Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation
Abstract
1. Introduction
2. Results and Discussions
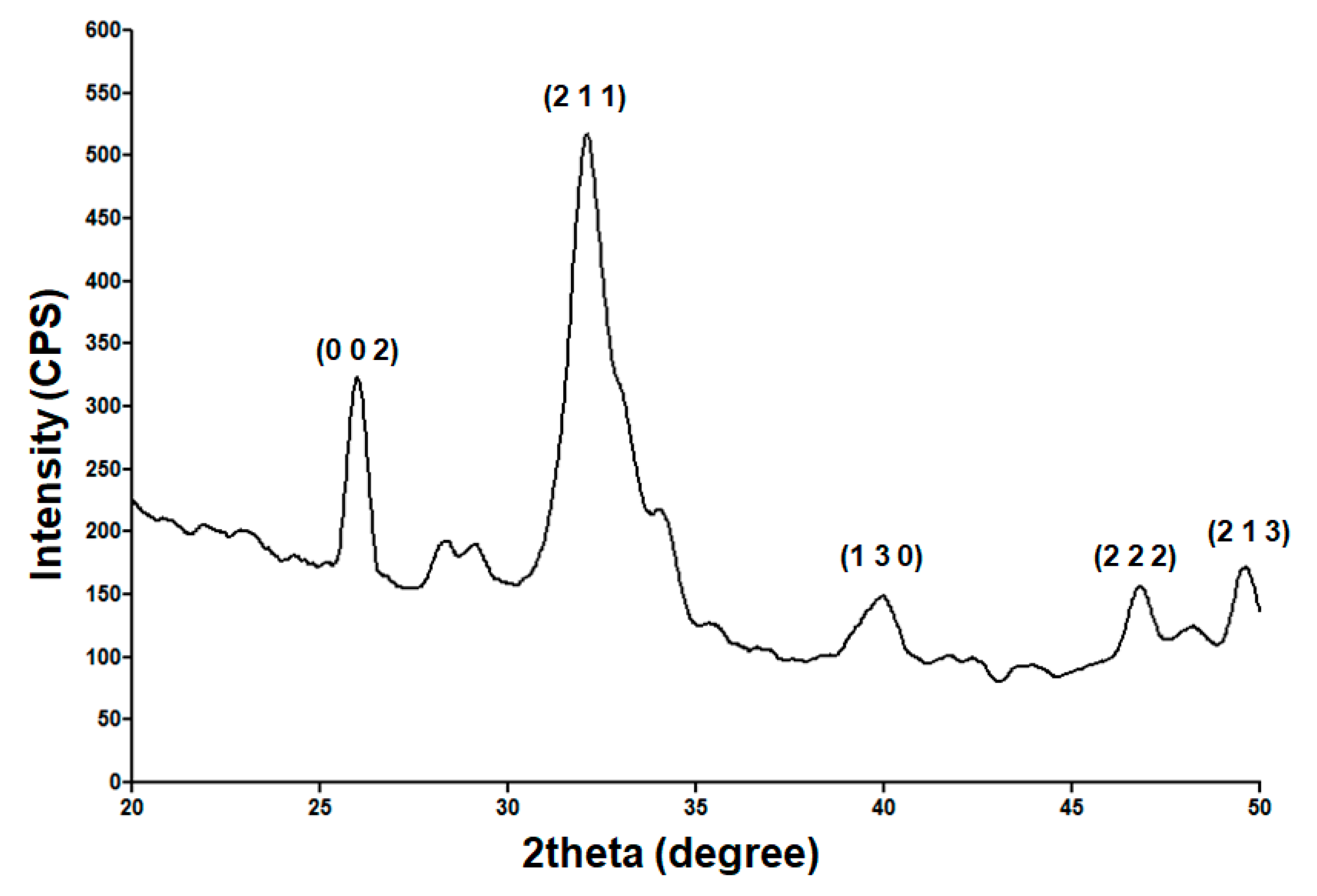
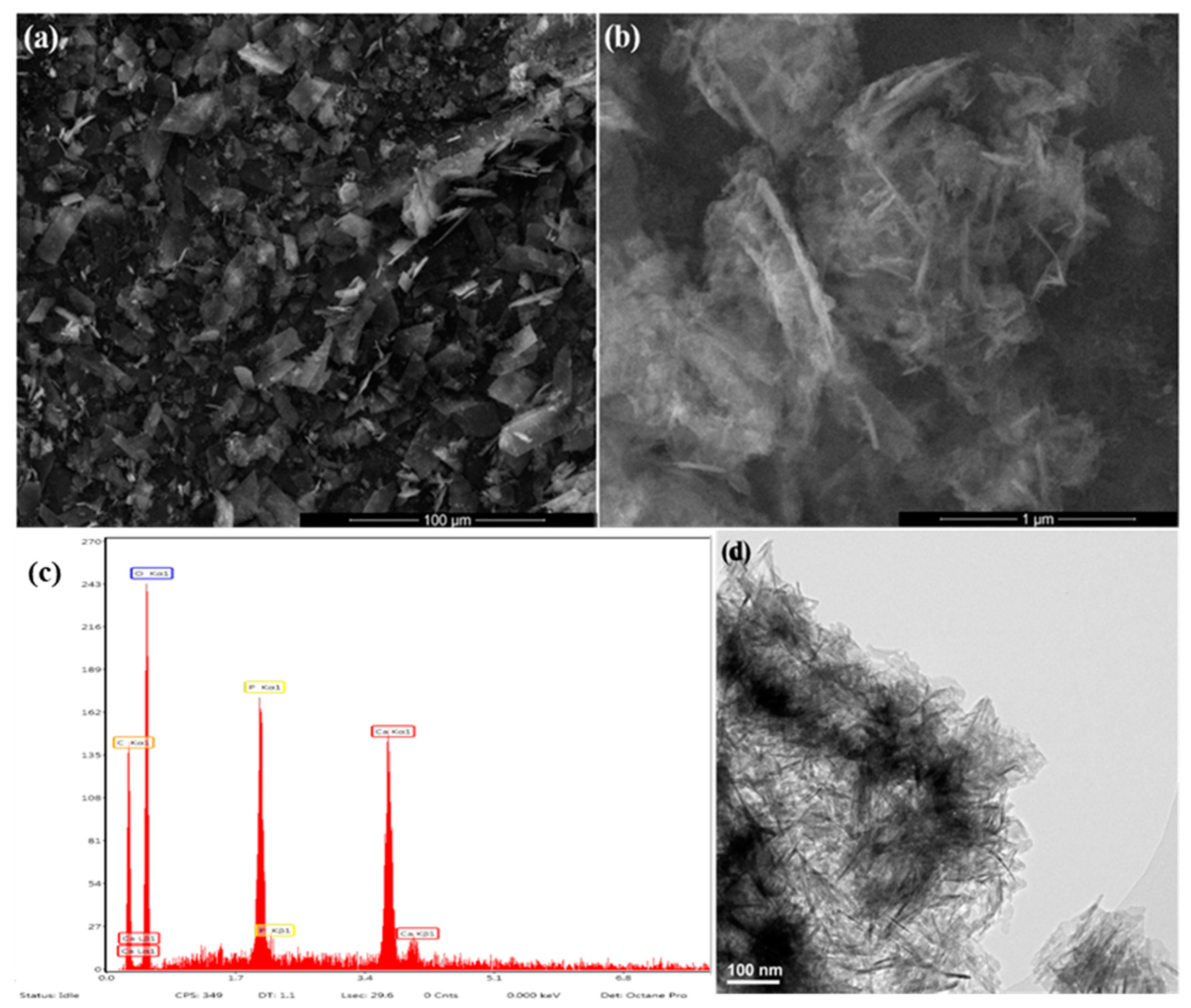
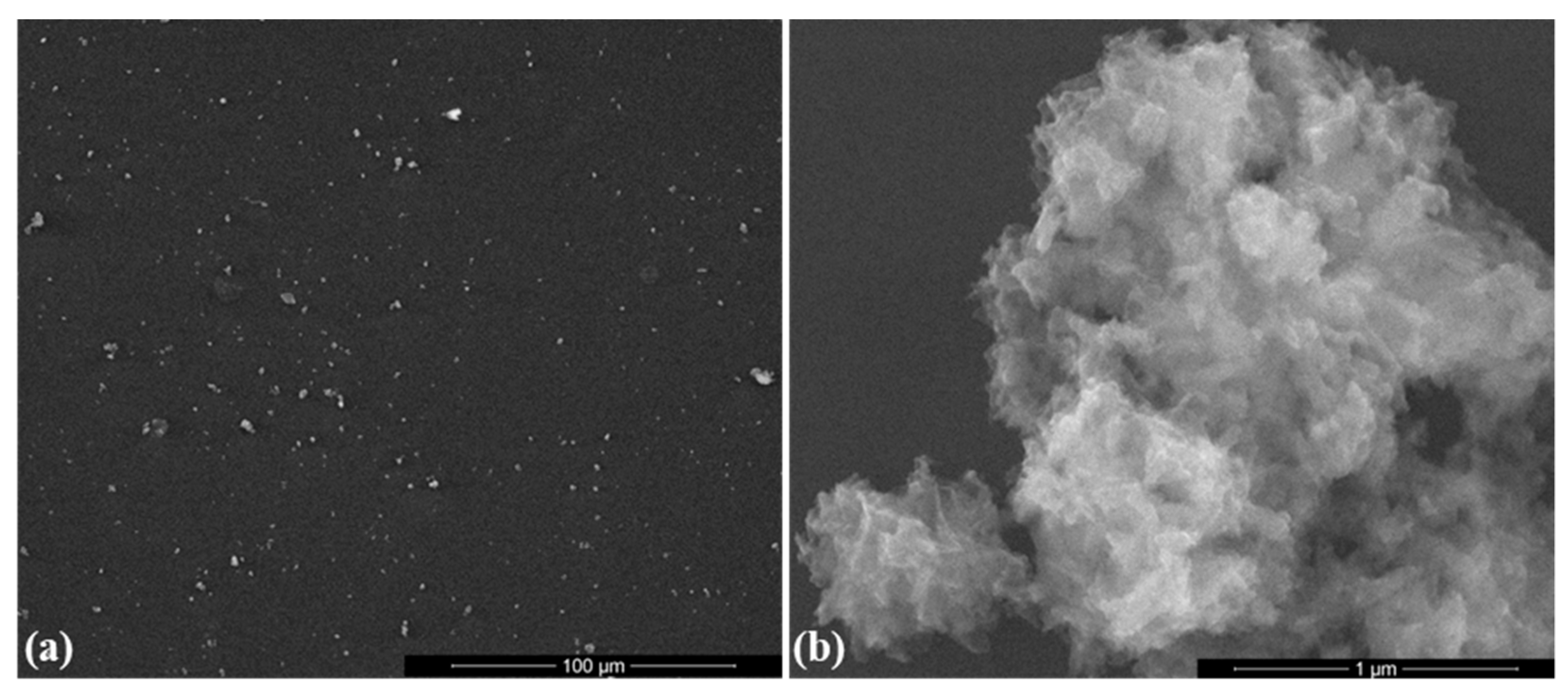
2.1. Physicochemical Investigation of HAp Powder
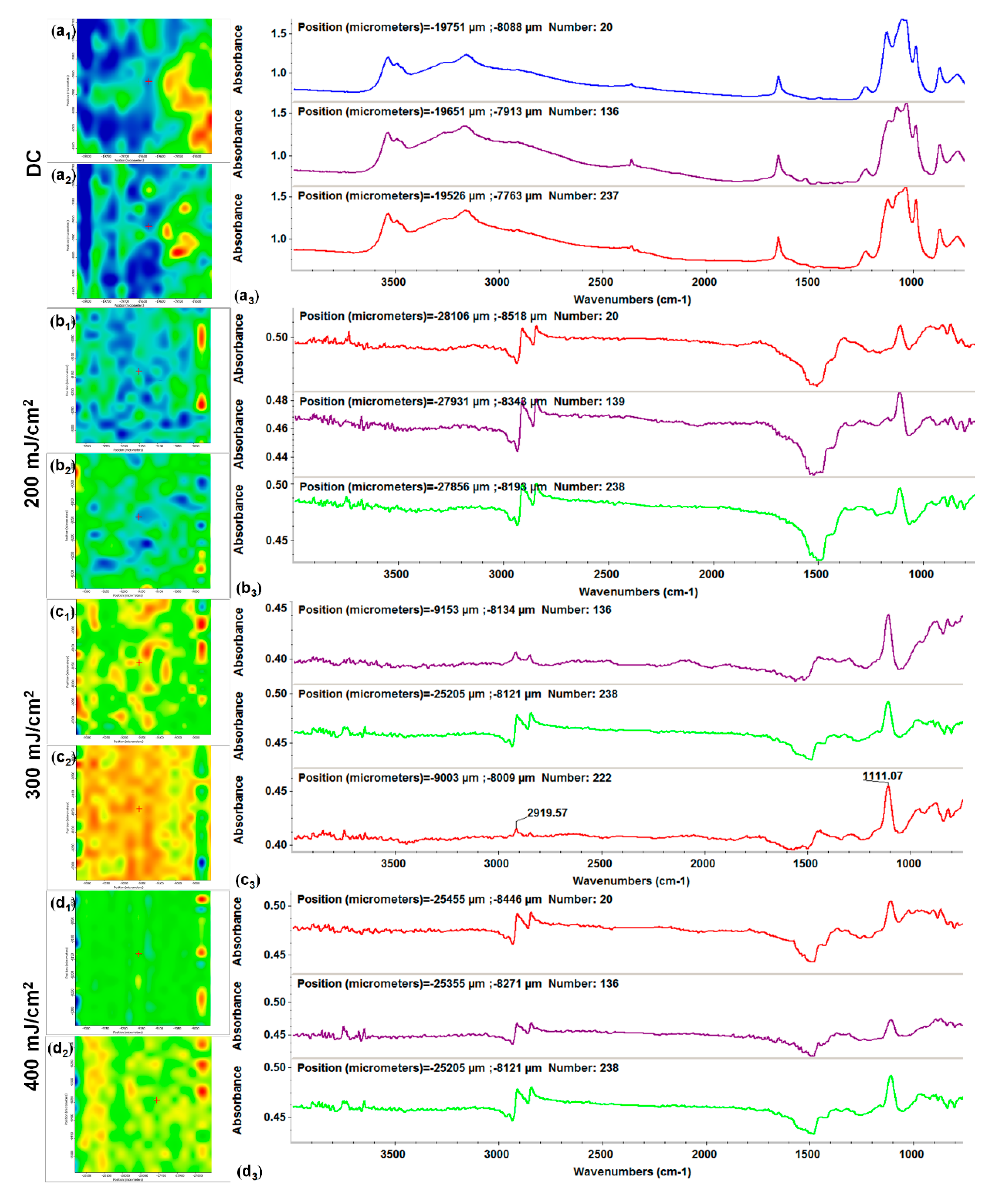
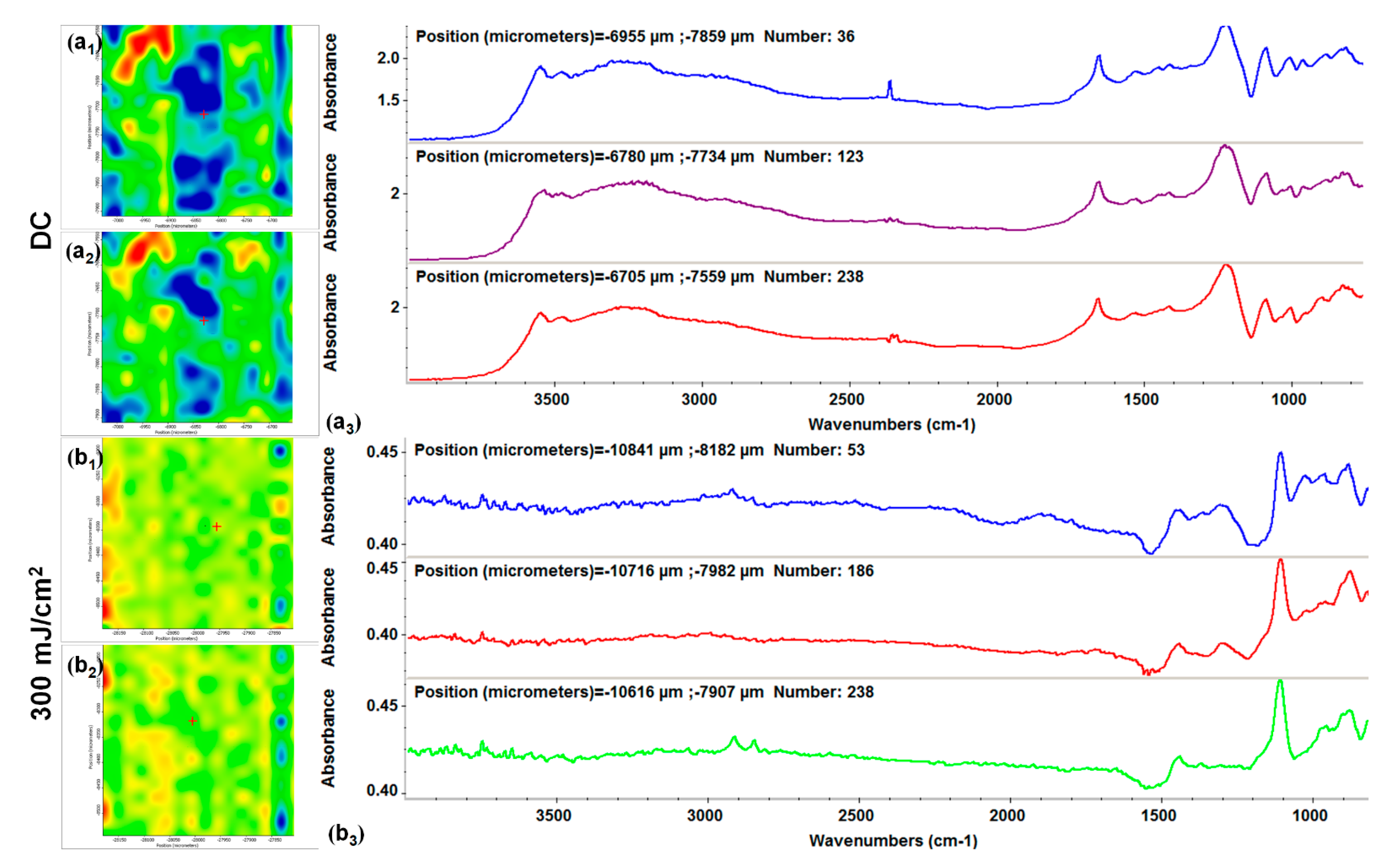
2.2. Physicochemical Investigation of HAp-Based Coatings
2.3. Biological Evaluation of HAp-Based Coatings
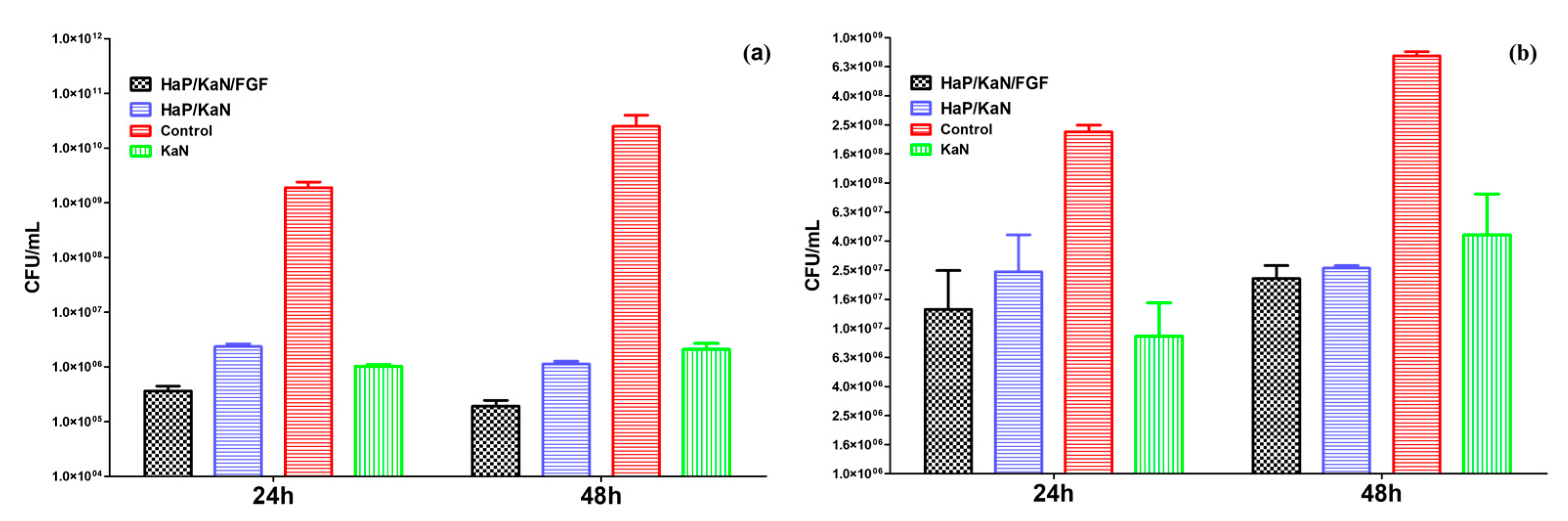
2.4. Microbiological Evaluation of HAp-Based Coatings
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.2.1. Hydroxyapatite (HAp) Synthesis
3.2.2. HAp-Based Coatings Synthesis
3.3. Physicochemical Investigation
3.3.1. X-ray Diffraction (XRD)
3.3.2. Transmission Electron Microscopy (TEM)
3.3.3. Infrared Microscopy (IRM)
3.3.4. Scanning Electron Microscopy (SEM)
3.4. Biocompatibility Evaluation
3.4.1. MTT Cell Viability Assay
3.4.2. Nitric Oxide (NO) Cell Cytotoxicity Assay
3.4.3. Fluorescence Microscopy
3.5. Microbiological Evaluation
3.6. Statistical Analysis of Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, S.-G.; Chen, B.; Lv, D.; Zhang, Y.; Nie, F.-F.; Li, W.; Lv, Y.; Zhao, H.-L.; Liu, H.-M. Evaluation of shoulder function in clavicular fracture patients after six surgical procedures based on a network meta-analysis. Disabil. Rehabil. 2017, 39, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yang, X.; Wang, Q.; Li, C.; Yin, Y.; Han, X. Skeletal stability following bioresorbable versus titanium fixation in orthognathic surgery: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 47, 141–151. [Google Scholar] [CrossRef]
- Chen, Z.; Han, D.; Wang, Q.; Li, L. Four interventions for pediatric femoral shaft fractures: Network meta-analysis of randomized trials. Int. J. Surg. 2020, 80, 53–60. [Google Scholar] [CrossRef]
- Brantley, W.A. Evolution, clinical applications, and prospects of nickel-titanium alloys for orthodontic purposes. J. World Fed. Orthod. 2020, 9, S19–S26. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J. Mech. Behav. Biomed. Mater. 2020, 105, 103671. [Google Scholar] [CrossRef] [PubMed]
- Narra, S.P.; Mittwede, P.N.; DeVincent Wolf, S.; Urish, K.L. Additive Manufacturing in Total Joint Arthroplasty. Orthop. Clin. N. Am. 2019, 50, 13–20. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Pei, X.; Han, J.; Wang, J. Network meta-analysis of survival rate and complications in implant-supported single crowns with different abutment materials. J. Dent. 2019, 88, 103115. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Kumar, K. Sustainability in bio-metallic orthopedic implants. Biointerface Res. Appl. Chem. 2019, 9, 3825–3829. [Google Scholar] [CrossRef]
- Babu, B.S.; Kumar, K. Temperature dependence of indentation behavior of Ti-6Al-4V alloy for bio medical applications. Biointerface Res. Appl. Chem. 2019, 9, 4629–4634. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J.J.P. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef]
- Dias Corpa Tardelli, J.; Bolfarini, C.; Cândido dos Reis, A. Comparative analysis of corrosion resistance between beta titanium and Ti-6Al-4V alloys: A systematic review. J. Trace Elem. Med. Biol. 2020, 62, 126618. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.-C.; Yue, B. Approaches to promoting bone marrow mesenchymal stem cell osteogenesis on orthopedic implant surface. World J. Stem. Cells 2020, 12, 545–561. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Dourado, N.; Pereira, F.; Alves, N.; Miranda, G.; Silva, F.S. Additive manufactured porous biomaterials targeting orthopedic implants: A suitable combination of mechanical, physical and topological properties. Mater. Sci. Eng. C 2020, 107, 110342. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2020, 13, 89. [Google Scholar] [CrossRef]
- Wang, S.; Ogawa, T.; Zheng, S.; Miyashita, M.; Tenkumo, T.; Gu, Z.; Lian, W.; Sasakia, K. The effect of low-magnitude high-frequency loading on peri-implant bone healing and implant osseointegration in Beagle dogs. J. Prosthodont. Res. 2018, 62, 497–502. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Mitra, I.; Bose, S. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar] [CrossRef]
- Kammerlander, C.; Hem, E.S.; Klopfer, T.; Gebhard, F.; Sermon, A.; Dietrich, M.; Bach, O.; Weil, Y.; Babst, R.; Blauth, M. Cement augmentation of the Proximal Femoral Nail Antirotation (PFNA)—A multicentre randomized controlled trial. Injury 2018, 49, 1436–1444. [Google Scholar] [CrossRef]
- Nam, D.; Lawrie, C.M.; Salih, R.; Nahhas, C.R.; Barrack, R.L.; Nunley, R.M. Cemented Versus Cementless Total Knee Arthroplasty of the Same Modern Design: A Prospective, Randomized Trial. J. Bone Jt. Surg. Am. 2019, 101, 1185–1192. [Google Scholar] [CrossRef]
- Parker, M.J.; Cawley, S. Cemented or uncemented hemiarthroplasty for displaced intracapsular fractures of the hip: A randomized trial of 400 patients. Bone Jt. J. 2020, 102, 11–16. [Google Scholar] [CrossRef]
- Zhuang, Y.; Gan, Y.; Shi, D.; Zhao, J.; Tang, T.; Dai, K. A novel cytotherapy device for rapid screening, enriching and combining mesenchymal stem cells into a biomaterial for promoting bone regeneration. Sci. Rep. 2017, 7, 15463. [Google Scholar] [CrossRef]
- Shadmanfar, S.; Labibzadeh, N.; Emadedin, M.; Jaroughi, N.; Azimian, V.; Mardpour, S.; Abbasi Kakroodi, F.; Bolurieh, T.; Hosseini, S.E.; Chehrazi, M.; et al. Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018, 20, 499–506. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chung, P.K.; Suh, D.S.; Heo, D.B.; Tak, D.H.; Koh, Y.G. Implantation of mesenchymal stem cells in combination with allogenic cartilage improves cartilage regeneration and clinical outcomes in patients with concomitant high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 544–554. [Google Scholar] [CrossRef]
- Johar, A.O. Ridge Augmentation with Autogenous Bone Graft and Expanded Polytetrafluoroethylene Membrane using Tenting Screw: A Randomized Controlled Clinical Trial. J. Contemp. Dent. Pract. 2019, 20, 409–416. [Google Scholar] [CrossRef]
- Atef, M.; Osman, A.H.; Hakam, M. Autogenous interpositional block graft vs onlay graft for horizontal ridge augmentation in the mandible. Clin. Implant. Dent. Relat. Res. 2019, 21, 678–685. [Google Scholar] [CrossRef]
- Mendoza-Azpur, G.; de la Fuente, A.; Chavez, E.; Valdivia, E.; Khouly, I. Horizontal ridge augmentation with guided bone regeneration using particulate xenogenic bone substitutes with or without autogenous block grafts: A randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Mariotti, G.; Pagliaro, U.; Mazzoni, A.; Moscatelli, M.; Nieri, M. The Fence Technique: 100% Autogenous Bone Graft vs 50% Deproteinized Bovine Bone Matrix and 50% Autogenous Bone Graft. A Histologic Randomized Controlled Trial. Int. J. Periodontics Restor. Dent. 2020, 40, 181–190. [Google Scholar] [CrossRef]
- Bruschi, G.B.; Crespi, R.; Capparè, P.; Gherlone, E. Transcrestal sinus floor elevation: A retrospective study of 46 patients up to 16 years. Clin. Implant Dent. Relat Res. 2012, 14, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Sinus floor elevation by osteotome: Hand mallet versus electric mallet. A prospective clinical study. Int. J. Oral. Maxillofac Implants. 2012, 27, 1144–1150. [Google Scholar] [PubMed]
- Bruschi, G.B.; Crespi, R.; Capparè, P.; Bravi, F.; Bruschi, E.; Gherlone, E. Localized Management of Sinus Floor Technique for Implant Placement in Fresh Molar Sockets. Clin. Implant Dent. Relat. Res. 2013, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- La Monaca, G.; Iezzi, G.; Cristalli, M.P.; Pranno, N.P.; Sfasciotti, G.L.; Vozza, I. Comparative Histological and Histomorphometric Results of Six Biomaterials Used in Two-Stage Maxillary Sinus Augmentation Model after 6-Month Healing. Biomed. Res. Int. 2018, 2018, 9430989. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2019, 714, 636–667. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Popa, M.; Hussien, M.D.; Cârstea, I.A.; Grigore, R.; Lazăr, V.; Bezirtzoglou, E.; Chifiriuc, M.C.; Sakizlian, M.; Berteşteanu, Ş. Insights on metal based dental implants and their interaction with the surrounding tissues. Curr. Top Med. Chem. 2015, 15, 1614–1621. [Google Scholar] [CrossRef]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Geesink, R.G.T.; Manley, M.T. (Eds.) Hydroxyapatite Coatings in Orthopaedic Surgery; Raven Press: New York, NY, USA, 1993. [Google Scholar]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Dizaj, S.M. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Maleki, A.; Lotfipour, F.; Sharifi, S.; Rezaie, F.; Samiei, M. Porous hydroxyapatite-gelatin nanoscaffolds for bone and teeth applications. Biointerface Res. Appl. Chem. 2018, 8, 3670–3673. [Google Scholar]
- Sharifi, S.; Mokhtarpour, M.; Jahangiri, A.; Dehghanzadeh, S.; Maleki-Dizaj, S.; Shahi, S. Hydroxyapatite nanofibers as beneficial nanomaterial in dental sciences. Biointerface Res. Appl. Chem. 2018, 8, 3695–3699. [Google Scholar]
- Surmenev, R.A.; Surmeneva, M.A. A critical review of decades of research on calcium phosphate–based coatings: How far are we from their widespread clinical application? Curr. Opin. Biomed. Eng. 2019, 10, 35–44. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence properties biomineralization pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Pires, L.C.; Capalbo da Silva, R.; Poli, P.P.; Esgalha, F.R.; Hadad, H.; Pitol Palin, L.; Piquera Santos, A.F.; Teixiera Colombo, L.; Kawamata de Jesus, L.; Farnezi Bassi, A.P.; et al. Evaluation of Osteoconduction of a Synthetic Hydroxyapatite/β-Tricalcium Phosphate Block Fixed in Rabbit Mandibles. Materials 2020, 13, 4902. [Google Scholar] [CrossRef]
- Hofmann, A.; Gorbulev, S.; Guehring, T.; Schulz, A.P.; Schupfner, R.; Raschke, M.; Huber-Wagner, S.; Rommens, P.M. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J. Bone Jt. Surg. Am. 2020, 102, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implant. 2011, 26, 866–872. [Google Scholar] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: A histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants 2011, 26, 1057–1062. [Google Scholar] [PubMed]
- Nam, J.W.; Khureltogtokh, S.; Choi, H.M.; Lee, A.R.; Park, Y.B.; Kim, H.J. Randomised controlled clinical trial of augmentation of the alveolar ridge using recombinant human bone morphogenetic protein 2 with hydroxyapatite and bovine-derived xenografts: Comparison of changes in volume. Br. J. Oral Maxillofac. Surg. 2017, 55, 822–829. [Google Scholar] [CrossRef]
- Oh, J.-S.; Seo, Y.-S.; Lee, G.-J.; You, J.-S.; Kim, S.-G. A Comparative Study with Biphasic Calcium Phosphate to Deproteinized Bovine Bone in Maxillary Sinus Augmentation: A Prospective Randomized and Controlled Clinical Trial. Int. J. Oral Maxillofac. Implants 2019, 34, 233–242. [Google Scholar] [CrossRef]
- Chen, F.-M.; Zhangm, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015, 29, 1463–1486. [Google Scholar] [CrossRef]
- Coffin, J.D.; Homer-Bouthiette, C.; Hurley, M.M. Fibroblast Growth Factor 2 and Its Receptors in Bone Biology and Disease. J. Endocr. Soc. 2018, 2, 657–671. [Google Scholar] [CrossRef]
- Narayanan, A.; Srinaath, N.; Rohini, M.; Selvamurugan, N. Regulation of Runx2 by MicroRNAs in osteoblast differentiation. Life Sci. 2019, 232, 116676. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. A Prospective Longitudinal Study on Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients with 1-Year Follow-Up: The Role of CD4+ Level, Smoking Habits, and Oral Hygiene. Clin. Implant Dent. Relat. Res. 2016, 18, 955–964. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients: A Prospective Longitudinal Study with 1-Year Follow-Up. Clin. Implant. Dent. Relat. Res. 2016, 18, 725–734. [Google Scholar] [CrossRef]
- Miwa, S.; Shirai, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Tada, K.; Kajino, Y.; Higuchi, T.; Abe, K.; Aiba, H.; et al. Risk factors for surgical site infection after malignant bone tumor resection and reconstruction. BMC Cancer 2019, 19, 33. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Cheng, M.; Liang, J.; Zhang, Y.; Hu, L.; Gong, P.; Cai, R.; Zhang, L.; Zhang, H.; Ge, J.; Ji, Y.; et al. The bacteriophage EF-P29 efficiently protects against lethal vancomycin-resistant Enterococcus faecalis and alleviates gut microbiota imbalance in a murine bacteremia model. Front. Microbiol. 2017, 8, 837. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Podgoreanu, P.; Negrea, S.M.; Buia, R.; Delcaru, C.; Trusca, S.B.; Lazar, V.; Chifiriuc, M.C. Alternative strategies for fighting multidrug resistant bacterial infections. Biointerface Res. Appl. Chem. 2019, 9, 3834–3841. [Google Scholar]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcona, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Yuan, Z.; He, Y.; Lin, C.; Liu, P.; Cai, K. Antibacterial surface design of biomedical titanium materials for orthopedic applications. J. Mater. Sci. Technol. 2021, 78, 51067. [Google Scholar] [CrossRef]
- Buranapanitkit, B.; Srinilta, V.; Ingviga, N.; Oungbho, K.; Geater, A.; Ovatlarnporn, C. The efficacy of a hydroxyapatite composite as a biodegradable antibiotic delivery system. Clin. Orthop. Relat. Res. 2004, 424, 244–252. [Google Scholar] [CrossRef]
- Predoi, D.; Popa, C.L.; Chapon, P.; Groza, A.; Iconaru, S.L. Evaluation of the Antimicrobial Activity of Different Antibiotics Enhanced with Silver-Doped Hydroxyapatite Thin Films. Materials 2016, 9, 778. [Google Scholar] [CrossRef]
- Macha, I.J.; Ben-Nissan, B.; Santos, J.; Cazalbou, S.; Milthorpe, B. Hydroxyapatite/PLA biocomposite thin films for slow drug delivery of antibiotics for the treatment of bone and implant-related infections. Key Eng. Mater. 2016, 696, 271–276. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Yates, M.Z. Hydroxyapatite Nanocrystal Deposited Titanium Dioxide Nanotubes Loaded with Antibiotics for Combining Biocompatibility and Antibacterial Properties. Biomater. Soft Mater. 2018, 3, 1703–1709. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanodimensional and nanocrystallineapatites and other calcium orthophosphates in biomedical engineering, biology and medicine. Materials 2009, 2, 1975–2045. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B. Calcium phosphate nano-coatings and nanocomposites, part I: Recent develop-ments and advancements in tissue engineering and bioimaging. Nanomedicine 2015, 10, 2249–2261. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Choi, A.H. Sol-gel production of bioactive nanocoatings for medical applications: Part I: Anintroduction. Nanomedicine 2006, 1, 311–319. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive Surfaces of Polylactide and Silver Nanoparticles for the Prevention of Microbial Contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef]
- Grumezescu, V.; Negut, I.; Gherasim, O.; Birca, A.C.; Grumezescu, A.M.; Hudita, A.; Galateanu, B.; Costache, M.; Andronescu, E.; Holban, A.M. Antimicrobial applications of MAPLE processed coatings based on PLGA and lincomycin functionalized magnetite nanoparticles. Appl. Surf. Sci. 2019, 484, 587–599. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, J.; Lee, K.M.; Jin, Y.Z.; Yun, H.-S.; Hyun, G.; Kim, G.H.; Lee, J.H. Enhanced healing of rat calvarial defects with 3D printed calcium-deficient hydroxyapatite/collagen/bone morphogenetic protein 2 scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 108, 103782. [Google Scholar] [CrossRef]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.; Jiang, K.; Yang, X.; Watanabe, N.; Mayo, K.H.; et al. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater. Sci. Eng. C 2020, 116, 111158. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhu, L.; Huang, X.; Tang, D.; He, D.; Shi, J.; Xu, G. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci. Rep. 2017, 7, 7814. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomed. Nanotech. Biol. Med. 2018, 14, 1987–1997. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Immobilization of BMP-2 and VEGF within Multilayered Polydopamine-Coated Scaffolds and the Resulting Osteogenic and Angiogenic Synergy of Co-Cultured Human Mesenchymal Stem Cells and Human Endothelial Progenitor Cells. Int. J. Molec. Sci. 2020, 21, 6418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Han, Y.; Li, J.; Cai, B.; Gao, H.; Feng, W.; Li, S.; Liu, J.; Li, D. BMP-2 immobilized PLGA/hydroxyapatite fibrous scaffold via polydopamine stimulates osteoblast growth. Mater. Sci. Eng. C 2017, 78, 658–666. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Fu, C.; Yang, F.; Jiao, Z.; Shi, X.; Ito, Y.; Wang, Z.; Liu, Q.; Zhang, P. Micro-porous polyetheretherketone implants decorated with BMP-2 via phosphorylated gelatin coating for enhancing cell adhesion and osteogenic differentiation. Colloid. Surf. B 2018, 169, 233–241. [Google Scholar] [CrossRef]
- Yun, Y.J.; Kim, H.-J.; Lee, D.-W.; Um, S.; Chun, H.J. Polydopamine-mediated surface modifications of poly l-lactic acid with hydroxyapatite, heparin and bone morphogenetic protein-2 and their effects on osseointegration. J. Ind. Eng. Chem. 2018, 67, 244–254. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.J.; Fernández-Villa, D.; Serrano, M.C.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomaterialia 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Ullah, I.; Li, W.; Lei, S.; Zhang, Y.; Zhang, W.; Farooq, U.; Ullah, S.; Ullah, M.W.; Zhang, X. Simultaneous co-substitution of Sr2+/Fe3+ in hydroxyapatite nanoparticles for potential biomedical applications. Ceram. Int. 2018, 44, 21338–21348. [Google Scholar] [CrossRef]
- Veerla, S.C.; Kim, D.R.; Kim, J.; Sohn, H.; Yang, S.Y. Controlled nanoparticle synthesis of Ag/Fe co-doped hydroxyapatite system for cancer cell treatment. Mater. Sci. Eng. C 2019, 98, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Hirota, K.; Mizutani, T.; Aoyama, Y. Co-precipitation of tapioca starch and hydroxyapatite. Effects of phosphorylation of starch on mechanical properties of the composites. Results Mater. 2019, 3, 100035. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Scolaro, C.; Giofré, S.V.; Romeo, R.; Visco, A. Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties. Polymers 2020, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.H.; Lin, Y.W.; Lin, F.H.; Sun, J.S.; Chao, Y.H.; Lin, H.Y.; Chang, Z.C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 6002. [Google Scholar] [CrossRef]
- Ciocca, L.; Lesci, I.G.; Ragazzini, S.; Gioria, S.; Valsesia, A.; Parrilli, A.; Spadari, A.; Dozza, B.; Mora, P.; Piattelli, A.; et al. Nanostructured surface bioactive composite scaffold for filling of bone defects. Biointerface Res. Appl. Chem. 2020, 10, 5038–5047. [Google Scholar] [CrossRef]
- Szcześ, A.; Yan, Y.; Chibowski, E.; Hołysz, L.; Banach, M. Properties of natural and synthetic hydroxyapatite and their surface free energy determined by the thin-layer wicking method. Appl. Surf. Sci. 2018, 434, 1232–1238. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Fan, F.; Yu, C.; Lu, W.; Du, M. Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts. Mater. Sci. Eng. C 2018, 90, 706–712. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Arcos Navarrete, D. Biological Apatites in Bone and Teeth. In Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; Vallet-Regi, M., Arcos Navarrete, D., Eds.; Royal Society of Chemistry: London, UK, 2015; pp. 1–29. [Google Scholar]
- Grumezescu, V.; Andronescu, E.; Holban, A.M.; Socol, G.; Grumezescu, A.M.; Ficai, A.; Lazar, V.; Chifiriuc, M.C.; Trusca, R.; Iordache, F. Fabrication and characterization of functionalized surfaces with 3-amino propyltrimethoxysilane films for anti-infective therapy applications. Appl. Surf. Sci. 2015, 336, 401–406. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.M.; Sima, L.E.; Chiritoiu, M.B.; Chiritoiu, G.N.; Grumezescu, A.M.; Ivan, L.; Safciuc, F.; Antohe, F.; Florica, C.; et al. Laser deposition of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid)—Lysozyme microspheres based coatings with anti-microbial properties. Int. J. Pharm. 2017, 521, 184–195. [Google Scholar] [CrossRef]
- Anghel, I.; Andronescu, E.; Rădulescu, D.; Rădulescu, M.; Iordache, F.; Vasile, B.Ș.; Surdu, A.V.; Albu, M.G.; Maniu, H.; Chifiriuc, M.C.; et al. Biocompatible 3D Matrix with Antimicrobial Properties. Molecules 2016, 21, 115. [Google Scholar] [CrossRef]
- Ardelean, I.L.; Gudovan, D.; Ficai, D.; Ficai, A.; Andronescu, E.; Albu-Kaya, M.G.; Neacsu, P.; Ion, R.N.; Cimpean, A.; Mitran, V. Collagen/hydroxyapatite bone grafts manufactured by homogeneous/heterogeneous 3D printing. Matter. Lett. 2018, 231, 179–182. [Google Scholar] [CrossRef]
- Rodriguez Chanfrau, J.E. Evaluation of the influence of microwaves radiation on a biomaterial composed of three phases of calcium phosphates. Biointerface Res. Appl. Chem. 2020, 10, 5141–5144. [Google Scholar] [CrossRef]
- Raya, I.; Mayasari, E.; Yahya, A.; Syahrul, M.; Latunra, A.I. Shynthesis and Characterizations of Calcium Hydroxyapatite Derived from Crabs Shells (Portunus pelagicus) and Its Potency in Safeguard against to Dental Demineralizations. Int. J. Biomater. 2015, 2015, 469176. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Wang, Q.; Tan, R. Kanamycin detection based on the catalytic ability enhancement of gold nanoparticles. Biosens. Bioelectron. 2017, 91, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M.T. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of Listeria monocytogenes. Biomed. Pharmacother. 2017, 86, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Guo, M.; Tan, J.; Huang, S.; Tang, Y.; Tan, L.; Liang, Y. A fluorescent molecularly imprinted polymer using aptamer as a functional monomer for sensing of kanamycin. Sens. Actuator. B Chem. 2018, 268, 47–54. [Google Scholar] [CrossRef]
- Nugrahani, I.; Fauzia, R. Quantitative vibrational methods development and its performance comparison to colorimetry on the assay of Kanamycin sulfate. Int. J. Appl. Pharm. 2019, 11, 426–435. [Google Scholar] [CrossRef]
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv. Drug Deliv. Rev. 2012, 64, 1257–1276. [Google Scholar] [CrossRef]
- Kyllönen, L.; D’Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomaterialia 2015, 11, 412–434. [Google Scholar] [CrossRef]
- Shim, I.K.; Chung, H.J.; Jung, M.R.; Nam, S.Y.; Lee, S.Y.; Lee, H.; Heo, S.J.; Lee, S.J. Biofunctional porous anodized titanium implants for enhanced bone regeneration. J. Biomed. Mater. Res. A 2014, 102, 3639–3648. [Google Scholar] [CrossRef]
- Murahashi, Y.; Yano, F.; Nakamoto, H.; Maenohara, Y.; Iba, K.; Yamashita, T.; Tanaka, S.; Ishihara, K.; Okamura, Y.; Moro, T.; et al. Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomaterialia 2019, 85, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, Y.; Ito, A.; Hara, Y.; Mutsuzaki, H.; Murai, S.; Fujii, K.; Sogo, Y.; Hirose, M.; Oyane, A.; Kobayashi, F.; et al. Initial clinical trial of pins coated with fibroblast growth factor-2–apatite composite layer in external fixation of distal radius fractures. J Orthop. 2019, 16, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Song, X.; Chen, Y.; Zheng, Y.; Yin, P.; Lei, T. Zn-HA/Bi-HA biphasic coatings on Titanium: Fabrication, characterization, antibacterial and biological activity. Colloids Surf. B Biointerfaces 2020, 189, 110813. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.Z.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Murugan, N.; Murugan, C.; Sundramoorthy, A.K. In vitro and in vivo characterization of mineralized hydroxyapatite/polycaprolactone-graphene oxide based bioactive multifunctional coating on Ti alloy for bone implant applications. Arabian. J. Chem. 2018, 11, 959–969. [Google Scholar] [CrossRef]
- Batebi, K.; Khazaei, B.A.; Afshar, A. Characterization of sol-gel derived silver/fluor-hydroxyapatite composite coatings on titanium substrate. Surf. Coat. Technol. 2018, 352, 522–528. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Sharonova, A.A.; Chernousova, S.; Prymak, O.; Loza, K.; Tkachev, M.S.; Shulepov, I.A.; Epple, M.; Surmenev, R.A. Incorporation of silver nanoparticles into magnetron-sputtered calcium phosphate layers on titanium as an antibacterial coating. Colloids Surf. B Biointerfaces 2017, 156, 104–113. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T., Jr.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candianid, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Sabzi, M.; Far, S.M.; Dezfuli, S.M. Characterization of bioactivity behavior and corrosion responses of hydroxyapatite-ZnO nanostructured coating deposited on NiTi shape memory alloy. Ceram. Int. 2018, 44, 21395–21405. [Google Scholar] [CrossRef]
- Yazici, H.; Habib, G.; Boone, K.; Urgen, M.; Utku, F.S.; Tamerler, C. Self-assembling antimicrobial peptides on nanotubular titanium surfaces coated with calcium phosphate for local therapy. Mater. Sci. Eng. C 2019, 94, 333–343. [Google Scholar] [CrossRef]
- Ji, X.-J.; Gao, L.; Liu, J.-C.; Wang, J.; Cheng, Q.; Li, J.-P.; Li, S.-Q.; Zhi, K.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and antibacterial properties of hydroxyapatite coating induced by gentamicin-loaded polymeric multilayers on magnesium alloys. Colloids Surf. B Biointerfaces 2019, 179, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Karacan, I.; Ben-Nissan, B.; Wang, H.A.; Juritz, A.; Swain, M.V.; Müller, W.H.; Chou, J.; Stamboulis, A.; Macha, I.J.; Taraschi, V. Mechanical testing of antimicrobial biocomposite coating on metallic medical implants as drug delivery system. Mater. Sci. Eng. C 2019, 104, 109757. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Chen, X.; Zhang, B.; Zhang, H.; Jin, P.; Li, H. Room-temperature deposition of hydroxyapatite/antibiotic composite coatings by vacuum cold spraying for antibacterial applications. Surf. Coat. Technol. 2017, 330, 87–91. [Google Scholar] [CrossRef]
- Rădulescu, D.; Grumezescu, V.; Andronescu, E.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Oprea, A.E.; Rădulescu, M.; Surdu, A.; Trusca, R.; et al. Biocompatible cephalosporin-hydroxyapatite-poly(lactic-co-glycolic acid)-coatings fabricated by MAPLE technique for the prevention of bone implant associated infections. Appl. Surf. Sci. 2016, 374, 387–396. [Google Scholar] [CrossRef]
- Balaure, P.C.; Grumezescu, A.M. Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part I: Molecular Basis of Biofilm Recalcitrance. Passive Anti-Biofouling Nanocoatings. Nanomaterials 2020, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Holban, A.M.; Iordanskii, A.; Grumezescu, A.M.; Bychkova, A.; Andronescu, E.; Mogoantă, L.; Mogoșanu, G.D.; Iordache, F. Prosthetic devices with nanostructurated surfaces for increased resistance to microbial colonization. Curr. Pharm. Biotechnol. 2015, 16, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, Y.; Hong, Y.; Zhao, X.; Reyes, P.I.; Lu, Y. Magnesium Zinc Oxide Nanostructure-Modified Multifunctional Sensors for Full-Scale Dynamic Monitoring of Pseudomonas aeruginosa Biofilm Formation. ECS J. Solid State Sci. Technol. 2020, 9, 115026. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Wang, J.; Wei, H.; Liu, X.; Yan, X.; Zhou, Y.; Liu, Y.; Yang, S. Surface proper-ties and MC3T3-E1 cell response of cortical bone allografts modified with low-concentration phosphoric acid. Cell Physiol. Biochem. 2017, 41, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chen, C.Y.; Ke, C.J.; Chao, Y.H.; Sun, J.S. Traumatic brain injury result in accelerated fracture healing: A Rat Model. J. Clin. Exp. Orthop. 2015, 1, 7. [Google Scholar] [CrossRef]
- Ning, C.; Wang, S.; Zhu, Y.; Zhong, M.; Lin, X.; Zhang, Y.; Tan, G.; Li, M.; Yin, Z.; Yu, P.; et al. Ti nanorod arrays with a medium density significantly promote osteogenesis and osteointegration. Sci. Rep. 2016, 6, 19047. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Negut, I.; Dumitrescu, M.F.; Stan, M.S.; Nica, I.C.; Holban, A.M.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. https://doi.org/10.3390/antibiotics10020160
Gherasim O, Grumezescu AM, Grumezescu V, Negut I, Dumitrescu MF, Stan MS, Nica IC, Holban AM, Socol G, Andronescu E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics. 2021; 10(2):160. https://doi.org/10.3390/antibiotics10020160
Chicago/Turabian StyleGherasim, Oana, Alexandru Mihai Grumezescu, Valentina Grumezescu, Irina Negut, Marius Florin Dumitrescu, Miruna Silvia Stan, Ionela Cristina Nica, Alina Maria Holban, Gabriel Socol, and Ecaterina Andronescu. 2021. "Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation" Antibiotics 10, no. 2: 160. https://doi.org/10.3390/antibiotics10020160
APA StyleGherasim, O., Grumezescu, A. M., Grumezescu, V., Negut, I., Dumitrescu, M. F., Stan, M. S., Nica, I. C., Holban, A. M., Socol, G., & Andronescu, E. (2021). Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics, 10(2), 160. https://doi.org/10.3390/antibiotics10020160










