The P.E.A.N.U.T. Method: Update on an Integrative System Approach for the Treatment of Chronic Otitis Media with Effusion and Adenoid Hypertrophy in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Treatment
2.3.1. P for Pneumatization Exercises
2.3.2. E for Education of Parents and Patients
2.3.3. A for Antiallergic Diet
2.3.4. N for Nasal Local Preparations
2.3.5. U for Useful Constitutional Medication
2.3.6. T for Thermal Interventions (Externally Applied Warm)
2.4. Outcome Variables
2.5. Statistical Analysis
2.6. Human Research Ethics Committee
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcomes Results
3.3. Frequency of Antibiotic Use
3.4. Frequency of Analgesic or Antipyretic Medication
3.5. Number of Surgical Interventions
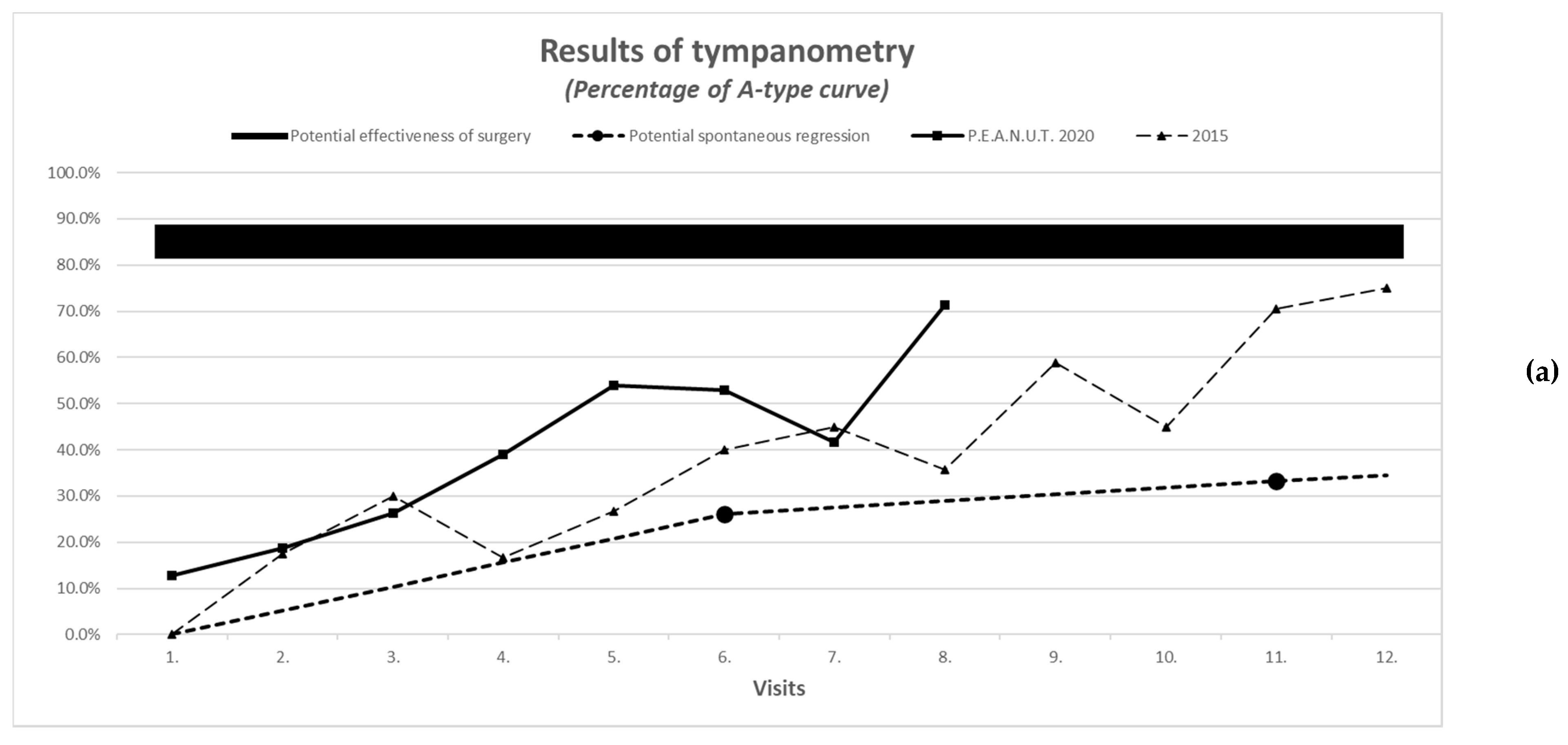
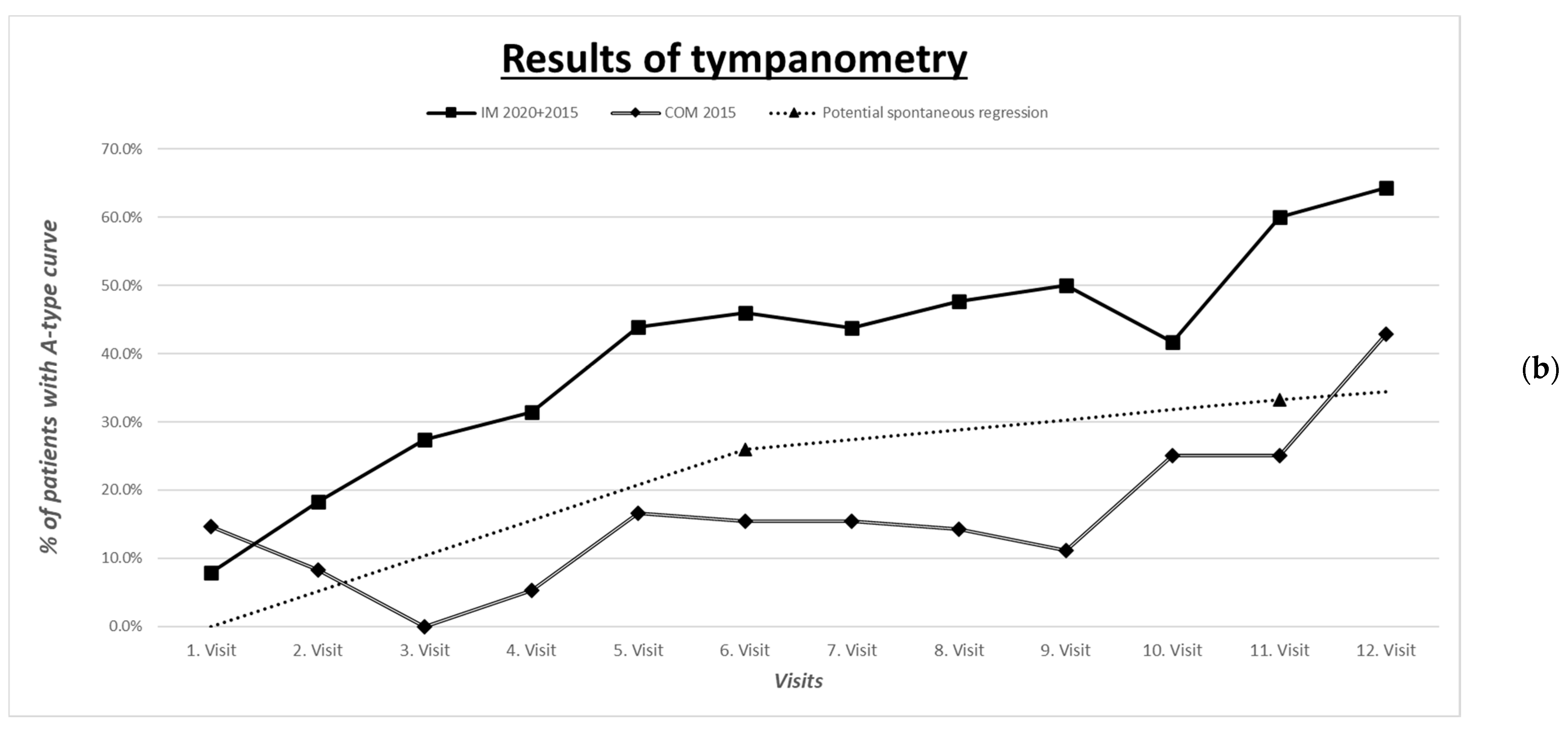
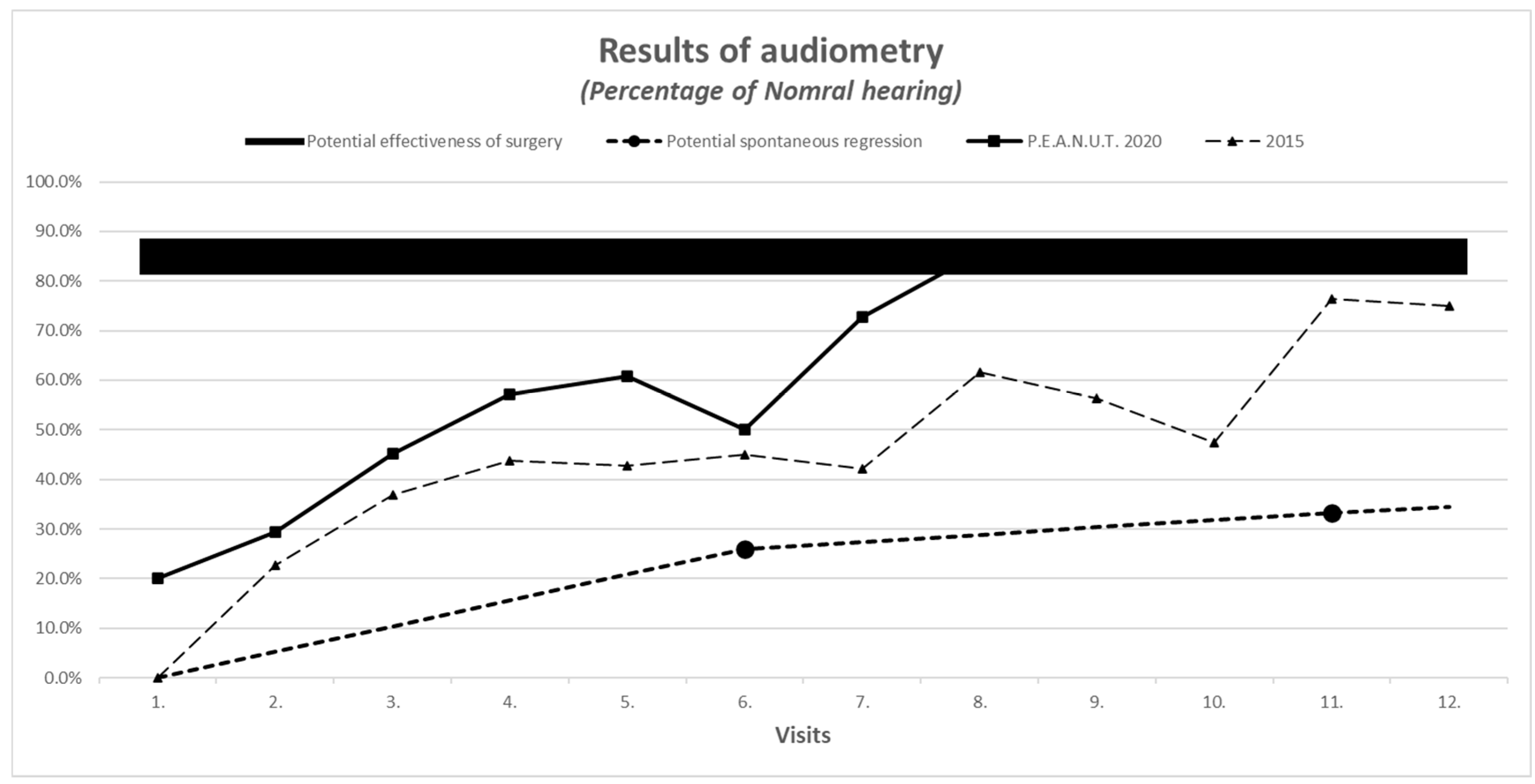
3.6. Improvement in Tympanometric and Audiometric Measurements
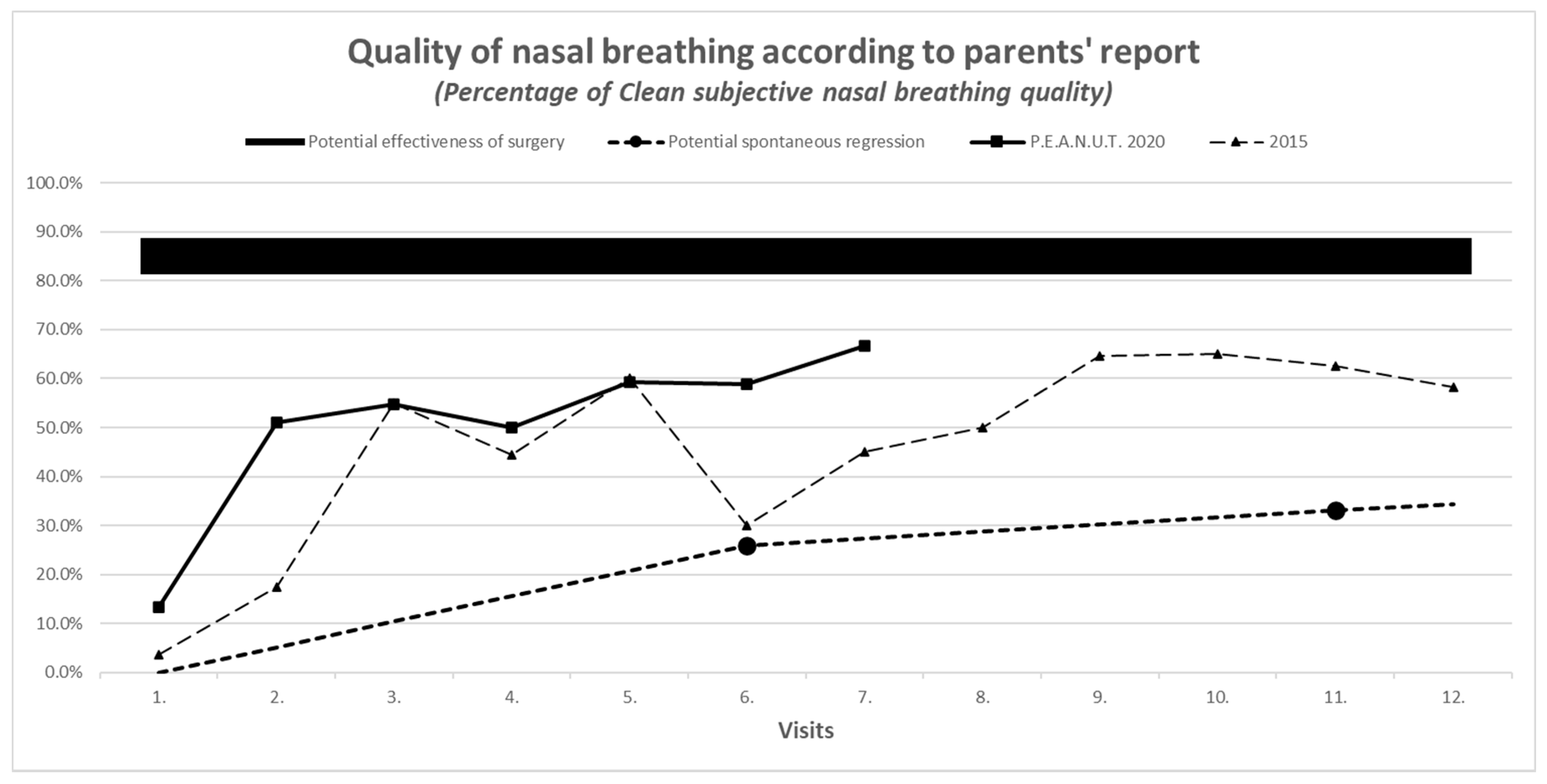
3.7. Results of Secondary Outcomes
4. Discussion
4.1. Main Findings
4.2. Additional Finding
4.3. Strengths and Limitations
4.4. Interpretation, Relation to Previously Published Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Patents
References
- Zhang, Y.; Xu, M.; Zhang, J.; Zeng, L.; Wang, Y.; Zheng, Q.Y. Risk Factors for Chronic and Recurrent Otitis Media—A Meta-Analysis. PLoS ONE 2014, 9, e86397. [Google Scholar] [CrossRef] [PubMed]
- Rodney MDonlan, J.W. Costerton Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Chan, C.L.; Wabnitz, D.; Bardy, J.J.; Bassiouni, A.; Wormald, P.-J.; Vreugde, S.; Psaltis, A.J. The microbiome of otitis media with effusion. Laryngoscope 2016, 126, 2844–2851. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gill, S.; Xu, L.; Gonzalez, E.; Pichichero, M. Comparative Analysis of Microbiome in Nasopharynx and Middle Ear in Young Children with Acute Otitis Media. Front. Genet. 2019. [Google Scholar] [CrossRef]
- Barron, C.L.; Kamel-Abusalha, L.B.; Sethia, R.; Goodman, S.D.; Elmaraghy, C.A.; Bakaletz, L.O. Identification of essential biofilm proteins in middle ear fluids of otitis media with effusion patients. Laryngoscope 2020, 130, 806–811. [Google Scholar] [CrossRef]
- Vanneste, P.; Page, C. Otitis media with effusion in children: Pathophysiology, diagnosis, and treatment. A review. J. Otol. 2019, 14, 33–39. [Google Scholar] [CrossRef]
- Wallace, I.F.; Berkman, N.D.; Lohr, K.N.; Harrison, M.F.; Kimple, A.J.; Steiner, M.J. Surgical treatments for otitis media with effusion: A systematic review. Pediatrics 2014, 133, 296–311. [Google Scholar] [CrossRef]
- Hao, J.; Chen, M.; Liu, B.; Yang, Y.; Liu, W.; Ma, N.; Han, Y.; Liu, Q.; Ni, X.; Zhang, J. Compare two surgical interventions for otitis media with effusion in young children. Eur. Arch. Otorhinolaryngol. 2019, 276, 2125–2131. [Google Scholar] [CrossRef]
- Yin, G.; Tan, J.; Li, P. Balloon dilation of Eustachian tube combined with tympanostomy tube insertion and middle ear pressure equalization therapy for recurrent secretory otitis media. J. Otol. 2019, 14, 101–105. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Shin, J.J.; Schwartz, S.R.; Coggins, R.; Gagnon, L.; Hackell, J.M.; Hoelting, D.; Hunter, L.L.; Kummer, A.W.; Payne, S.C.; et al. Clinical Practice Guideline: Otitis Media with Effusion Executive Summary (Update). Otolaryngol. Head Neck Surg 2016, 154, 201–214. [Google Scholar] [CrossRef]
- Szőke, H.; Maródi, M.; Sallay, Z.; Székely, B.; Sterner, M.G.; Hegyi, G. Integrative versus Conventional Therapy of Chronic Otitis Media with Effusion and Adenoid Hypertrophy in Children: A Prospective Observational Study. Forsch. Komplementmed. 2016, 23, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, L.; Chobaut, J.-C. Eustachian tube rehabilitation therapy: Indications, techniques, and results. Fr ORL 2006, 2006, 241–248. [Google Scholar]
- Leunig, A.; Mees, K. Middle ear ventilation with the Otovent latex membrane system. Laryngo Rhino Otol. 1995, 74, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Blanshard, J.; Maw, A.; Bawden, R. Conservative treatment of otitis media with effusion by autoinflation of the middle ear. Clin. Otolaryngol. Allied Sci. 1993, 18, 189. [Google Scholar] [CrossRef]
- Stangerup, S.; Sederberg-Olsen, J.; Balle, V. Treatment with the Otovent device in tubal dysfunction and secretory otitis media in children. Ugeskr. Laeger 1991, 153, 3008–3009. [Google Scholar]
- Lee, H.J.; Park, S.K.; Choi, K.Y.; Park, S.E.; Chun, Y.M.; Kim, K.S.; Park, S.N.; Cho, Y.S.; Kim, Y.J.; Kim, H.J. Korean clinical practice guidelines: Otitis media in children. J. Korean Med. Sci. 2012, 27, 835–848. [Google Scholar] [CrossRef]
- Juntti, H.; Tikkanen, S.; Kokkonen, J.; Alho, O.P.; Niinimäki, A. Cow’s milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol. 1999, 119, 867–873. [Google Scholar] [CrossRef]
- Baars, E.W. A mixed-method approach in reviewing the effects, safety and working principles of Citrus/Cydonia on hay fever. Eur. J. Integr. Med. 2012, 70. [Google Scholar] [CrossRef]
- Gründemann, C.; Papagiannopoulos, M.; Lamy, E.; Mersch-Sundermann, V.; Huber, R. Immunomodulatory properties of a lemon-quince preparation (Gencydo®) as an indicator of anti-allergic potency. Phytomedicine 2011, 18, 760–768. [Google Scholar] [CrossRef]
- Baars, E.W.; Savelkoul, H.F.J. Savelkoul Citrus/Cydonia Comp. Can Restore the Immunological Balance in Seasonal Allergic Rhinitis-Related Immunological Parameters In Vitro. Mediat. Inflamm. 2008, 2008, 496467. [Google Scholar] [CrossRef]
- Baars, E.W.; Jong, M.C.; Boers, I.; Nierop, A.F.M.; Savelkoul, H.F.J. A Comparative In Vitro Study of the Effects of Separate and Combined Products of Citrus e fructibus and Cydonia e fructibus on Immunological Parameters of Seasonal Allergic Rhinitis. Mediat. Inflamm. 2012, 2012, 109829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial Activity of Tunisian Quince (Cydonia oblonga Miller) Pulp and Peel Polyphenolic Extracts. J. Agric. Food Chem. 2007, 55, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Baars, E.W.; Jong, M.C.; Nierop, A.F.M.; Boers, I.; Savelkoul, H. Subcutaneous Injections Versus Nasal Spray for Seasonal Allergic Rhinitis: A Randomized Controlled Trial on Efficacy and Safety. ISRN Allergy 2011, 2011, 11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Huber Efficacy of a Nasal Spray from Citrus limon and Cydonia oblonga for the Treatment of Hay Fever Symptoms—A Randomized, Placebo Controlled Cross-Over Study. Phytother. Res. 2016, 30, 1481–1486. [Google Scholar] [CrossRef]
- Huber, R.; Stintzing, F.C.; Briemle, D.; Beckmann, C.; Meyer, U.; Gründemann, C. In Vitro Antiallergic Effects of Aqueous Fermented Preparations from Citrus and Cydonia fruits. Planta Med. 2011, 78, 334–340. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef]
- Vagedes, J.; Helmert, E.; Kuderer, S.; Müller, V.; Voege, P.; Szőke, H.; Valentini, J.; Joos, S.; Kohl, M.; Andrasik, F. Effects of Footbaths with Mustard, Ginger, or Warm Water Only on Objective and Subjective Warmth Distribution in Healthy Subjects: A Randomized Controlled Trial. Complementary Ther. Med. 2018, 41, 287–294. [Google Scholar] [CrossRef]
- Venekamp, R.; Burton, M.J.; Van Dongen, T.M.A.; Van Der Heijden, G.J.; Van Zon, A.; Schilder, A.G.M. Antibiotics for otitis media with effusion in children. Cochrane Database Syst. Rev. 2016, 2016, CD009163. [Google Scholar] [CrossRef]
- Grijalva, C.G.; Nuorti, J.P.; Griffin, M.R. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009, 302, 758–766. [Google Scholar] [CrossRef]
- Hamre, H.J.; Fischer, M.; Heger, M.; Riley, D.; Haidvogl, M.; Baars, E.W.; Bristol, E.; Evans, M.; Schwarz, R.; Kiene, H. Anthroposophic vs. conventional therapy of acute respiratory and ear infections. Wien. Klin. Wochenschr. 2005, 117, 256–268. [Google Scholar] [CrossRef]
- Hamre, H.J.; Glockmann, A.; Schwarz, R.; Riley, D.S.; Baars, E.W.; Kiene, H.; Kienle, G.S. Antibiotic Use in Children with Acute Respiratory or Ear Infections: Prospective Observational Comparison of Anthroposophic and Conventional Treatment under Routine Primary Care Conditions. Evid. Based Complement. Altern. Med. 2014, 2014, 243801. [Google Scholar] [CrossRef] [PubMed]
- Baars, E.W.; Zoen, E.B.-V.; Breitkreuz, T.; Martin, D.; Matthes, H.; Von Schoen-Angerer, T.; Soldner, G.; Vagedes, J.; Van Wietmarschen, H.; Patijn, O.; et al. The Contribution of Complementary and Alternative Medicine to Reduce Antibiotic Use: A Narrative Review of Health Concepts, Prevention, and Treatment Strategies. Evid. Based Complement. Altern. Med. 2019, 2019, 5365608. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, E.; Lüke, C.; Ostermann, T.; Tabali, M.; Hübner, J.; Matthes, H. Prescribing practices in the treatment of upper respiratory tract infections in anthroposophic medicine. Forsch. Komplementärmedizin 2007, 14, 207–215. [Google Scholar] [CrossRef]
- MacKay, D. Can CAM therapies help reduce antibiotic resistance? (Antibiotic Resistance). Altern. Med. Rev. 2003, 8, 28–43. [Google Scholar]
- Sarrell, E.M.; Cohen, H.A.; Kahan, E. Naturopathic treatment for ear pain in children. Pediatrics 2003, 111, e574–e579. [Google Scholar] [CrossRef]
- Levi, J.R.; Brody, R.M.; McKee-Cole, K.; Pribitkin, E.; O’Reilly, R.C. Complementary and alternative medicine for pediatric otitis media. Int. J. Pediatric Otorhinolaryngol. 2013, 77, 926–931. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Kay, D. Natural history of untreated otitis media. Laryngoscope 2003, 113, 1645–1657. [Google Scholar] [CrossRef]
- Williamson, I.G.; Vennik, J.; Harnden, A.; Voysey, M.; Perera, R.; Kelly, S.; Yao, G.; Raftery, J.; Mant, D.; Little, P. Effect of nasal balloon autoinflation in children with otitis media with effusion in primary care: An open randomized controlled trial. CMAJ 2015, 187, 961–969. [Google Scholar] [CrossRef]
- Bidarian-Moniri, A.; Ramos, M.-J.; Ejnell, H. Autoinflation for treatment of persistent otitis media with effusion in children: A cross-over study with a 12-month follow-up. Int. J. Pediatric Otorhinolaryngol. 2014, 78, 1298–1305. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, M.; Zheng, W.; Wei, R.; Zhang, B.; Tong, B.; Qiu, J. Myringotomy and tube insertion combined with balloon eustachian tuboplasty for the treatment of otitis media with effusion in children. Eur. Arch Otorhinolaryngol. 2020, 277, 1281–1287. [Google Scholar] [CrossRef]
| (1) Number of invasive surgical interventions | Count |
| (2) Number of acute infection episodes needing antibiotic therapy | Number during the observation period |
| (3) Number of acute infection episodes needing analgesic therapy (local and systemic) | Number during the observation period |
| (4) Tympanometric measurement with evaluation of the worse ear | A-type curve: normal pressure C- or D-type curve: under- or overpressure B-type curve: low admittance |
| (5) Audiometric measurement with evaluation of the worse ear | Normal hearing: −10 to −20 dB Light hearing loss: −30 to −40 dB Middle hearing loss: −50 to −60 dB Severe hearing loss: >−60 dB |
| (1) Frequency of acute otitis media during the observed time period | Count |
| (2) Parents’ report on treatment outcome by subjective scales: (a) nasal congestion;(b) hearing | (a) 1 = no, 2 = moderate, 3 = severe (b) 1 = good, 2 = moderate, 3 = bad |
| (3) Adherence to therapy by subjective scale | 1 = good, 2 = moderate, 3 = bad |
| (4) Median time of follow-ups | Days |
| (5) Mean number of days counted from the first visit during the follow-up period | Days |
| (6) Participation in the visits | Number of patients, % |
| 2015 | 2020 | p-Value | |
|---|---|---|---|
| Number of patients evaluated | 28 | 48 | - |
| Mean baseline age in months | 56.2 | 58.5 | Mann–Whitney test 0.76 |
| Tympanometry: abnormal findings (B + C + D type curves at least 2 times during the first 3 visits) | 75% | 87.5% | Mann–Whitney test 0.166 |
| Audiometry: hearing loss at baseline | minimal = 50% moderate = 32% severe = 11% | minimal = 37% moderate = 43% severe = 0% | Chi-squared test 0.001 |
| Parents’ initial report on nasal obstruction (abnormal = 2 and 3 on subjective scale 1–3) | 96% | 70% | Mann–Whitney test 0.006 |
| Parents’ initial self-report on hearing (abnormal = 2 and 3 on subjective scales 1–3) | 86% | 77% | Mann–Whitney test 0.34 |
| Prior number of acute otitis media/year/patient | 1.6 | 1.5 | Mann–Whitney test 0.46 |
| Prior number of antibiotic treatments/year | 1.6 | 1.7 | Mann–Whitney test 0.71 |
| Prior number of analgesic treatments/year | 1.4 | 2.0 | Mann–Whitney test 0.35 |
| Indication for adenoidectomy present at baseline | 7 out of 28 = 25% | 27 out of 48 = 56.3% | Mann–Whitney test 0.01 |
| 2015 | 2020 | p-Value | |
|---|---|---|---|
| Frequency of antibiotic use, number of patients | 5 out of 28 = 17.9% | 10 out of 48 = 20.8% | Chi-squared test 0.753 |
| Number of antibiotic treatments/year/patient | 0.21 | 0.48 | Mann–Whitney test 0.545 |
| Use of analgesic or antipyretic medication, number of patients | 6 out of 28 = 21.4% | 16 out of 48 = 33.3% | Chi-squared test 0.27 |
| Number of analgesic treatments/year/patient | 0.36 | 0.63 | Mann–Whitney test 0.257 |
| Number of invasive surgical interventions | 1 out of 28 = 3.6% | 3 out of 48 = 6.25% | Mann–Whitney test 0.616 |
| Improvement in tympanometric measurement with evaluation of the worse ear | A-type curve: normal pressure C- or D-type curve: under- or overpressure B-type curve: low admittance | See comparison in Figure 1a,b | |
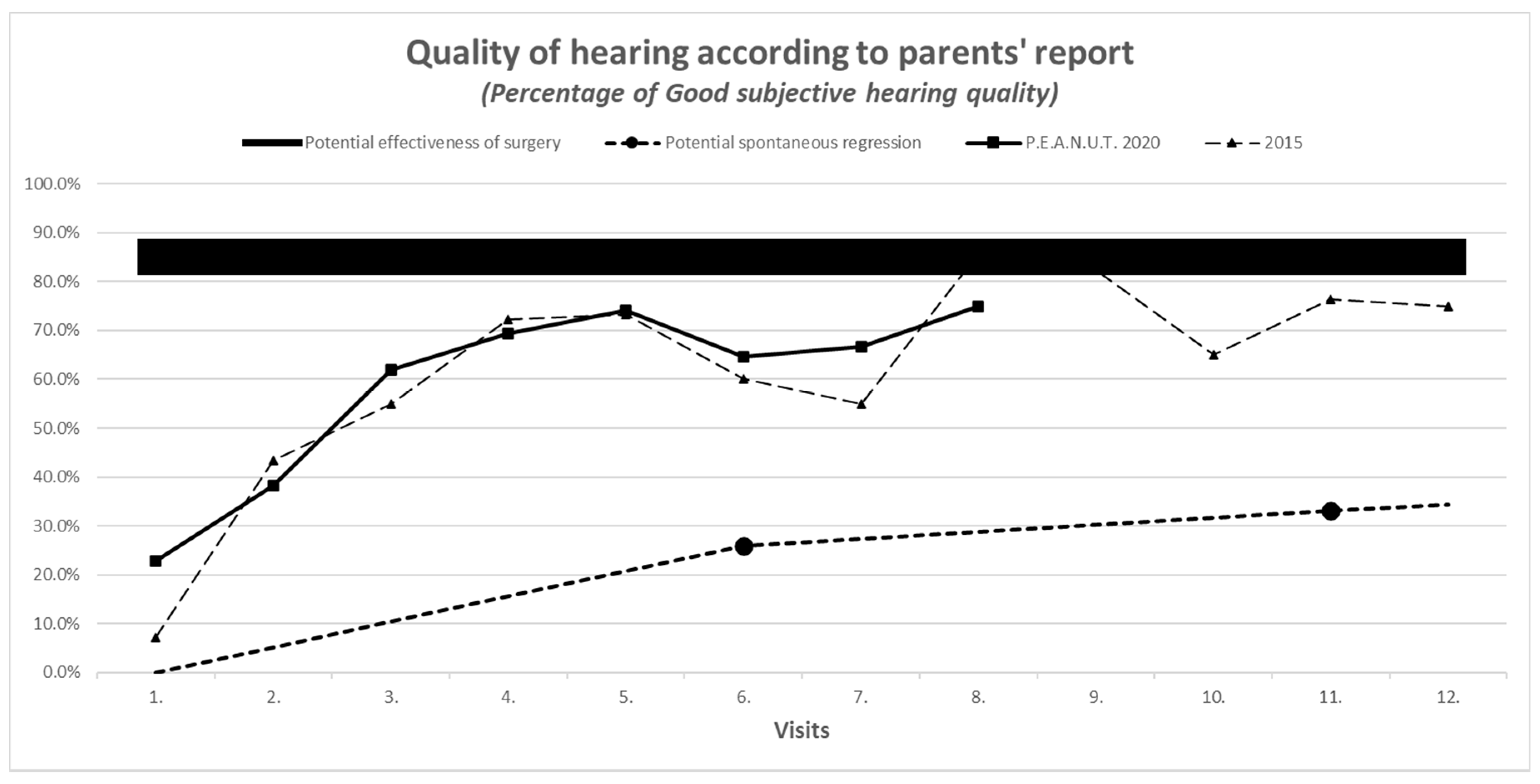
| Improvement in audiometric measurement with evaluation of the worse ear | Normal hearing: −10 to −20 dB Minimal hearing loss: −30 to −40 dB Middle hearing loss: −50 to −60 dB Severe hearing loss: >−60 dB | See the comparison in Figure 2 | |
| 2015 | 2020 | p-Value | |
|---|---|---|---|
| Number of acute otitis media during the observation period, count of patients | 13 out of 28 = 46.4% | 21 out of 48 = 43.8% | Chi-squared test 0.82 |
| Number of acute otitis media/year/patient during the observation period | 0.86 | 0.71 | Mann–Whitney test 0.877 |
| Adherence to prescribed therapies | 1 (good) = 67.3 % 2 (intermediate) = 29.1% 3 (bad) = 3.6% | 1 (good) = 56.2% 2 (intermediate) = 38.4% 3 (bad) = 5.4% | - |
| Average days between follow-up visits (visit 1–12), days, mean | 49.4 | 48.7 | Test for median differences and Mann–Whitney test 0.000 |
| Length of observation period visit 1–12, days, mean | 353 | 208 | Mann–Whitney test 0.000 |
| Adverse reactions | 2% | 2% | - |
| Reference | Mean Age (Years Range) | Diagnostic Method | Intervention | Time of Follow-Up (Months) | Effect |
|---|---|---|---|---|---|
| Rosenfeld 2016, 2003 [10,37] | NA (0–18) | NA | No intervention = Natural resolution | 6 vs. 12 | 26% vs. 33% |
| Williamson 2015 [38] | 5.4 (4–11) | Tympanometry, quality of life | Usual care alone ------------ Usual care plus autoinflation | 1 vs. 3 ----------- 1 vs. 3 | 36% vs. 38% ----------- 47% vs. 50% |
| Bidarian-Moniri 2014 [39] | 5 (2–8) | Tympanometry middle ear pressure (daPa) for both ears --------------- Audiometry mean, median hearing level (dB) for best ear | Balloon autoinflation | Inclusion 2 6 12 ------------ Inclusion 2 6 12 | −389, −400 −189, −182 −196, −185 −151, −123 ------------ 22,22 16,13 16,13 14,12 |
| Szőke [11] | 4.7 (1–8) | Tympanometry, audiometry | P.E.A.N.U.T. method | 12 | 70–80% |
| Chen 2020 [40] | NA | Audiometry (air-bone gap) | Surgery | 6–18 | 80–90% (range 60–94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szőke, H.; Maródi, M.; Vagedes, J.; Székely, B.; Magyarosi, I.; Bedő, A.; Fellegi, V.; Somogyvári, K.; Móricz, P. The P.E.A.N.U.T. Method: Update on an Integrative System Approach for the Treatment of Chronic Otitis Media with Effusion and Adenoid Hypertrophy in Children. Antibiotics 2021, 10, 134. https://doi.org/10.3390/antibiotics10020134
Szőke H, Maródi M, Vagedes J, Székely B, Magyarosi I, Bedő A, Fellegi V, Somogyvári K, Móricz P. The P.E.A.N.U.T. Method: Update on an Integrative System Approach for the Treatment of Chronic Otitis Media with Effusion and Adenoid Hypertrophy in Children. Antibiotics. 2021; 10(2):134. https://doi.org/10.3390/antibiotics10020134
Chicago/Turabian StyleSzőke, Henrik, Márta Maródi, Jan Vagedes, Balázs Székely, István Magyarosi, Adél Bedő, Veronika Fellegi, Krisztina Somogyvári, and Péter Móricz. 2021. "The P.E.A.N.U.T. Method: Update on an Integrative System Approach for the Treatment of Chronic Otitis Media with Effusion and Adenoid Hypertrophy in Children" Antibiotics 10, no. 2: 134. https://doi.org/10.3390/antibiotics10020134
APA StyleSzőke, H., Maródi, M., Vagedes, J., Székely, B., Magyarosi, I., Bedő, A., Fellegi, V., Somogyvári, K., & Móricz, P. (2021). The P.E.A.N.U.T. Method: Update on an Integrative System Approach for the Treatment of Chronic Otitis Media with Effusion and Adenoid Hypertrophy in Children. Antibiotics, 10(2), 134. https://doi.org/10.3390/antibiotics10020134






