbert, W. A new method for sequencing DNA. Proc. Natl. Acad. Sci. USA 1977, 74, 560–564. [Google Scholar] [CrossRef]
- Fleischmann, R.D.; Adams, M.D.; White, O.; Clayton, R.A.; Kirkness, E.F.; Kerlavage, A.R.; Bult, C.J.; Tomb, J.F.; Dougherty, B.A.; Merrick, J.M.; et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995, 269, 496. [Google Scholar] [CrossRef]
- Margulies, M.; Egholm, M.; Altman, W.E.; Attiya, S.; Bader, J.S.; Bemben, L.A.; Berka, J.; Braverman, M.S.; Chen, Y.-J.; Chen, Z.; et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005, 437, 376–380. [Google Scholar] [CrossRef]
- Teng, J.L.L.; Yeung, M.L.; Chan, E.; Jia, L.; Lin, C.H.; Huang, Y.; Tse, H.; Wong, S.S.Y.; Sham, P.C.; Lau, S.K.P.; et al. PacBio But Not Illumina Technology Can Achieve Fast, Accurate and Complete Closure of the High GC, Complex Burkholderia pseudomallei Two-Chromosome Genome. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Levene, M.J.; Korlach, J.; Turner, S.W.; Foquet, M.; Craighead, H.G.; Webb, W.W. Zero-Mode Waveguides for Single-Molecule Analysis at High Concentrations. Science 2003, 299, 682. [Google Scholar] [CrossRef]
- Clarke, J.; Wu, H.-C.; Jayasinghe, L.; Patel, A.; Reid, S.; Bayley, H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 2009, 4, 265–270. [Google Scholar] [CrossRef]
- Ruppé, E.; Bengtsson-Palme, J.; Charretier, Y.; Schrenzel, J. How next-generation sequencing can address the antimicrobial resistance challenge. AMR Control 2019, 20, 60–66. [Google Scholar]
- Malmberg, C.; Yuen, P.; Spaak, J.; Cars, O.; Tängdén, T.; Lagerbäck, P. A Novel Microfluidic Assay for Rapid Phenotypic Antibiotic Susceptibility Testing of Bacteria Detected in Clinical Blood Cultures. PLoS ONE 2016, 11, e0167356. [Google Scholar] [CrossRef]
- Köser, C.U.; Ellington, M.J.; Peacock, S.J. Whole-genome sequencing to control antimicrobial resistance. Trends Genet. 2014, 30, 401–407. [Google Scholar] [CrossRef]
- Oniciuc, E.A.; Likotrafiti, E.; Alvarez-Molina, A.; Prieto, M.; Santos, J.A.; Alvarez-Ordóñez, A. The Present and Future of Whole Genome Sequencing (WGS) and Whole Metagenome Sequencing (WMS) for Surveillance of Antimicrobial Resistant Microorganisms and Antimicrobial Resistance Genes across the Food Chain. Genes 2018, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Bortolaia, V.; Tate, H.; Tyson, G.H.; Aarestrup, F.M.; McDermott, P.F. Using Genomics to Track Global Antimicrobial Resistance. Front. Public Health 2019, 7. [Google Scholar] [CrossRef]
- Boolchandani, M.; D’Souza, A.W.; Dantas, G. Sequencing-based methods and resources to study antimicrobial resistance. Nat. Rev. Genet. 2019, 20, 356–370. [Google Scholar] [CrossRef]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Mason, A.; Foster, D.; Bradley, P.; Golubchik, T.; Doumith, M.; Gordon, N.C.; Pichon, B.; Iqbal, Z.; Staves, P.; Crook, D.; et al. Accuracy of Different Bioinformatics Methods in Detecting Antibiotic Resistance and Virulence Factors from Staphylococcus aureus Whole-Genome Sequences. J. Clin. Microbiol. 2018, 56, e01815-17. [Google Scholar] [CrossRef] [PubMed]
- Lauener, F.N.; Imkamp, F.; Lehours, P.A.-O.; Buissonnière, A.; Benejat, L.; Zbinden, R.; Keller, P.A.-O.; Wagner, K. Genetic Determinants and Prediction of Antibiotic Resistance Phenotypes in Helicobacter pylori. J. Clin. Med. 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Satola, S.W.; Read, T.D. Genome-Based Prediction of Bacterial Antibiotic Resistance. J. Clin. Microbiol. 2019, 57, e01405–e01418. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Long, S.W.; McDermott, P.F.; Olsen, R.J.; Olson, R.; Stevens, R.L.; Tyson, G.H.; Zhao, S.; Davis, J.J. Using Machine Learning to Predict Antimicrobial MICs and Associated Genomic Features for Nontyphoidal Salmonella. J. Clin. Microbiol. 2019, 57, e01260-18. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.C.; Kavvas, E.S.; Monk, J.M.; Palsson, B.O. Machine learning with random subspace ensembles identifies antimicrobial resistance determinants from pan-genomes of three pathogens. PLoS Comput. Biol. 2020, 16, e1007608. [Google Scholar] [CrossRef] [PubMed]
- González-Escalona, N.A.O.; Allard, M.A.; Brown, E.W.; Sharma, S.; Hoffmann, M. Nanopore sequencing for fast determination of plasmids, phages, virulence markers, and antimicrobial resistance genes in Shiga toxin-producing Escherichia coli. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Amoako, K.K.; Thomas, M.C.; Kong, F.; Janzen, T.W.; Hahn, K.R.; Shields, M.J.; Goji, N. Rapid detection and antimicrobial resistance gene profiling of Yersinia pestis using pyrosequencing technology. J. Microbiol. Methods 2012, 90, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ajbani, K.; Lin, S.Y.; Rodrigues, C.; Nguyen, D.; Arroyo, F.; Kaping, J.; Jackson, L.; Garfein, R.S.; Catanzaro, D.; Eisenach, K.; et al. Evaluation of pyrosequencing for detecting extensively drug-resistant Mycobacterium tuberculosis among clinical isolates from four high-burden countries. Antimicrob. Agents Chemother. 2015, 59, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Farheen, H.; Chandrappa, C.P.; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-genome sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515. [Google Scholar] [CrossRef]
- Vélez, J.R.; Cameron, M.; Rodríguez-Lecompte, J.C.; Xia, F.; Heider, L.C.; Saab, M.; McClure, J.T.; Sánchez, J. Whole-Genome Sequence Analysis of Antimicrobial Resistance Genes in Streptococcus uberis and Streptococcus dysgalactiae Isolates from Canadian Dairy Herds. Front. Vet. Sci. 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tyson, G.H.; Chen, Y.; Li, C.; Mukherjee, S.; Young, S.; Lam, C.; Folster, J.P.; Whichard, J.M.; McDermott, P.F. Whole-Genome Sequencing Analysis Accurately Predicts Antimicrobial Resistance Phenotypes in Campylobacter Spp. Appl. Environ. Microbiol. 2016, 82, 459. [Google Scholar] [CrossRef]
- Alghoribi, M.F.; Balkhy, H.H.; Woodford, N.; Ellington, M.J. The role of whole genome sequencing in monitoring antimicrobial resistance: A biosafety and public health priority in the Arabian Peninsula. J. Infect. Public Health 2018, 11, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Argimón, S.; Masim, M.A.L.; Gayeta, J.M.; Lagrada, M.L.; Macaranas, P.K.V.; Cohen, V.; Limas, M.T.; Espiritu, H.O.; Palarca, J.C.; Chilam, J.; et al. Integrating whole-genome sequencing within the National Antimicrobial Resistance Surveillance Program in the Philippines. Nat. Commun. 2020, 11, 2719. [Google Scholar] [CrossRef] [PubMed]
- Fasciana, T.; Gentile, B.; Aquilina, M.; Ciammaruconi, A.; Mascarella, C.; Anselmo, A.; Fortunato, A.; Fillo, S.; Petralito, G.; Lista, F.; et al. Co-existence of virulence factors and antibiotic resistance in new Klebsiella pneumoniae clones emerging in south of Italy. BMC Infect. Dis. 2019, 19, 928. [Google Scholar] [CrossRef]
- Berbers, B.; Saltykova, A.; Garcia-Graells, C.; Philipp, P.; Arella, F.; Marchal, K.; Winand, R.; Vanneste, K.; Roosens, N.H.C.; De Keersmaecker, S.C.J. Combining short and long read sequencing to characterize antimicrobial resistance genes on plasmids applied to an unauthorized genetically modified Bacillus. Sci. Rep. 2020, 10, 4310. [Google Scholar] [CrossRef]
- Quick, J.; Loman, N.J.; Duraffour, S.; Simpson, J.T.; Severi, E.; Cowley, L.; Bore, J.A.; Koundouno, R.; Dudas, G.; Mikhail, A.; et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 2016, 530, 228–232. [Google Scholar] [CrossRef]
- Giordano, F.; Aigrain, L.; Quail, M.A.; Coupland, P.; Bonfield, J.K.; Davies, R.M.; Tischler, G.; Jackson, D.K.; Keane, T.M.; Li, J.; et al. De novo yeast genome assemblies from MinION, PacBio and MiSeq platforms. Sci. Rep. 2017, 7, 3935. [Google Scholar] [CrossRef]
- Loman, N.J.; Quick, J.; Simpson, J.T. A complete bacterial genome assembled de novo using only nanopore sequencing data. Nat. Methods 2015, 12, 733–735. [Google Scholar] [CrossRef]
- Greninger, A.L.; Naccache, S.N.; Federman, S.; Yu, G.; Mbala, P.; Bres, V.; Stryke, D.; Bouquet, J.; Somasekar, S.; Linnen, J.M.; et al. Rapid metagenomic identification of viral pathogens in clinical samples by real-time nanopore sequencing analysis. Genome Med. 2015, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.L.; Watson, M.; Minot, S.S.; Rivera, M.C.; Franklin, R.B. MinION™ nanopore sequencing of environmental metagenomes: A synthetic approach. GigaScience 2017, 6. [Google Scholar] [CrossRef]
- Judge, K.; Harris, S.R.; Reuter, S.; Parkhill, J.; Peacock, S.J. Early insights into the potential of the Oxford Nanopore MinION for the detection of antimicrobial resistance genes. J. Antimicrob. Chemother. 2015, 70, 2775–2778. [Google Scholar] [CrossRef] [PubMed]
- Ashton, P.M.; Nair, S.; Dallman, T.; Rubino, S.; Rabsch, W.; Mwaigwisya, S.; Wain, J.; O’Grady, J. MinION nanopore sequencing identifies the position and structure of a bacterial antibiotic resistance island. Nat. Biotechnol. 2015, 33, 296–300. [Google Scholar] [CrossRef]
- Greig, D.R.; Dallman, T.J.; Hopkins, K.L.; Jenkins, C. MinION nanopore sequencing identifies the position and structure of bacterial antibiotic resistance determinants in a multidrug-resistant strain of enteroaggregative Escherichia coli. Microb. Genom. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Mwaigwisya, S.; Crossman, L.C.; Doumith, M.; Munroe, D.; Pires, C.; Khan, A.M.; Woodford, N.; Saunders, N.J.; Wain, J.; et al. Identification of bacterial pathogens and antimicrobial resistance directly from clinical urines by nanopore-based metagenomic sequencing. J. Antimicrob. Chemother. 2016, 72, 104–114. [Google Scholar] [CrossRef]
- Kamathewatta, K.I.; Bushell, R.N.; Young, N.D.; Stevenson, M.A.; Billman-Jacobe, H.; Browning, G.F.; Marenda, M.S. Exploration of antibiotic resistance risks in a veterinary teaching hospital with Oxford Nanopore long read sequencing. PLoS ONE 2019, 14, e0217600. [Google Scholar] [CrossRef]
- Taxt, A.M.; Avershina, E.; Frye, S.A.; Naseer, U.; Ahmad, R. Rapid identification of pathogens, antibiotic resistance genes and plasmids in blood cultures by nanopore sequencing. Sci. Rep. 2020, 10, 7622. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Dvorak, C.M.T.; Estrada, A.A.; Gebhart, C.; Marthaler, D.G.; Murtaugh, M.P. MinION sequencing of Streptococcus suis allows for functional characterization of bacteria by multilocus sequence typing and antimicrobial resistance profiling. J. Microbiol. Methods 2020, 169, 105817. [Google Scholar] [CrossRef]
- Lim, A.; Naidenov, B.; Bates, H.; Willyerd, K.; Snider, T.; Couger, M.B.; Chen, C.; Ramachandran, A. Nanopore ultra-long read sequencing technology for antimicrobial resistance detection in Mannheimia haemolytica. J. Microbiol. Methods 2019, 159, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Angers, A.; Petrillo, M.; Patak, D.A.; Querci, M.; Guy, V.D.E. The Role and Implementation of Next-Generation Sequencing Technologies in the Coordinated Action Plan against Antimicrobial Resistance; Publications Office of the EU: Luxembourg, 2017; pp. 1831–9424. [Google Scholar]
- Gajdács, M.; Spengler, G.; Urbán, E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik’s Cube of Clinical Microbiology? Antibiotics 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U.S.; Acar, Z.; Sydenham, T.V.; Johansson, Å. Antimicrobial susceptibility testing of Bacteroides fragilis using the MALDI Biotyper antibiotic susceptibility test rapid assay (MBT-ASTRA). Anaerobe 2018, 54, 236–239. [Google Scholar] [CrossRef]
- Zürcher, S.; Mooser, C.; Lüthi, A.U.; Mühlemann, K.; Barbani, M.T.; Mohacsi, P.; Garzoni, C.; Gorgievski-Hrisoho, M.; Schaller, A.; Flatz, L. Sensitive and rapid detection of ganciclovir resistance by PCR based MALDI-TOF analysis. J. Clin. Virol. 2012, 54, 359–363. [Google Scholar] [CrossRef]
- Liu, N.; Wang, L.; Cai, G.; Zhang, D.; Lin, J. Establishment of a simultaneous detection method for ten duck viruses using MALDI-TOF mass spectrometry. J. Virol. Methods 2019, 273, 113723. [Google Scholar] [CrossRef]
- Paul, S.; Singh, P.; As, S.; Rudramurthy, S.M.; Chakrabarti, A.; Ghosh, A.K. Rapid detection of fluconazole resistance in Candida tropicalis by MALDI-TOF MS. Med. Mycol. 2017, 56, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J. Microbiol. Methods 2017, 138, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; van Belkum, A. One System for All: Is Mass Spectrometry a Future Alternative for Conventional Antibiotic Susceptibility Testing? Front. Microbiol. 2019, 10, 2711. [Google Scholar] [CrossRef]
- Vrioni, G.; Tsiamis, C.; Oikonomidis, G.; Theodoridou, K.; Kapsimali, V.; Tsakris, A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: Current achievements and future perspectives. Ann. Transl. Med. 2018, 6, 240. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Mallecot, Y.; Riazzo, C.; Miranda-Casas, C.; Rojo-Martín, M.D.; Gutiérrez-Fernández, J.; Navarro-Marí, J.M. Rapid detection and identification of strains carrying carbapenemases directly from positive blood cultures using MALDI-TOF MS. J. Microbiol. Methods 2014, 105, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, B.; Cercenado, E.; Coste, A.T.; Greub, G. Review of the impact of MALDI-TOF MS in public health and hospital hygiene, 2018. Eurosurveill 2019, 24, 1800193. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, S.; Petersen, M.; Wang, S.; Lu, X. Detection and Characterization of Antibiotic-Resistant Bacteria Using Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 762. [Google Scholar] [CrossRef]
- Byun, J.H.; Yu, A.R.; Kim, M.S.; Lee, K. Performance of Microflex LT Biotyper and VITEK MS for Routine Identification of Yeasts. Ann. Lab Med. 2018, 38, 487–489. [Google Scholar] [CrossRef]
- Martiny, D.; Busson, L.; Wybo, I.; El Haj, R.A.; Dediste, A.; Vandenberg, O. Comparison of the Microflex LT and Vitek MS systems for routine identification of bacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2012, 50, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.C.; Saffert, R.T.; Cunningham, S.A.; Hyman, J.; Walsh, J.; Arbefeville, S.; Howard, W.; Pruessner, J.; Safwat, N.; Cockerill, F.R.; et al. Evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of nonfermenting gram-negative bacilli isolated from cultures from cystic fibrosis patients. J. Clin. Microbiol. 2012, 50, 2034–2039. [Google Scholar] [CrossRef]
- Dortet, L.; Bonnin, R.A.; Pennisi, I.; Gauthier, L.; Jousset, A.B.; Dabos, L.; Furniss, R.C.D.; Mavridou, D.A.I.; Bogaerts, P.; Glupczynski, Y.; et al. Rapid detection and discrimination of chromosome- and MCR-plasmid-mediated resistance to polymyxins by MALDI-TOF MS in Escherichia coli: The MALDIxin test. J Antimicrob Chemother 2018. [Google Scholar] [CrossRef]
- Furniss, R.A.-O.; Dortet, L.A.-O.; Bolland, W.; Drews, O.; Sparbier, K.; Bonnin, R.A.; Filloux, A.A.-O.; Kostrzewa, M.; Mavridou, D.A.-O.; Larrouy-Maumus, G. Detection of Colistin Resistance in Escherichia coli by Use of the MALDI Biotyper Sirius Mass Spectrometry System. J. Clin. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, Y.; Kosai, K.; Yamakawa, H.; Kaku, N.; Uno, N.; Morinaga, Y.; Hasegawa, H.; Yanagihara, K. Detection of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae using the MALDI Biotyper Selective Testing of Antibiotic Resistance–β-Lactamase (MBT STAR-BL) assay. J. Microbiol. Methods 2019, 160, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Nisa, S.; Bercker, C.; Midwinter, A.C.; Bruce, I.; Graham, C.F.; Venter, P.; Bell, A.; French, N.P.; Benschop, J.; Bailey, K.M.; et al. Combining MALDI-TOF and genomics in the study of methicillin resistant and multidrug resistant Staphylococcus pseudintermedius in New Zealand. Sci. Rep. 2019, 9, 1271. [Google Scholar] [CrossRef]
- Tang, W.; Ranganathan, N.; Shahrezaei, V.; Larrouy-Maumus, G. MALDI-TOF mass spectrometry on intact bacteria combined with a refined analysis framework allows accurate classification of MSSA and MRSA. PLoS ONE 2019, 14, e0218951. [Google Scholar] [CrossRef]
- Vatanshenassan, M.; Boekhout, T.; Meis, J.F.; Berman, J.; Chowdhary, A.; Ben-Ami, R.; Sparbier, K.; Kostrzewa, M. Candida auris Identification and Rapid Antifungal Susceptibility Testing Against Echinocandins by MALDI-TOF MS. Front. Cell. Infect. Microbiol. 2019, 9, 20. [Google Scholar] [CrossRef]
- Cordovana, M.; Pranada, A.B.; Ambretti, S.; Kostrzewa, M. MALDI-TOF bacterial subtyping to detect antibiotic resistance. Clin. Mass Spectrom. 2019, 14, 3–8. [Google Scholar] [CrossRef]
- Ota, Y.; Furuhashi, K.; Nagao, Y.; Nanba, T.; Yamanaka, K.; Ishikawa, J.; Nagura, O.; Iwaizumi, M.; Hamada, E.; Maekawa, M. Detection of extended-spectrum β-lactamases producing Enterobacteriaceae using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry based MBT STAR-BL software module with β-lactamase inhibition assay depends on the bacterial strains. J. Microbiol. Methods 2019, 167, 105734. [Google Scholar] [CrossRef]
- Neonakis, I.K.; Spandidos, D.A. Detection of carbapenemase producers by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS). Eur. J. Clin. Microbiol. Infect Dis. 2019, 38, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, W.; Li, M.; Deng, S.; Huang, Q.; Lu, W. Evaluation of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for identifying VIM- and SPM-type metallo-β-lactamase-producing Pseudomonas aeruginosa clinical isolates. Infect. Drug Resist. 2019, 12, 2781–2788. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Kyritsi, M.; Sakka, M.; Chatzinikolaou, K.; Donos, S.; Boziaris, I.S.; Hadjichristodoulou, C.; Athanassiou, C.G. Matrix-assisted laser desorption ionization–time of flight mass spectrometry reveals Enterococcus and Enterobacter spp. in major insect species involved in food security with resistance to common antibiotics. J. Pest Sci. 2020, 93, 159–170. [Google Scholar] [CrossRef]
- Rocco, V.G.; Intra, J.; Sarto, C.; Tiberti, N.; Savarino, C.; Brambilla, M.; Brambilla, P. Rapid Identification of Carbapenemase-producing Klebsiella pneumoniae strains by Matrix-Assisted Laser Desorption/Ionization-Time of Flight using Vitek(®) Mass Spectrometry System. Eurasian J. Med. 2019, 51, 209–213. [Google Scholar] [CrossRef]
- Dortet, L.A.-O.; Potron, A.; Bonnin, R.A.-O.; Plesiat, P.; Naas, T.A.-O.; Filloux, A.A.-O.; Larrouy-Maumus, G. Rapid detection of colistin resistance in Acinetobacter baumannii using MALDI-TOF-based lipidomics on intact bacteria. Sci. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, C.; Rehnstam-Holm, A.-S.; Nilson, B. Rapid detection of antibiotic resistance in positive blood cultures by MALDI-TOF MS and an automated and optimized MBT-ASTRA protocol for Escherichia coli and Klebsiella pneumoniae. Infect. Dis. 2020, 52, 45–53. [Google Scholar] [CrossRef]
- Horseman, T.S.; Lustik, M.B.; Fong, K.S.K. Rapid qualitative antibiotic resistance characterization using VITEK MS. Diagn. Microbiol. Infect. Dis. 2020, 97, 115093. [Google Scholar] [CrossRef]
- Oviaño, M.; Gato, E.; Bou, G. Rapid Detection of KPC-Producing Enterobacterales Susceptible to Imipenem/Relebactam by Using the MALDI-TOF MS MBT STAR-Carba IVD Assay. Front. Microbiol. 2020, 11, 328. [Google Scholar] [CrossRef]
- Nix, I.D.; Idelevich, E.A.; Storck, L.M.; Sparbier, K.; Drews, O.; Kostrzewa, M.; Becker, K. Detection of Methicillin Resistance in Staphylococcus aureus From Agar Cultures and Directly From Positive Blood Cultures Using MALDI-TOF Mass Spectrometry-Based Direct-on-Target Microdroplet Growth Assay. Front. Microbiol. 2020, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Löffler, K.; Dinkelacker, A.G.; Bader, B.; Autenrieth, I.B.; Peter, S.; Liese, J. Fourier-Transform Infrared (FTIR) Spectroscopy for Typing of Clinical Enterobacter cloacae Complex Isolates. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Salman, A.; Sharaha, U.; Rodriguez-Diaz, E.; Shufan, E.; Riesenberg, K.; Bigio, I.J.; Huleihel, M. Detection of antibiotic resistant Escherichia Coli bacteria using infrared microscopy and advanced multivariate analysis. Analyst 2017, 142, 2136–2144. [Google Scholar] [CrossRef]
- Zwielly, A.; Gopas, J.; Brkic, G.; Mordechai, S. Discrimination between drug-resistant and non-resistant human melanoma cell lines by FTIR spectroscopy. Analyst 2009, 134, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Novais, Â.; Freitas, A.R.; Rodrigues, C.; Peixe, L. Fourier transform infrared spectroscopy: Unlocking fundamentals and prospects for bacterial strain typing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, L.; Urbaniak, M.; Adamus-Białek, W.; Kaca, W. The use of infrared spectroscopy and artificial neural networks for detection of uropathogenic Escherichia coli strains’ susceptibility to cephalothin. Acta Biochim. Pol. 2013, 60, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Sharaha, U.; Rodriguez-Diaz, E.; Riesenberg, K.; Bigio, I.J.; Huleihel, M.; Salman, A. Using Infrared Spectroscopy and Multivariate Analysis to Detect Antibiotics’ Resistant Escherichia coli Bacteria. Anal. Chem. 2017, 89, 8782–8790. [Google Scholar] [CrossRef]
- Kochan, K.; Nethercott, C.; Perez−Guaita, D.; Jiang, J.-H.; Peleg, A.Y.; Wood, B.R.; Heraud, P. Detection of Antimicrobial Resistance-Related Changes in Biochemical Composition of Staphylococcus aureus by Means of Atomic Force Microscopy-Infrared Spectroscopy. Anal. Chem. 2019, 91, 15397–15403. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.; Pantazis, A.K.; Ntogka, M.; Parasyris, K.; Theodosi, G.-I.; Kaprou, G.; Gizeli, E. 3D-printed Point-of-Care Platform for Genetic Testing of Infectious Diseases Directly in Human Samples Using Acoustic Sensors and a Smartphone. ACS Sens. 2019, 4, 1329–1336. [Google Scholar] [CrossRef]
- Tsougeni, K.; Kastania, A.S.; Kaprou, G.D.; Eck, M.; Jobst, G.; Petrou, P.S.; Kakabakos, S.E.; Mastellos, D.; Gogolides, E.; Tserepi, A. A modular integrated lab-on-a-chip platform for fast and highly efficient sample preparation for foodborne pathogen screening. Sens. Actuators B Chem. 2019, 288, 171–179. [Google Scholar] [CrossRef]
- Fernández-Gavela, A.; Herranz, S.; Chocarro, B.; Falke, F.; Schreuder, E.; Leeuwis, H.; Heideman, R.G.; Lechuga, L.M. Full integration of photonic nanoimmunosensors in portable platforms for on-line monitoring of ocean pollutants. Sens. Actuators B Chem. 2019, 297, 126758. [Google Scholar] [CrossRef]
- Yılmaz, B.; Yılmaz, F. Chapter 8—Lab-on-a-Chip Technology and Its Applications. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–153. [Google Scholar] [CrossRef]
- Jung, W.; Han, J.; Choi, J.-W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Papadopoulos, V.; Papageorgiou, D.P.; Kefala, I.; Papadakis, G.; Gizeli, E.; Chatzandroulis, S.; Kokkoris, G.; Tserepi, A. Ultrafast, low-power, PCB manufacturable, continuous-flow microdevice for DNA amplification. Anal. Bioanal. Chem. 2019, 411, 5297–5307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zheng, G.; Zhang, Y.; Ma, R.; Kang, X. Evaluation of a micro/nanofluidic chip platform for the high-throughput detection of bacteria and their antibiotic resistance genes in post-neurosurgical meningitis. Int. J. Infect. Dis. 2018, 70, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.C.; Spoto, G. Integration of isothermal amplification methods in microfluidic devices: Recent advances. Biosens. Bioelectron. 2017, 90, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Sakakihara, S.; Grushnikov, A.; Kikuchi, K.; Noji, H.; Yamaguchi, A.; Iino, R.; Yagi, Y.; Nishino, K. A Microfluidic Channel Method for Rapid Drug-Susceptibility Testing of Pseudomonas aeruginosa. PLoS ONE 2016, 11, e0148797. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, X. Rapid Identification and Susceptibility Testing of Uropathogenic Microbes via Immunosorbent ATP-Bioluminescence Assay on a Microfluidic Simulator for Antibiotic Therapy. Anal. Chem. 2015, 87, 2410–2418. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, J.; Lee, M.; Kim, E.G.; Lee, J.S.; Lee, S.; Joo, S.; Song, S.H.; Kim, E.C.; Lee, J.C.; et al. A rapid antimicrobial susceptibility test based on single-cell morphological analysis. Sci. Transl. Med. 2014. [Google Scholar] [CrossRef]
- Pitruzzello, G.; Thorpe, S.; Johnson, S.; Evans, A.; Gadêlha, H.; Krauss, T.F. Multiparameter antibiotic resistance detection based on hydrodynamic trapping of individual E. coli. Lab Chip 2019, 19, 1417–1426. [Google Scholar] [CrossRef]
- Galvan, D.D.; Yu, Q. Surface-Enhanced Raman Scattering for Rapid Detection and Characterization of Antibiotic-Resistant Bacteria. Adv. Healthc. Mater. 2018, 7, 1701335. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, M.; Liu, Y.; Huang, J.; Liu, Z.; Tian, H.; Zheng, Y.; de la Chapelle, M.L.; Zhang, Y.; Fu, W. Surface-enhanced Raman scattering method for the identification of methicillin-resistant Staphylococcus aureus using positively charged silver nanoparticles. Microchim. Acta 2019, 186, 102. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Samuelson, D.R.; Xu, Y.; Zhang, H.; Wang, S.; Rasco, B.A.; Xu, J.; Konkel, M.E. Detecting and tracking nosocomial methicillin-resistant Staphylococcus aureus using a microfluidic SERS biosensor. Anal. Chem. 2013, 85, 2320–2327. [Google Scholar] [CrossRef]
- Chang, K.-W.; Cheng, H.-W.; Shiue, J.; Wang, J.-K.; Wang, Y.-L.; Huang, N.-T. Antibiotic Susceptibility Test with Surface-Enhanced Raman Scattering in a Microfluidic System. Anal. Chem. 2019, 91, 10988–10995. [Google Scholar] [CrossRef]
- Liao, C.; Huang, H.; Chen, Y.; Huang, N. The Microfluidic Microwell Device Integrating Surface Enhanced Raman Scattering for Bacteria Enrichment and in Situ Antibiotic Susceptibility Test. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 1048–1051. [Google Scholar]
- Bodelón, G.; Montes-García, V.; Pérez-Juste, J.; Pastoriza-Santos, I. Surface-Enhanced Raman Scattering Spectroscopy for Label-Free Analysis of P. aeruginosa Quorum Sensing. Front. Cell. Infect. Microbiol. 2018, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-B.; Chien, C.-C.; You, H.-L.; Kuo, F.-C.; Lee, M.S.; Lee, G.-B. An integrated microfluidic system for antimicrobial susceptibility testing with antibiotic combination. Lab Chip 2019, 19, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Petersen, M.; Lu, X. Identification and Antimicrobial Susceptibility Testing of Campylobacter Using a Microfluidic Lab-on-a-Chip Device. Appl. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- Tang, Y.; Zhen, L.; Liu, J.; Wu, J. Rapid antibiotic susceptibility testing in a microfluidic pH sensor. Anal. Chem. 2013, 85, 2787–2794. [Google Scholar] [CrossRef]
- Hu, C.; Kalsi, S.; Zeimpekis, I.; Sun, K.; Ashburn, P.; Turner, C.; Sutton, J.M.; Morgan, H. Ultra-fast electronic detection of antimicrobial resistance genes using isothermal amplification and Thin Film Transistor sensors. Biosens. Bioelectron. 2017, 96, 281–287. [Google Scholar] [CrossRef]
- Xu, B.; Du, Y.; Lin, J.; Qi, M.; Shu, B.; Wen, X.; Liang, G.; Chen, B.; Liu, D. Simultaneous Identification and Antimicrobial Susceptibility Testing of Multiple Uropathogens on a Microfluidic Chip with Paper-Supported Cell Culture Arrays. Anal. Chem. 2016, 88, 11593–11600. [Google Scholar] [CrossRef] [PubMed]
- He, P.J.W.; Katis, I.N.; Kumar, A.J.U.; Bryant, C.A.; Keevil, C.W.; Somani, B.K.; Mahobia, N.; Eason, R.W.; Sones, C.L. Laser-patterned paper-based sensors for rapid point-of-care detection and antibiotic-resistance testing of bacterial infections. Biosens. Bioelectron. 2020, 152, 112008. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Amicizia, D.; Panatto, D.; Tramalloni, D.; Valle, I.; Gasparini, R. Chapter Six—Quartz-Crystal Microbalance (QCM) for Public Health: An Overview of Its Applications. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 101, pp. 149–211. [Google Scholar]
- Reyes, P.I.; Yang, K.; Zheng, A.; Li, R.; Li, G.; Lu, Y.; Tsang, C.K.; Zheng, S.X.F. Magnesium Zinc Oxide Nanostructure-modified Quartz Crystal Microbalance for Dynamic Monitoring of Antibiotic Effects and Antimicrobial Resistance. Procedia Technol. 2017, 27, 46–47. [Google Scholar] [CrossRef]
- Reyes, P.I.; Yang, K.; Zheng, A.; Li, R.; Li, G.; Lu, Y.; Tsang, C.K.; Zheng, S.X.F. Dynamic monitoring of antimicrobial resistance using magnesium zinc oxide nanostructure-modified quartz crystal microbalance. Biosens. Bioelectron. 2017, 93, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Toosky, M.N.; Grunwald, J.T.; Pala, D.; Shen, B.; Zhao, W.; D’Agostini, C.; Coghe, F.; Angioni, G.; Motolese, G.; Abram, T.J.; et al. A rapid, point-of-care antibiotic susceptibility test for urinary tract infections. J. Med. Microbiol. 2020, 69, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Abram, T.J.; Cherukury, H.; Ou, C.Y.; Vu, T.; Toledano, M.; Li, Y.; Grunwald, J.T.; Toosky, M.N.; Tifrea, D.F.; Slepenkin, A.; et al. Rapid bacterial detection and antibiotic susceptibility testing in whole blood using one-step, high throughput blood digital PCR. Lab Chip 2020, 20, 477–489. [Google Scholar] [CrossRef]
- Wistrand-Yuen, P.; Malmberg, C.; Fatsis-Kavalopoulos, N.; Lübke, M.; Tängdén, T.; Kreuger, J. A Multiplex Fluidic Chip for Rapid Phenotypic Antibiotic Susceptibility Testing. mBio 2020, 11, e03109–e03119. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jung, Y.-G.; Kim, J.; Kim, S.; Jung, Y.; Na, H.; Kwon, S. Rapid antibiotic susceptibility testing by tracking single cell growth in a microfluidic agarose channel system. Lab Chip 2013, 13, 280–287. [Google Scholar] [CrossRef]
- Baltekin, Ö.; Boucharin, A.; Tano, E.; Andersson, D.I.; Elf, J. Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging. Proc. Natl. Acad. Sci. USA 2017, 114, 9170. [Google Scholar] [CrossRef]
- Li, H.; Torab, P.; Mach, K.E.; Surrette, C.; England, M.R.; Craft, D.W.; Thomas, N.J.; Liao, J.C.; Puleo, C.; Wong, P.K. Adaptable microfluidic system for single-cell pathogen classification and antimicrobial susceptibility testing. Proc. Natl. Acad. Sci. USA 2019, 116, 10270–10279. [Google Scholar] [CrossRef]
- Adagio™. Adagio™ Antimicrobial Susceptibility Testing System. Available online: https://www.diagnostics-bio-rad.com/wp-content/uploads/2016/11/2015-Adagio-Brochure-EN.pdf (accessed on 17 July 2020).
- Strauss, M.; Zoabi, K.; Sagas, D.; Reznik-Gitlitz, B.; Colodner, R. Evaluation of Bio-Rad® discs for antimicrobial susceptibility testing by disc diffusion and the ADAGIO™ system for the automatic reading and interpretation of results. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 375–384. [Google Scholar] [CrossRef]
- Idelevich, E.A.; Becker, K.; Schmitz, J.; Knaack, D.; Peters, G.; Köck, R. Evaluation of an Automated System for Reading and Interpreting Disk Diffusion Antimicrobial Susceptibility Testing of Fastidious Bacteria. PLoS ONE 2016, 11, e0159183. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.; Di Martino, T. Effective implementation of the Accelerate Pheno™ system for positive blood cultures. J. Antimicrob. Chemother. 2019, 74, i40–i43. [Google Scholar] [CrossRef] [PubMed]
- Van Belkum, A.; Burnham, C.-A.D.; Rossen, J.W.A.; Mallard, F.; Rochas, O.; Dunne, W.M. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Barman, P.; Chopra, S.; Thukral, T. Direct testing by VITEK(®) 2: A dependable method to reduce turnaround time in Gram-negative bloodstream infections. J. Lab. Phys. 2018, 10, 260–264. [Google Scholar] [CrossRef]
- Lutgring, J.D.; Bittencourt, C.; McElvania TeKippe, E.; Cavuoti, D.; Hollaway, R.; Burd, E.M. Evaluation of the Accelerate Pheno System: Results from Two Academic Medical Centers. J. Clin. Microbiol. 2018, 56, e01672-17. [Google Scholar] [CrossRef]
- Anton-Vazquez, V.; Adjepong, S.; Suarez, C.; Planche, T. Evaluation of a new Rapid Antimicrobial Susceptibility system for Gram-negative and Gram-positive bloodstream infections: Speed and accuracy of Alfred 60AST. BMC Microbiol. 2019, 19, 268. [Google Scholar] [CrossRef] [PubMed]
- Kost, G.J. Geospatial Spread of Antimicrobial Resistance, Bacterial and Fungal Threats to COVID-19 Survival, and Point-of-Care Solutions. Arch. Pathol. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Burns, J.L.; Saiman, L.; Whittier, S.; Krzewinski, J.; Liu, Z.; Larone, D.; Marshall, S.A.; Jones, R.N. Comparison of two commercial systems (Vitek and MicroScan-WalkAway) for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Diagn. Microbiol. Infect. Dis. 2001, 39, 257–260. [Google Scholar] [CrossRef]
- Yamakawa, H.; Kosai, K.; Kawamoto, Y.; Akamatsu, N.; Matsuda, J.; Kaku, N.; Uno, N.; Morinaga, Y.; Hasegawa, H.; Yanagihara, K. Performance evaluation of BD Phoenix™, an automated microbiology system, for the screening of IMP-producing Enterobacteriaceae. J. Microbiol. Methods 2018, 145, 47–49. [Google Scholar] [CrossRef]
- Dickenson, R.A.; Chapin, K.C. Comparative evaluation of the Sensititre ARIS 2X and the BD Phoenix automated identification and antimicrobial susceptibility test systems. In Proceedings of the American Society for Microbiology Annual Meeting, Orlando, FL, USA, 21–25 May 2006. [Google Scholar]
- MarketsandMarkets™. Antimicrobial Susceptibility Testing Market by Product (Manual, Automated Susceptibility Testing System), Type (Antibacterial, Antifungal), Application (Clinical Diagnostics), Method (ETEST, Disk Diffusion), End-User—Global Forecasts to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/antimicrobial-susceptibility-testing-market-206359984.html?gclid=Cj0KCQiA6t6ABhDMARIsAONIYyxnArdZQp3cfWqttKKKLOJw8d40ilYzt_KfHs0h_T9VYTzh652A6jMaAqgzEALw_wcB (accessed on 15 January 2021).
- MarketResearch.com. Antimicrobial Susceptibility Testing Market by Product (Manual Testing, Automated AST, Consumable), Method (Disk Diffusion, Dilution), Application (Clinical Diagnosis, Epidemiology), End User (Diagnostic Laboratories, Hospital)—Forecast to 2027. Available online: https://www.meticulousresearch.com/pressrelease/78/antimicrobial-susceptibility-testing-market-2027 (accessed on 15 January 2021).
- El-Bouri, K.; Johnston, S.; Rees, E.; Thomas, I.; Bome-Mannathoko, N.; Jones, C.; Reid, M.; Ben-Ismaeil, B.; Davies, A.P.; Harris, L.G.; et al. Comparison of bacterial identification by MALDI-TOF mass spectrometry and conventional diagnostic microbiology methods: Agreement, speed and cost implications. Br. J. Biomed. Sci. 2012, 69, 47–55. [Google Scholar] [CrossRef]
- Moschou, D.; Tserepi, A. The lab-on-PCB approach: Tackling the μTAS commercial upscaling bottleneck. Lab Chip 2017, 17, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Sifakis, F.; Harbarth, S.; Schrijver, R.; van Mourik, M.; Voss, A.; Sharland, M.; Rajendran, N.B.; Rodríguez-Baño, J. Surveillance for control of antimicrobial resistance. Lancet Infect Dis. 2018, 18, e99–e106. [Google Scholar] [CrossRef]
| Name | Type of Tool | Link |
|---|---|---|
| RGI | Assembly-based | https://card.mcmaster.ca/analyze/rgi (accessed on 15 January 2021) |
| CARD | Assembly-based | https://card.mcmaster.ca/ (accessed on 15 January 2021) |
| ARGs-OAP (v2 | Assembly-based | https://galaxyproject.org/use/args-oap/ (accessed on 15 January 2021) |
| ARIBA | Assembly-based | https://github.com/sanger-pathogens/ariba (accessed on 15 January 2021) |
| NCBI–AMRFinder | Assembly-based | https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/AMRFinder/ (accessed on 15 January 2021) |
| PointFinder | Assembly-based | https://cge.cbs.dtu.dk/services/ResFinder/ (accessed on 15 January 2021) |
| ShortBRED | Read-based | http://huttenhower.sph.harvard.edu/shortbred (accessed on 15 January 2021) |
| SEAR | Read-based | https://github.com/will-rowe/SEAR (accessed on 15 January 2021) |
| KmerResistance | Read-based | https://cge.cbs.dtu.dk/services/KmerResistance/ (accessed on 15 January 2021) |
| PATRIC | Read-based | www.patricbrc.org (accessed on 15 January 2021) |
| SSTAR | Read-based | https://github.com/tomdemanbio/Sequence-Search-Toolfor-Antimicrobial-Resistance-SSTAR (accessed on 15 January 2021) |
| DeepArgs | Read-based | https://bench.cs.vt.edu/deeparg (accessed on 15 January 2021) |
| GROOT | Read-based | https://github.com/will-rowe/groot (accessed on 15 January 2021) |
| Organism | Antibiotic | Year (Reference) |
|---|---|---|
| E. coli | Polymyxins | 2018 [122] |
| E. coli | Colistin | 2019 [123] |
| E. coli Klebsiella pneumoniae | Beta-lactams (ESBL-producing isolates) | 2019 [124] |
| Staphylococcus aureus S. intermedius S. pseudintermedius | Novobiocin Polymyxin B Acriflavine | 2019 [125] |
| S. aureus | Methicillin | 2019 [126] |
| Candida auris | Echinocandins | 2019 [127] |
| K. pneumoniae Bacteroides fragilis S. aureus | Carbapenems (carbapenemase-producing isolates) Methicillin | 2019 [128] |
| Enterobacteriaceae | Carbapenems (carbapenemase-producing isolates) | 2019 [129] |
| Enterobacteriaceae | Carbapenem | 2019 [130] |
| Pseudomonas aeruginosa | Beta-lactams (MBL) | 2019 [131] |
| Enterococcus faecium | Vancomycin | 2019 [132] |
| K. pneumoniae | Carbapenems (carbapenemase-producing isolates) | 2019 [133] |
| Acinetobacter baumannii | Colistin | 2020 [134] |
| E. coli K. pneumoniae | Cefotaxime Meropenem, Ciprofloxacin | 2020 [135] |
| S. aureus Enterococcus species E. coli K. pneumoniae | Oxacillin (methicillin) Vancomycin Ceftriaxone Meropenem | 2020 [136] |
| Enterobacterales | Imipenem/Relebactam | 2020 [137] |
| S. aureus | Methicillin | 2020 [138] |
| Category | Method | LoD 1 | TAT 2 | Reference |
|---|---|---|---|---|
| Microfluidic | Optical (laser) | 20-25 cells | 3-5 h | [15] |
| Microfluidic | Colorimetric | 100 CFU/mL | 24 h | [166] |
| Microfluidic | Colorimetric | N/A 3 | 24 h | [165] |
| Microfluidic | Colorimetric | N/A | 15 h | [169] |
| Microfluidic | Colorimetric | N/A | Overnight | [170] |
| Microfluidic | Microscopy | N/A | 4 h | [177] |
| Microfluidic | Microscopy | N/A | 30 min | [178] |
| Microfluidic | Microscopy | N/A | 33 min | [179] |
| Microfluidic | Microscopy | N/A | 5 h | [176] |
| Cuvette | Microscopy | 50 CFU/mL | 2 h | [174] |
| Microfluidic | Electrochemical (pH) | 100 cells | 3 min | [168] |
| Microfluidic | FT-RIFS | N/A | 2 h | [167] |
| Microfluidic | QCM | N/A | 10 min | [172] |
| Microfluidic | Digital PCR | 10 CFU/mL | 1 h | [175] |
| Microfluidic | SERS | 10 3 CFU/mL | N/A | [162] |
| Microfluidic | SERS | 10 3 CFU/mL | N/A | [163] |
| Name | Link | Detection Method | TAT 1 | ID 2 | AST 3 | MIC 4 | Reference |
|---|---|---|---|---|---|---|---|
| VITEK® 2 Compact (bioMérieux SA) | https://www.biomerieux-diagnostics.com/vitekr-2-compact-0 (accessed on 15 January 2021) | Turbidity | 2–18 h | • | • | [185] | |
| Adagio™ Antimicrobial Susceptibility Testing System (Bio-Rad Laboratories) | https://www.diagnostics-bio-rad.com/wp-content/uploads/2016/11/2015-Adagio-Brochure-EN.pdf (accessed on 15 January 2021) | Imaging device measuring the size of the inhibition zone around antibiotic discs | • | • | [180] | ||
| Accelerate Pheno™ (Accelerate Diagnostics, Inc.) | https://acceleratediagnostics.com/products/accelerate-pheno-system/ (accessed on 15 January 2021) | Fluorescence in-situ hybridization (FISH) | ≈ 7 | • | • | • | [186] |
| Alfred 60AST system (Alifax S.r.l.) | https://www.alifax.com/products/bacteriology-line/show/alfred-60 (accessed on 15 January 2021) | Light Scattering Technology/ Turbidity | 4–6 h | • | [187] | ||
| ProMax ® (GeneFluidics, Inc.) | http://genefluidics.com/20151123/wp-content/uploads/2018/08/ProMax.pdf (accessed on 15 January 2021) | Electrochemical-based sensors based on sandwich hybridization of capture and detector probes with target 16S rRNA | 3 h | • | |||
| UtiMax® (GeneFluidics, Inc.) | http://genefluidics.com/20151123/wp-content/uploads/2018/08/UtiMax.pdf (accessed on 15 January 2021) | Electrochemical-based sensors based on sandwich hybridization of capture and detector probes with target 16S rRNA | 3 h | • | • | [27] | |
| BsiMax® (GeneFluidics, Inc.) | http://genefluidics.com/20151123/wp-content/uploads/2018/08/BsiMax.pdf (accessed on 15 January 2021) | Electrochemical-based sensors based on sandwich hybridization of capture and detector probes with target 16S rRNA | 9.5 h | • | • | [188] | |
| MicroScan WalkAway plus System (Beckman Coulter, Inc.) | https://www.beckmancoulter.com/es/products/microbiology/microscan-walkaway-plus-system (accessed on 15 January 2021) | Turbidity | 4 h–overnight | • | • | • | [189] |
| BD Phoenix™ (Becton, Dickinson and Company) | https://www.bd.com/en-us/offerings/capabilities/microbiology-solutions/identification-and-susceptibility-testing/bd-phoenix-automated-identification-and-susceptibility-testing-system (accessed on 15 January 2021) | Turbidity and colorimetric change | 4.5 h | • | • | [190] | |
| Sensititre™ ARIS™ 2X (Thermo Scientific™) | https://www.fishersci.com/shop/products/sensititre-aris-2x-id-ast-inst/stv3090 (accessed on 15 January 2021) | Fluorescence measurement | Overnight (18 h–24 h) | • | • | [191] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Conventional methods | ||
| Phenotypic methods |
|
|
| Molecular-based assays |
|
|
| Non-conventional methods | ||
| WGS 4, WMS 5 |
|
|
| MALDI-TOF MS 6 |
|
|
| FT-IR 7 spectroscopy |
|
|
| Technology | ||
| Microfluidics and Lab-on-a-chip (LoC 9) |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics 2021, 10, 209. https://doi.org/10.3390/antibiotics10020209
Kaprou GD, Bergšpica I, Alexa EA, Alvarez-Ordóñez A, Prieto M. Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics. 2021; 10(2):209. https://doi.org/10.3390/antibiotics10020209
Chicago/Turabian StyleKaprou, Georgia D., Ieva Bergšpica, Elena A. Alexa, Avelino Alvarez-Ordóñez, and Miguel Prieto. 2021. "Rapid Methods for Antimicrobial Resistance Diagnostics" Antibiotics 10, no. 2: 209. https://doi.org/10.3390/antibiotics10020209
APA StyleKaprou, G. D., Bergšpica, I., Alexa, E. A., Alvarez-Ordóñez, A., & Prieto, M. (2021). Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics, 10(2), 209. https://doi.org/10.3390/antibiotics10020209









