Elicitation of Stress-Induced Phenolic Metabolites for Antimicrobial Applications against Foodborne Human Bacterial Pathogens
Abstract
1. Introduction/Background: Food Safety and Public Health Challenges Related to Bacterial Foodborne Pathogens
2. Phenolic Metabolites of Plants
3. Role and Mechanism of Phenolic Metabolites as Antimicrobials
4. Biosynthesis of Stress-Inducible Phenolics in Plant Systems
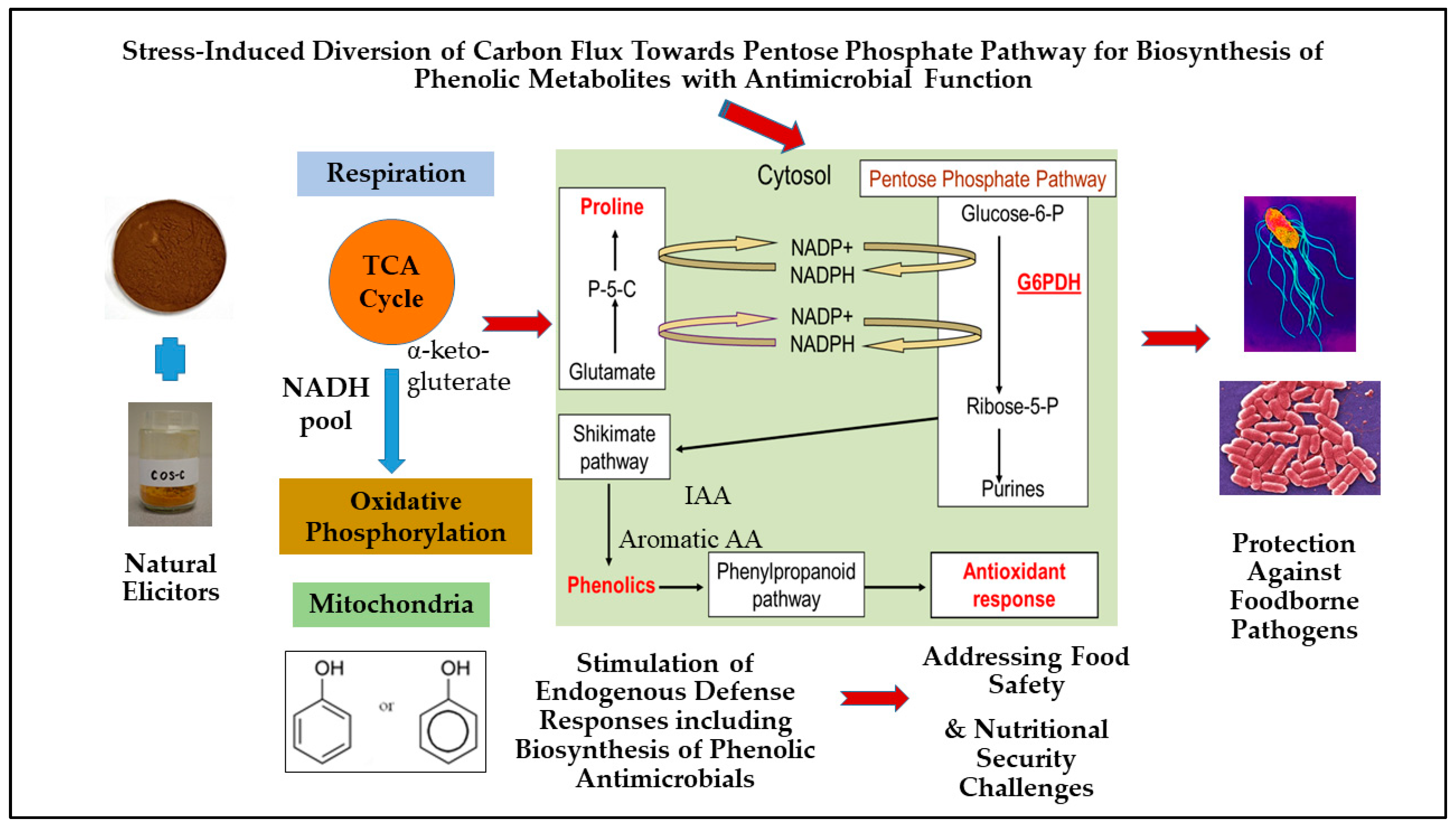
5. Role of Pentose Phosphate Pathway (PPP) Regulation in Plants for Biosynthesis of Stress-Inducible Phenolic Metabolites
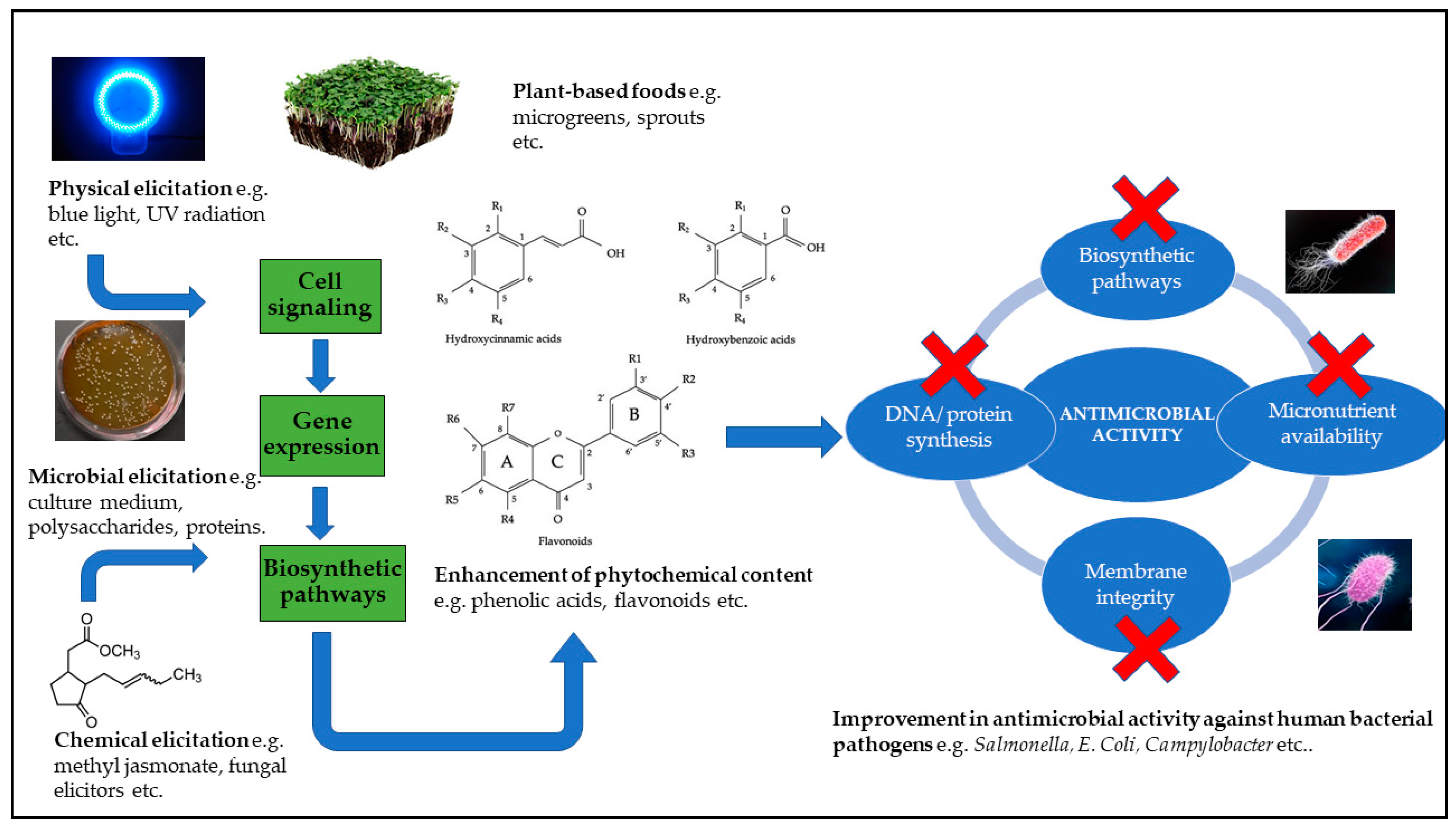
6. Elicitation Strategies to Enhance Stress-Inducible Phenolic Metabolites for Antimicrobial Applications
6.1. Physical Elicitation Strategies
6.2. Chemical Elicitation Strategies
6.3. Microbial Elicitation Strategies
6.4. Phytohormone Elicitation Strategies
6.5. Other Strategies
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanning, I.B.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Food safety and food security. Nat. Educ. Knowl. 2012, 3, 9. [Google Scholar]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Shetty, K. Metabolic and Microbiome Innovations for Improving Phenolic Bioactives for Health. In Advances in Plant Phenolics: From Chemistry to Human Health; Jayaprakasha, G.K., Patil, B.S., Gattusso, G., Eds.; American Chemical Society: Washington, DC, USA, 2018; Volume 1286, pp. 261–281. ISBN 9780841232969. [Google Scholar]
- Sarkar, D.; Shetty, K. Metabolic stimulation of plant phenolics for food preservation and health. Annu. Rev. Food Sci. Technol. 2014, 5, 395–413. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Safety. Available online: https://vlibrary.emro.who.int/idr_records/food-safety-2/ (accessed on 26 August 2020).
- World Health Organization. Food Safety. Available online: http://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 3 July 2020).
- Centers for Disease Control and Prevention. Estimation of Foodborne Illness in the United States. Available online: https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 4 October 2020).
- United States Department of Agriculture Economic Research Service. Cost Estimation of Foodborne Illness. Available online: https://www.ers.usda.gov/data-products/cost-estimates-of-foodborne-illnesses/ (accessed on 4 October 2020).
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, 1–21. [Google Scholar] [CrossRef]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef]
- Riggio, G.; Wang, Q.; Kniel, K.; Gibson, K. Microgreens—A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Aruscavage, D.; Lee, K.; Miller, S.; LeJeune, J.T. Interactions affecting the proliferation and control of human pathogens on edible plants. J. Food Sci. 2006, 71, 89–99. [Google Scholar] [CrossRef]
- United States Food & Drug Administration. Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-guide-minimize-microbial-food-safety-hazards-fresh-fruits-and-vegetables (accessed on 11 January 2021).
- World Health Organization. Five Keys to Growing Safer Fruits and Vegetables: Promoting Health by Decreasing Microbial Contamination; WHO Department of Food Safety and Zoonoses: Geneva, Switzerland, 2012; pp. 1–36. ISBN 978-92-4-150400-3. [Google Scholar]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. R. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function, and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.; Wahlqvist, M. A model for the role of the proline-linked pentose-phosphate pathway in phenolic phytochemical biosynthesis and mechanism of action for human health and environmental applications. Asia Pac. J. Clin. Nutr. 2004, 13, 1–24. [Google Scholar]
- Vardhan, P.V.; Shukla, L.I. Gamma irradiation of medicinally important plants and the enhancement of secondary metabolite production. Int. J. Radiat. Biol. 2017, 93, 967–979. [Google Scholar] [CrossRef]
- Verpoorte, R.; Memelink, J. Engineering secondary metabolite production in plants. Curr. Opin. Biotechnol. 2002, 13, 181–187. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef]
- Vattem, D.A.; Lin, Y.T.; Labbe, R.G.; Shetty, K. Antimicrobial activity against select food-borne pathogens by phenolic antioxidants enriched in cranberry pomace by solid-state bioprocessing using the food grade fungus Rhizopus oligosporus. Process Biochem. 2004, 39, 1939–1946. [Google Scholar] [CrossRef]
- Christopher, A.; Sarkar, D.; Zwinger, S.; Shetty, K. Ethnic food perspective of North Dakota common emmer wheat and relevance for health benefits targeting type 2 diabetes. J. Ethn. Foods 2018, 5, 66–74. [Google Scholar] [CrossRef]
- Christopher, A.; Orwat, J.; Sarkar, D.; Hatterman-Valenti, H.; Shetty, K. Ozone elicited phenolic bioactives in grapes and health relevant screening targeted for type 2 diabetes using in vitro assay models. J. Med. Active Plants 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Acamovic, T.; Brooker, J.D. Biochemistry of plant secondary metabolites and their effects in animals. Proc. Nutr. Soc. 2005, 64, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Lu, Y.; Wu, Q.; Liu, Y.; Xia, Y.; Xia, K.; Cui, J. UV-B-induced anthocyanin accumulation in hypocotyls of radish sprouts continues in the dark after irradiation. J. Sci. Food Agric. 2016, 96, 886–892. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Tian, J.; Li, N.; Jia, L.; Shen, W.; Cui, J. Enhanced anthocyanin accumulation of immature radish microgreens by hydrogen-rich water under short wavelength light. Sci. Hortic. 2019, 247, 75–85. [Google Scholar] [CrossRef]
- Bruce, T.J.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Garcia, S.; González, G.A.; Dávila-Aviña, J.; García-Heredia, A.; Heredia, N. Natural antimicrobials from plants for food applications. In Functional Foods and Biotechnology: Biotransformation and Analysis of Functional Foods and Ingredients; Shetty, K., Sarkar, D., Eds.; CRC Press, Taylor & Frances Group: Boca Raton, FL, USA, 2020; pp. 407–420. ISBN 9780367429218. [Google Scholar]
- Jayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556–568. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sakharkar, K.R.; Sing, L.C.; Chow, V.T.; Sakharkar, M.K. Insights into antifolate activity of phytochemicals against Pseudomonas aeruginosa. J. Drug Target 2011, 19, 179–188. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Matlawska, I.; Pilewski, N.A. Natural flavonoids as antimicrobial agents. JANA 2004, 7, 24–31. [Google Scholar]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlajac, K.; Vuorelaa, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; de Ornelas-Paz, J.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.M.; Ayala-Zavala, F.; Márquez-Ríos, E. Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Matsushima, M.; Suzuki, T.; Masui, A.; Kasai, K.; Kouchi, T.; Takagi, A.; Shirai, T.; Mine, T. Growth inhibitory action of cranberry on Helicobacter pylori. J. Gastroenterol. Hepatol. 2008, 23, S175–S180. [Google Scholar] [CrossRef]
- Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 2004, 70, 5672–5678. [Google Scholar] [CrossRef]
- Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Vibrio parahaemolyticus in seafood systems using oregano and cranberry phytochemical synergies and lactic acid. Innov. Food Sci. Emerg. Technol. 2005, 6, 453–458. [Google Scholar] [CrossRef]
- Seaberg, A.C.; Labbe, R.G.; Shetty, K. Inhibition of Listeria monocytogenes by elite clonal extracts of oregano (Origanum vulgare). Food Biotechnol. 2003, 17, 129–149. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Šimat, V.; Smole Možina, S.; Katalinić, V. In vitro antioxidant and antibacterial activity of Lamiaceae phenolic extracts: A correlation study. Food Technol. Biotechnol. 2014, 52, 119–127. [Google Scholar]
- Generalić Mekinić, I.; Ljubenkov, I.; Smole Možina, S.; Abramović, H.; Šimat, V.; Katalinić, A.; Novak, T.; Skroza, D. Abiotic factors during a one-year vegetation period affect sage phenolic metabolites, antioxidants and antimicrobials. Ind. Crops Prod. 2019, 141, 111741. [Google Scholar] [CrossRef]
- Abukakar, M.G.; Ukwuani, A.N.; Shehu, R.A. Phytochemical screening and antibacterial activity of Tamarindus indica pulp extract. Asian J. Biochem. 2008, 3, 134–138. [Google Scholar] [CrossRef]
- Sibi, G.; Chatly, P.; Adhikari, S.; Ravikumar, K.R. Phytoconstituents and their influence on antimicrobial properties of Morinda citrifolia L. Res. J. Med. Plant. 2012, 6, 441–448. [Google Scholar] [CrossRef]
- Munyendo, W.L.L.; Orwa, J.A.; Rukunga, G.M.; Bii, C.C. Bacteriostatic and bactericidal activities of Aspilia mossambicensis, Ocimum gratissimum and Toddalia asiatica extracts on selected pathogenic bacteria. Res. J. Med. Plant. 2011, 5, 717–727. [Google Scholar] [CrossRef]
- Oboh, G. Antioxidant and antimicrobial properties of ethanolic extract of Ocimum gratissimum leaves. J. Pharmacol. Toxicol. 2010, 5, 396–402. [Google Scholar]
- Zhao, X.; Zhang, C.; Guigas, C.; Ma, Y.; Corrales, M.; Tauscher, B.; Hu, X. Composition, antimicrobial activity, and antiproliferative capacity of anthocyanin extracts of purple corn (Zea mays L.) from China. Eur. Food Res. Technol. 2009, 228, 759–765. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simões, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef]
- Díaz-Gómez, R.; López-Solís, R.; Obreque-Slier, E.; Toledo-Araya, H. Comparative antibacterial effect of gallic acid and catechin against Helicobacter pylori. LWT Food Sci. Technol. 2013, 54, 331–335. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Roccaro, A.S.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Gradišar, H.; Pristovšek, P.; Plaper, A.; Jerala, R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In vitro antimicrobial activity of some phenolic compounds (coumarin and quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 2012, 183–187. [Google Scholar] [CrossRef]
- Lee, P.; Tan, K.S. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch. Oral Biol. 2015, 60, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Compean, K.L.; Ynalvez, R.A. Antimicrobial activity of plant secondary metabolites: A review. Res. J. Med. Plant. 2014, 8, 204–213. [Google Scholar] [CrossRef]
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arabidopsis Book 2011, 9, e0152. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Shetty, K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: A review. Process Biochem. 2004, 39, 789–804. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 2010, 8, e0140. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.; McCue, P. Phenolic antioxidant biosynthesis in plants for functional food application: Integration of systems biology and biotechnological approaches. Food Biotechnol. 2003, 17, 67–97. [Google Scholar] [CrossRef]
- Limón, R.I.; Peñas, E.; Martínez-Villaluenga, C.; Frias, J. Role of elicitation on the health-promoting properties of kidney bean sprouts. LWT Food Sci. Technol. 2014, 56, 328–334. [Google Scholar] [CrossRef]
- Sarkar, D.; Bhowmik, P.C.; Shetty, K. Antioxidant enzyme response of creeping bentgrass clonal lines with marine peptide and chitosan oligosaccharide. Agron. J. 2010, 102, 981–989. [Google Scholar] [CrossRef]
- Yang, R.; Shetty, K. Stimulation of rosmarinic acid in shoot cultures of oregano (Origanum vulgare) clonal line in response to proline, proline analogue, and proline precursors. J. Agric. Food Chem. 1998, 46, 888–2893. [Google Scholar] [CrossRef]
- Orwat, J. Phenolic Antioxidant-Linked Bioactive Enrichment in Black Beans (Phaseolus vulgaris L.) to Screen for Health Benefits and Enhancement of Salinity Resilience. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2016. [Google Scholar]
- Ramakrishna, R.; Sarkar, D.; Manduri, A.; Iyer, S.G.; Shetty, K. Improving phenolic bioactive-linked anti-hyperglycemic functions of dark germinated barley sprouts (Hordeum vulgare L.) using seed elicitation strategy. J. Food Sci. Technol. 2017, 54, 3666–3678. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Shetty, K. Metabolic stimulation of phenolic biosynthesis and antioxidant enzyme response in dark germinated barley (Hordeum vulgare L.) sprouts using bioprocessed elicitors. Food Sci. Biotechnol. 2019, 28, 1093–1106. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Jahangir, M.; Abdel-Farid, I.B.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 2009, 67, 23–33. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Gilbert, A.R.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. Photo irradiated caffeic acid as an antimicrobial treatment for fresh produce. FEMS Microbiol. Lett. 2018, 365, fny132. [Google Scholar] [CrossRef]
- Hussein, E.A.; Taj-Eldeen, A.M.; Al-Zubairi, A.S.; Elhakimi, A.S.; Al-Dubaie, A.R. Phytochemical screening, total phenolics and antioxidant and antibacterial activities of callus from Brassica nigra L. hypocotyl explants. Int. J. Pharmacol. 2010, 6, 464–471. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Rahamooz-Haghighi, S.; Bagheri, K.; Sharafi, A.; Danafar, H. Establishment and elicitation of transgenic root culture of Plantago lanceolata and evaluation of its anti-bacterial and cytotoxicity activity. Prep. Biochem. Biotechnol. 2020, 1–18. [Google Scholar] [CrossRef]
- Poulev, A.; O’Neal, J.M.; Logendra, S.; Pouleva, R.B.; Timeva, V.; Garvey, A.S.; Gleba, D.; Jenkins, I.S.; Halpern, B.T.; Kneer, R.; et al. Elicitation, a new window into plant chemodiversity and phytochemical drug discovery. J. Med. Chem. 2003, 46, 2542–2547. [Google Scholar] [CrossRef]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Helicobacter pylori by phenolic extracts of sprouted peas (Pisum sativum L.). J. Food Biochem. 2006, 30, 21–34. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Singh, S.; Nag, T.N. Antimicrobial activity of flavonoids from in vitro tissue culture and seeds of Gossypium species. Rom. Biotechnol. Lett. 2010, 15, 4959–4963. [Google Scholar]
- Al Khateeb, W.; Hussein, E.; Qouta, L.; Alu’datt, M.; Al-Shara, B.; Abu-Zaiton, A. In vitro propagation and characterization of phenolic content along with antioxidant and antimicrobial activities of Cichorium pumilum Jacq. Plant Cell Tissue Organ Cult. 2012, 110, 103–110. [Google Scholar] [CrossRef]
- Debnath, M. Clonal propagation and antimicrobial activity of an endemic medicinal plant Stevia rebaudiana. J. Med. Plant Res. 2007, 2, 45–51. [Google Scholar] [CrossRef]
- Ncube, B.; Ngunge, V.N.P.; Finnie, J.F.; Van Staden, J. A comparative study of the antimicrobial and phytochemical properties between outdoor grown and micropropagated Tulbaghia violacea Harv. plants. J. Ethnopharmacol. 2011, 134, 775–780. [Google Scholar] [CrossRef]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Ravanfar, S.A.; Karimi, E.; Mehrabanjoubani, P.; Ebrahimi, M. Enhancement of phenolic and flavonoids compounds, antioxidant and cytotoxic effects in regenerated red cabbage by application of Zeatin. Nat. Prod. Res. 2018, 34, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.S.; de Melo, W.C.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light based anti-infectives: Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Atallah, M.T.; Shetty, K. Effects of UV treatment on the proline-linked pentose phosphate pathway for phenolics and L-DOPA synthesis in dark germinated Vicia faba. Process Biochem. 2002, 37, 1285–1295. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J. Appl. Phycol. 1999, 11, 559. [Google Scholar] [CrossRef]
- Sarkar, D.; Bhowmik, P.C.; Shetty, K. The role of proline-associated pentose phosphate pathway in cool-season turfgrasses after UV-B exposure. Environ. Exp. Botany 2011, 70, 251–258. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Zavaleta-Gatica, R.; Tiznado-Hernández, M.E. Improving postharvest quality of mango ‘Haden’ by UV-C treatment. Postharvest Biol. Technol. 2007, 45, 108–116. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Maraei, R.W.; Elsawy, K.M. Chemical quality and nutrient composition of strawberry fruits treated by γ-irradiation. J. Radiat. Res. Appl. Sci. 2017, 10, 80–87. [Google Scholar] [CrossRef]
- Oufedjikh, H.; Mahrouz, M.; Amiot, M.J.; Lacroix, M. Effect of γ-irradiation on phenolic compounds and phenylalanine ammonia-lyase activity during storage in relation to peel injury from peel of Citrus clementina Hort. Ex. Tanaka. J. Agric. Food Chem. 2000, 48, 559–565. [Google Scholar] [CrossRef] [PubMed]
- McCue, P.; Shetty, K. Clonal herbal extracts as elicitors of phenolic synthesis in dark-germinated mungbeans for improving nutritional value with implications for food safety. J. Food Biochem. 2002, 26, 209–232. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A.A. Ozone-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov. Food Sci. Emerg. Technol. 2010, 11, 666–671. [Google Scholar] [CrossRef]
- Yin, H.; Fretté, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- McCue, P.; Shetty, K. A biochemical analysis of mungbean (Vigna radiata) response to microbial polysaccharides and potential phenolic-enhancing effects for nutraceutical applications. Food Biotechnol. 2002, 16, 57–79. [Google Scholar] [CrossRef]
- Kalli, S.; Araya-Cloutier, C.; de Bruijn, W.J.; Chapman, J.; Vincken, J.P. Induction of promising antibacterial prenylated isoflavonoids from different subclasses by sequential elicitation of soybean. Phytochemistry 2020, 179, 112496. [Google Scholar] [CrossRef]
- Szabo, E.; Thelen, A.; Petersen, M. Fungal elicitor preparations and methyl jasmonate enhance rosmarinic acid accumulation in suspension cultures of Coleus blumei. Plant Cell Rep. 1999, 18, 485–489. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Vilcinskas, A.; Rahnamaeian, M. Cooperative interaction of antimicrobial peptides with the interrelated immune pathways in plants. Mol. Plant Pathol. 2016, 17, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.M.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arabidopsis Book 2011, e0156. [Google Scholar] [CrossRef] [PubMed]
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Dempsey, D.M.A.; Shah, J.; Klessig, D.F. Salicylic acid and disease resistance in plants. Crit. Rev. Plant Sci. 1999, 18, 547–575. [Google Scholar] [CrossRef]
- Durner, J.; Shah, J.; Klessig, D.F. Salicylic acid and disease resistance in plants. Trends Plant Sci. 1997, 2, 266–274. [Google Scholar] [CrossRef]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Naz, S.; Ali, A.; Iqbal, J. Phenolic content in vitro cultures of chickpea (Cicer arietinum L.) during callogenesis and organogenesis. Pak J. Bot. 2008, 40, 2525–2539. [Google Scholar]
- Verpoorte, R.; van der Heijden, R.; Memelink, J. Engineering the plant cell factory for secondary metabolite production. Transgenic Res. 2000, 9, 323–343. [Google Scholar] [CrossRef]
- do Nascimento, N.C.; Fett-Neto, A.G. Plant secondary metabolism and challenges in modifying its operation: An overview. In Plant Secondary Metabolism Engineering. Methods in Molecular Biology (Methods and Protocols); do Nascimento, N.C., Fett-Neto, A.G., Eds.; Humana Press: Totowa, NJ, USA, 2010; Volume 643, pp. 1–13. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Klöti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Watanabe, M.; Choi, S.K.; Teramoto, M.; Ohyama, K.; Misawa, N. Enrichment of carotenoids in flaxseed (Linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. J. Biosci. Bioeng. 2008, 105, 636–641. [Google Scholar] [CrossRef]
- Siebert, M.; Sommer, S.; Li, S.M.; Wang, Z.X.; Severin, K.; Heide, L. Genetic engineering of plant secondary metabolism (accumulation of 4-hydroxybenzoate glucosides as a result of the expression of the bacterial ubic gene in tobacco). Plant Physiol. 1996, 112, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Memelink, J.; Kijne, J.W.; van der Heijden, R.; Verpoorte, R. Genetic modification of plant secondary metabolite pathways using transcriptional regulators. In Plant Cells. Advances in Biochemical Engineering/Biotechnology; Zhong, J.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 72, pp. 103–125. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.A.; Jakoby, M.; Werber, M.; Martin, C.; Weisshaar, B.; Bailey, P.C. The basic helix–loop–helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003, 20, 735–747. [Google Scholar] [CrossRef]
- Dron, M.; Clouse, S.D.; Dixon, R.A.; Lawton, M.A.; Lamb, C.J. Glutathione and fungal elicitor regulation of a plant defense gene promoter in electroporated protoplasts. Proc. Natl. Acad. Sci. USA 1988, 85, 6738–6742. [Google Scholar] [CrossRef]
- Lois, R.; Dietrich, A.; Hahlbrock, K.; Schulz, W. A phenylalanine ammonia-lyase gene from parsley: Structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO 1989, 8, 1641–1648. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Dangl, J.L.; Becker-André, M.; Hahlbrock, K.; Schulz, W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO 1989, 8, 651–656. [Google Scholar] [CrossRef]
- Feldbrügge, M.; Sprenger, M.; Hahlbrock, K.; Weisshaar, B. PcMYB1, a novel plant protein containing a DNA-binding domain with one MYB repeat, interacts in vivo with a light-regulatory promoter unit. Plant J. 1997, 11, 1079–1093. [Google Scholar] [CrossRef]
| Plant Extracts | Antimicrobial Activity a,b | Bacterial Pathogen | Reference |
|---|---|---|---|
| Andromeda polifolia | 1–3 mm | S. aureus DSM 20231 c | [42] |
| 1–3 mm | E. coli ATCC 8739 d | ||
| Calluna vulgaris | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Epilobium angustifolium | 4–10 mm | S. aureus DSM 20231 | |
| 4–10 mm | E. coli ATCC 8739 | ||
| Filipendula ulmaria | 4–10 mm | S. aureus DSM 20231 | |
| 4–10 mm | E. coli ATCC 8739 | ||
| Lythrum salicaria | 1–3 mm | S. aureus DSM 20231 | |
| 4–10 mm | E. coli ATCC 8739 | ||
| Matricaria chamomilla | 1–3 mm | S. aureus DSM 20231 | |
| 3–4 mm | E. coli ATCC 8739 | ||
| Tanacetum vulgare | n.a | S. aureus DSM 20231 | |
| 3–4 mm | E. coli ATCC 8739 | ||
| Thymus vulgaris | 1–3 mm | S. aureus DSM 20231 | |
| 4–10 mm | E. coli ATCC 8739 | ||
| Allium cepa | 1–3 mm | S. aureus DSM 20231 | |
| 4–10 mm | E. coli ATCC 8739 | ||
| Avena sativa | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Beta vulgaris var. rubra | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Betula pubescens | 4–10 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Picea abies | 1–3 mm | S. aureus DSM 20231 | |
| n.a | E. coli ATCC 8739 | ||
| Pinus sylvestris | 4–10 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Salix caprea | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Secale cereale | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Solanum tuberosum | 3–4 mm | S. aureus DSM 20231 | |
| 1–3 mm | E. coli ATCC 8739 | ||
| Aronia melanocarpa | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | B. subtilis ATCC 9372 | ||
| 4–10 mm | M. luteus YMBL e | ||
| 1–3 mm | E. coli ATCC 8739 | ||
| Empetrum nigrum | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | S. epidermis ATCC 12228 | ||
| 3–4 mm | B. subtilis ATCC 9372 | ||
| 4–10 mm | M. luteus YMBL | ||
| 1–3 mm | E. coli ATCC 8739 | ||
| Malus pumila | 1–3 mm | S. aureus DSM 20231 | |
| n.a | S. epidermis ATCC 12228 | ||
| 1–3 mm | B. subtilis ATCC 9372 | ||
| n.a | M. luteus YMBL | ||
| n.a | E. coli ATCC 8739 | ||
| Ribes nigrum | 1–3 mm | S. aureus DSM 20231 | |
| n.a | S. epidermis ATCC 12228 | ||
| 1–3 mm | B. subtilis ATCC 9372 | ||
| 3–4 mm | M. luteus YMBL | ||
| n.a | E. coli ATCC 8739 | ||
| Rubus chamaemorus | 1–3 mm | S. aureus DSM 20231 | |
| 3–4 mm | S. epidermis ATCC 12228 | ||
| 3–4 mm | B. subtilis ATCC 9372 | ||
| 1–3 mm | M. luteus YMBL | ||
| 1–3 mm | E. coli ATCC 8739 | ||
| Rubus idaeus | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | S. epidermis ATCC 12228 | ||
| 3–4 mm | B. subtilis ATCC 9372 | ||
| 1–3 mm | M. luteus YMBL | ||
| 1–3 mm | E. coli ATCC 8739 | ||
| Sorbus aucuparia | 1–3 mm | S. aureus DSM 20231 | |
| n.a | S. epidermis ATCC 12228 | ||
| 1–3 mm | B. subtilis ATCC 9372 | ||
| 1–3 mm | M. luteus YMBL | ||
| n.a | E. coli ATCC 8739 | ||
| Vaccinium myrtillus | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | S. epidermis ATCC 12228 | ||
| 1–3 mm | B. subtilis ATCC 9372 | ||
| 4–10 mm | M. luteus YMBL | ||
| 1–3 mm | E. coli ATCC 8739 | ||
| Vaccinium oxycoccus | 3–4 mm | S. aureus DSM 20231 | |
| n.a | S. epidermis ATCC 12228 | ||
| n.a | B. subtilis ATCC 9372 | ||
| n.a | M. luteus YMBL | ||
| 4–10 mm | E. coli ATCC 8739 | ||
| Vaccinium uliginosum | 1–3 mm | S. aureus DSM 20231 | |
| 1–3 mm | S. epidermis ATCC 12228 | ||
| 1–3 mm | B. subtilis ATCC 9372 | ||
| 1–3 mm | M. luteus YMBL | ||
| n.a | E. coli ATCC 8739 | ||
| Vaccinium vitis-idaea | n.a | S. aureus DSM 20231 | |
| n.a | S. epidermis ATCC 12228 | ||
| 1–3 mm | B. subtilis ATCC 9372 | ||
| 1–3 mm | M. luteus YMBL | ||
| n.a | E. coli ATCC 8739 | ||
| Tomato cv. Pitenza | [43] | ||
| Stem | 10.0 mm | S. Typhimurium ATCC 14028 | |
| 8.6 mm | E. coli O157:H7 ATCC 43890 | ||
| 10.3 mm | S. aureus ATCC 65384 | ||
| 11.1 mm | L. ivanovii ATCC 19119 | ||
| Leaf | 10.8 mm | S. Typhimurium ATCC 14028 | |
| 9.4 mm | E. coli O157:H7 ATCC 43890 | ||
| 11.3 mm | S. aureus ATCC 65384 | ||
| 12.9 mm | L. ivanovii ATCC 19119 | ||
| Root | 8.7 mm | S. Typhimurium ATCC 14028 | |
| 8.3 mm | E. coli O157:H7 ATCC 43890 | ||
| 9.0 mm | S. aureus ATCC 65384 | ||
| 8.0 mm | L. ivanovii ATCC 19119 | ||
| Whole plant | 8.0 mm | S. Typhimurium ATCC 14028 | |
| 8.2 mm | E. coli O157:H7 ATCC 43890 | ||
| 9.9 mm | S. aureus ATCC 65384 | ||
| 8.9 mm | L. ivanovii ATCC 19119 | ||
| Tomato cv. Floradade | |||
| Stem | n.a | S. Typhimurium ATCC 14028 | |
| n.a | E. coli O157:H7 ATCC 43890 | ||
| 10.3 mm | S. aureus ATCC 65384 | ||
| n.a | L. ivanovii ATCC 19119 | ||
| Leaf | 10.0 mm | S. typhimurium ATCC 14028 | |
| 9.4 mm | E. coli O157:H7 ATCC 43890 | ||
| 9.3 mm | S. aureus ATCC 65384 | ||
| 9.0 mm | L. ivanovii ATCC 19119 | ||
| Root | n.a | S. Typhimurium ATCC 14028 | |
| n.a | E. coli O157:H7 ATCC 43890 | ||
| n.a | S. aureus ATCC 65384 | ||
| n.a | L. ivanovii ATCC 19119 | ||
| Whole plant | n.a | S. Typhimurium ATCC 14028 | |
| 7.7 mm | E. coli O157:H7 ATCC 43890 | ||
| 8.2 mm | S. aureus ATCC 65384 | ||
| n.a | L. ivanovii ATCC 19119 | ||
| Cranberry | 3.3 mg/mL | H. pylori NCTC 11637 f | [44] |
| 3.3 mg/mL | H. pylori NCTC 11638 | ||
| Oregano-cranberry combination | 1.4–2.5 mm | L. monocytogenes Scott A 4b g | [45] |
| Oregano-cranberry combination | 1.5–3.0 mm | V. parahaemolyticusg | [46] |
| Oregano extracts | 1.2 mg/mL | L. monocytogenes 4b g | [47] |
| Sage | 0.82 mg/mL | C. coli ATCC 33,559 | [48] |
| 6.72 mg/mL | E. coli O157:H7 ZM370 h | ||
| 6.72 mg/mL | Salmonella Infantis ZM9 | ||
| 1.68 mg/mL | B. cereus WSBC 10530 i | ||
| 1.68 mg/mL | L. monocytogenes ZM58 | ||
| 0.34 mg/mL | S. aureus ATCC 25923 | ||
| Thyme | 3.40 mg/mL | C. coli ATCC 33,559 | |
| 6.73 mg/mL | E. coli O157:H7 ZM370 | ||
| 6.73 mg/mL | Salmonella Infantis ZM9 | ||
| 6.73 mg/mL | B. cereus WSBC 10530 | ||
| 6.73 mg/mL | L. monocytogenes ZM58 | ||
| 6.48 mg/mL | S. aureus ATCC 25923 | ||
| Lemon balm | 3.40 mg/mL | C. coli ATCC 33,559 | |
| 6.73 mg/mL | E. coli O157:H7 ZM370 | ||
| 6.73 mg/mL | Salmonella Infantis ZM9 | ||
| 6.73 mg/mL | B. cereus WSBC 10530 | ||
| 6.73 mg/mL | L. monocytogenes ZM58 | ||
| 2.43 mg/mL | S. aureus ATCC 25923 | ||
| Peppermint | 1.71 mg/mL | C. coli ATCC 33,559 | |
| 6.71 mg/mL | E. coli O157:H7 ZM370 | ||
| 6.71 mg/mL | Salmonella Infantis ZM9 | ||
| 6.71 mg/mL | B. cereus WSBC 10530 | ||
| 6.71 mg/mL | L. monocytogenes ZM58 | ||
| 6.85 mg/mL | S. aureus ATCC 25923 | ||
| Oregano | 1.70 mg/mL | C. coli ATCC 33,559 | |
| 6.72 mg/mL | E. coli O157:H7 ZM370 | ||
| 6.72 mg/mL | Salmonella Infantis ZM9 | ||
| 3.36 mg/mL | B. cereus WSBC 10530 | ||
| 6.72 mg/mL | L. monocytogenes ZM58 | ||
| 5.60 mg/mL | S. aureus ATCC 25923 | ||
| Sage | 0.010 mg GAE/mL | B. cereus WSBC 10,530 | [49] |
| 0.020 mg GAE/mL | S. aureus ATCC 25923 | ||
| 0.161 mg GAE/mL | E. coli O157:H7 ZM370 | ||
| 0.161 mg GAE/mL | Salmonella Infantis ZMJ9 | ||
| Tamarindus indica | 0.6 mm | E. colij | [50] |
| 1.0 mm | S. aureusj | ||
| 0.8 mm | P. aeruginosaj | ||
| n.a | S. typhij | ||
| Morinda citrifolia | [51] | ||
| Leaf | 10 mm | S. aureusk | |
| 11 mm | S. epidermisk | ||
| 9 mm | Streptococcus pyogenesk | ||
| 9 mm | E. colik | ||
| 10 mm | Serratia marcescensk | ||
| 11 mm | P. aeruginosak | ||
| 8 mm | K. pneumoniaek | ||
| Stem | 9 mm | S. aureus | |
| 9 mm | S. epidermis | ||
| 8 mm | Streptococcus pyogenes | ||
| 7 mm | E. coli | ||
| 7 mm | Serratia marcescens | ||
| 8 mm | P. aeruginosa | ||
| 7 mm | K. pneumoniae | ||
| Root | 9 mm | S. aureus | |
| 12 mm | S. epidermis | ||
| 11 mm | Streptococcus pyogenes | ||
| 9 mm | E. coli | ||
| 8 mm | Serratia marcescens | ||
| 11 mm | P. aeruginosa | ||
| 11 mm | K. pneumoniae | ||
| Aspilia mossambicensisl | [52] | ||
| Leaf | 10–14 mm | S. aureusm | |
| 10–14 mm | P. aeruginosa ATCC 27853 | ||
| Stem bark | 10–14 mm | S. aureus | |
| 8–9 mm | P. aeruginosa ATCC 27853 | ||
| Root | 8–9 mm | S. aureus | |
| n.a | P. aeruginosa ATCC 27853 | ||
| Ocimum gratissimum | |||
| Leaf | n.a | S. aureus | |
| n.a | P. aeruginosa ATCC 27853 | ||
| Stem bark | 10–14 mm | S. aureus | |
| 8–9 mm | P. aeruginosa ATCC 27853 | ||
| Root | 10–14 mm | S. aureus | |
| 10–14 mm | P. aeruginosa ATCC 27853 | ||
| Toddalia asiatica | |||
| Leaf | n.a | S. aureus | |
| n.a | P. aeruginosa ATCC 27853 | ||
| Stem bark | 15–19 mm | S. aureus | |
| 10–14 mm | P. aeruginosa ATCC 27853 | ||
| Root | 10–14 mm | S. aureus | |
| n.a | P. aeruginosa ATCC 27853 | ||
| Ocimum gratissimum | 20 mm | P. aeruginosan | [53] |
| 29 mm | S. dysenteriae | ||
| 8.5 mm | Proteus sp. | ||
| 18 mm | S. aureus | ||
| Black pearl purple corn | 13.33 mm | S. enteritidis ATCC13076 | [54] |
| 10.33 mm | S. aureus ATCC 6538 | ||
| n.a | E. coli ATCC 11775 | ||
| Jinheiyu purple corn | 11 mm | S. enteritidis ATCC13076 | |
| 10.17 mm | S. aureus ATCC 6538 | ||
| n.a | E. coli ATCC 11775 | ||
| Jingheinuo purple corn | 11.5 mm | S. enteritidis ATCC13076 | |
| 9.17 mm | S. aureus ATCC 6538 | ||
| n.a | E. coli ATCC 11775 | ||
| Shijiazhuang purple corn | 13.33 mm | S. enteritidis ATCC13076 | |
| 12.17 mm | S. aureus ATCC 6538 | ||
| n.a | E. coli ATCC 11775 | ||
| Zhuozhou purple corn | 14.33 mm | S. enteritidis ATCC13076 | |
| 12.33 mm | S. aureus ATCC 6538 | ||
| n.a | E. coli ATCC 11775 |
| Phenolic Compound | Antimicrobial Activity a,b | Bacterial Pathogen | Reference |
|---|---|---|---|
| Protocatechuic acid | 2000 (µg/mL) | P. aeruginosa ATCC 15692 c | [35] |
| 2000 (µg/mL) | P. aeruginosa PA01 d | ||
| 2000 (µg/mL) | P. aeruginosa PT121 d | ||
| 2000 (µg/mL) | P. aeruginosa DB5218 e | ||
| 2000 (µg/mL) | P. aeruginosa DR3062 e | ||
| n.a | S. aureus DSM 2031 f | [42] | |
| n.a | S. epidermis ATCC 12228 | ||
| n.a | S. epidermis FOMK g | ||
| n.a | B. subtilis ATCC 9372 | ||
| n.a | B. subtilis ATCC 6633 | ||
| 3–4 mm | M. luteus YMBL h | ||
| n.a | E. coli ATCC 8739 | ||
| n.a | E. coli ATCC 11775 | ||
| 4–10 mm | P. aeruginosa ATCC 9027 | ||
| Gallic acid | 2000 (µg/mL) | P. aeruginosa ATCC 15692 c | [35] |
| 2000 (µg/mL) | P. aeruginosa PA01 d | ||
| 2000 (µg/mL) | P. aeruginosa PT121 d | ||
| 2000 (µg/mL) | P. aeruginosa DB5218 e | ||
| 2000 (µg/mL) | P. aeruginosa DR3062 e | ||
| 9.0 mm | E. coli CECT 434 i | [55] | |
| 10.0 mm | P. aeruginosa ATCC 10145 | ||
| 7.75 mm | L. monocytogenes ATCC 15313 | ||
| 8.0 mm | S. aureus CECT 976 | ||
| 14 mm | H. pylori ATCC 700392 | [56] | |
| 14 mm | H. pylori ATCC 43504 | ||
| n.a | S. aureus DSM 2031 f | [42] | |
| n.a | S. epidermis ATCC 12228 | ||
| 1–3 mm | S. epidermis FOMK g | ||
| n.a | B. subtilis ATCC 9372 | ||
| n.a | B. subtilis ATCC 6633 | ||
| 1–3 mm | M. luteus YMBL h | ||
| n.a | E. coli ATCC 8739 | ||
| n.a | E. coli ATCC 11775 | ||
| 4–10 mm | P. aeruginosa ATCC 9027 | ||
| Rutin | 4000 (µg/mL) | P. aeruginosa ATCC 15692 c | [35] |
| 4000 (µg/mL) | P. aeruginosa PA01 d | ||
| 4000 (µg/mL) | P. aeruginosa PT121 d | ||
| 4000 (µg/mL) | P. aeruginosa DB5218 e | ||
| 4000 (µg/mL) | P. aeruginosa DR3062 e | ||
| 0.01 mg/mL | E. coli O157:H7 ATCC 43895 | [57] | |
| 0.015 mg/mL | E. coli O157:H7 ATCC 35150 | ||
| 0.02 mg/mL | S. paratyphi UK Micro29 A j | ||
| 0.02 mg/mL | S. cholerasuis subsp. cholerasuis ATCC 10708 | ||
| 0.02 mg/mL | S. Enteritidis, UK(-) H2S j | ||
| Caffeic acid | 9.50 mm | E. coli CECT 434 i | [55] |
| 9.0 mm | P. aeruginosa ATCC 10145 | ||
| 10.3 mm | L. monocytogenes ATCC 15313 | ||
| 9.75 mm | S. aureus CECT 976 | ||
| n.a | S. aureus DSM 2031 f | [42] | |
| n.a | S. epidermis ATCC 12228 | ||
| 1–3 mm | S. epidermis FOMK g | ||
| n.a | B. subtilis ATCC 9372 | ||
| n.a | B. subtilis ATCC 6633 | ||
| n.a | M. luteus YMBL h | ||
| n.a | E. coli ATCC 8739 | ||
| n.a | E. coli ATCC 11775 | ||
| 1–3 mm | P. aeruginosa ATCC 9027 | ||
| Chlorogenic acid | 7.25 mm | E. coli CECT 434 i | [55] |
| 8.25 mm | P. aeruginosa ATCC 10145 | ||
| n.a | L. monocytogenes ATCC 15313 | ||
| n.a | S. aureus CECT 976 | ||
| 0.02 mg/mL | E. coli O157:H7 ATCC 43895 | [57] | |
| 0.015 mg/mL | E. coli O157:H7 ATCC 35150 | ||
| 0.005 mg/mL | S. paratyphi UK Micro29A j | ||
| 0.015 mg/mL | S. cholerasuis subsp. cholerasuis ATCC 10708 | ||
| 0.02 mg/mL | S. Enteritidis, UK(-) H2S j | ||
| (-) Epicatechin | 7.25 mm | E. coli CECT 434 i | [55] |
| 6.50 mm | P. aeruginosa ATCC 10145 | ||
| n.a | L. monocytogenes ATCC 15313 | ||
| n.a | S. aureus CECT 976 | ||
| 0.02 mg/mL | E. coli O157:H7 ATCC 43895 | [57] | |
| 0.015 mg/mL | E. coli O157:H7 ATCC 35150 | ||
| 0.01 mg/mL | S. paratyphi UK Micro29A | ||
| 0.02 mg/mL | S. cholerasuis subsp. cholerasuis ATCC 10708 | ||
| 0.02 mg/mL | S. Enteritidis, UK(-) H2S | ||
| Catechin | 11 mm | H. pylori ATCC 700392 | [56] |
| 16 mm | H. pylori ATCC 43504 | ||
| 1–3 mm | S. aureus DSM 2031 | [42] | |
| n.a | S. epidermis ATCC 12228 | ||
| 1–3 mm | S. epidermis FOMK | ||
| n.a | B. subtilis ATCC 9372 | ||
| n.a | B. subtilis ATCC 6633 | ||
| n.a | M. luteus YMBL | ||
| n.a | E. coli ATCC 8739 | ||
| n.a | E. coli ATCC 11775 | ||
| 1–3 mm | P. aeruginosa ATCC 9027 | ||
| Quercetin | 0.02 mg/mL | E. coli O157:H7 ATCC 43895 | [57] |
| 0.005 mg/mL | E. coli O157:H7 ATCC 35150 | ||
| 0.02 mg/mL | S. paratyphi UK Micro29A | ||
| 0.015 mg/mL | S. cholerasuis subsp. cholerasuis ATCC 10708 | ||
| 0.02 mg/mL | S. Enteritidis, UK(-) H2S | ||
| 4–10 mm | S. aureus DSM 2031 f | [42] | |
| 4–10 mm | S. epidermis ATCC 12228 | ||
| 4–10 mm | S. epidermis FOMK g | ||
| 1–3 mm | B. subtilis ATCC 9372 | ||
| 1–3 mm | B. subtilis ATCC 6633 | ||
| 4–10 mm | M. luteus YMBL h | ||
| 1–3 mm | E. coli ATCC 8739 | ||
| 3–4 mm | E. coli ATCC 11775 | ||
| 1–3 mm | P. aeruginosa ATCC 9027 | ||
| Ellagic acid | 4000 (µg/mL) | P. aeruginosa ATCC 15692 c | [35] |
| 4000 (µg/mL) | P. aeruginosa PA01 d | ||
| 4000 (µg/mL) | P. aeruginosa PT121 d | ||
| 4000 (µg/mL) | P. aeruginosa DB5218 e | ||
| 4000 (µg/mL) | P. aeruginosa DR3062 e | ||
| Tyrosol | n.a | E. coli CECT 434 i | [55] |
| 8.0 mm | P. aeruginosa ATCC 10145 | ||
| n.a | L. monocytogenes ATCC 15313 | ||
| n.a | S. aureus CECT 976 | ||
| Ferulic acid | 9.25 mm | E. coli CECT 434 | |
| 9.0 mm | P. aeruginosa ATCC 10145 | ||
| 11.5 mm | L. monocytogenes ATCC 15313 | ||
| 10.5 mm | S. aureus CECT 976 | ||
| Phloridzin | n.a | E. coli CECT 434 | |
| n.a | P. aeruginosa ATCC 10145 | ||
| n.a | L. monocytogenes ATCC 15313 | ||
| n.a | S. aureus CECT 976 | ||
| Epigallocatechin gallate/tetracycline combination | 0.5 (µg/mL) | S. epidermis (resistant) k | [58] |
| 0.5 (µg/mL) | S. aureus (resistant) l | ||
| 0.0625 (µg/mL) | S. epidermis (susceptible) k | ||
| 0.125 (µg/mL) | S. aureus (susceptible) l | ||
| Catechin- gallic acid combination | 13 mm | H. pylori ATCC 700392 | [56] |
| 11 mm | H. pylori ATCC 43504 | ||
| Curcumin | 0.02 mg/mL | E. coli O157:H7 ATCC 43895 | [57] |
| 0.005 mg/mL | E. coli O157:H7 ATCC 35150 | ||
| 0.015 mg/mL | S. paratyphi UK Micro29A j | ||
| 0.015 mg/mL | S. cholerasuis subsp. cholerasuis ATCC 10708 | ||
| 0.005 mg/mL | S. Enteritidis, UK(-) H2S j | ||
| Eugenol | 0.02 mg/mL | E. coli O157:H7 ATCC 43895 | |
| 0.02 mg/mL | E. coli O157:H7 ATCC 35150 | ||
| 0.02 mg/mL | S. paratyphi UK Micro29A | ||
| 0.02 mg/mL | S. cholerasuis subsp. cholerasuis ATCC 10708 | ||
| 0.015 mg/mL | S. Enteritidis, UK(-) H2S | ||
| Myricetin | 0.01 mg/mL | E. coli O157:H7 ATCC 43895 | |
| 0.01 mg/mL | E. coli O157:H7 ATCC 35150 | ||
| 0.015 mg/mL | S. paratyphi UK Micro29A | ||
| 0.02 mg/mL | S. cholerasuis subsp. cholerasuis ATCC 10708 | ||
| 0.01 mg/mL | S. Enteritidis, UK(-) H2S | ||
| Flavone | 4–10 mm | S. aureus DSM 2031 f | [42] |
| 1–3 mm | S. epidermis ATCC 12228 | ||
| 4–10 mm | S. epidermis FOMK g | ||
| 4–10 mm | B. subtilis ATCC 9372 | ||
| 3–4 mm | B. subtilis ATCC 6633 | ||
| >10 mm | M. luteus YMBL h | ||
| 3–4 mm | E. coli ATCC 8739 | ||
| 4–10 mm | E. coli ATCC 11775 | ||
| 1–3 mm | P. aeruginosa ATCC 9027 | ||
| Naringenin | >10 mm | S. aureus DSM 2031 | |
| >10 mm | S. epidermis ATCC 12228 | ||
| >10 mm | S. epidermis FOMK | ||
| >10 mm | B. subtilis ATCC 9372 | ||
| 4–10 mm | B. subtilis ATCC 6633 | ||
| >10 mm | M. luteus YMBL | ||
| 3–4 mm | E. coli ATCC 8739 | ||
| 4–10 mm | E. coli ATCC 11775 | ||
| 1–3 mm | P. aeruginosa ATCC 9027 | ||
| Naringin | n.a | S. aureus DSM 2031 | |
| n.a | S. epidermis ATCC 12228 | ||
| n.a | S. epidermis FOMK | ||
| n.a | B. subtilis ATCC 9372 | ||
| n.a | B. subtilis ATCC 6633 | ||
| 4–10 mm | M. luteus YMBL | ||
| n.a | E. coli ATCC 8739 | ||
| n.a | E. coli ATCC 11775 | ||
| 4–10 mm | P. aeruginosa ATCC 9027 | ||
| Methyl gallate | n.a | S. aureus DSM 2031 | |
| n.a | S. epidermis ATCC 12228 | ||
| n.t | S. epidermis FOMK | ||
| n.a | B. subtilis ATCC 9372 | ||
| n.t | B. subtilis ATCC 6633 | ||
| >10 mm | M. luteus YMBL | ||
| 4–10 mm | E. coli ATCC 8739 | ||
| n.t | E. coli ATCC 11775 | ||
| n.t | P. aeruginosa ATCC 9027 |
| Elicitor/Other Strategy Used | Target Plant/Plant Family | Phytochemicals Analyzed a | Target Bacterial Pathogen | References |
|---|---|---|---|---|
| Blue light | Spinach | Caffeic acid | E. coli O157:H7 ATCC b 700,728 L. innocua c | [83] |
| Light or dark incubated callus culture. | Black mustard | Flavonoids, tannins & volatile oils. | E. coli ATCC 11229 P. aeruginosa d K. pneumoniae ATCC 13,883 S. aureus NCTC 7447 e | [84] |
| Fish protein hydrolysate, oregano extract & lactoferrin. | Mung bean | Total phenolics | H. pylori ATCC 43579 | [85] |
| AgNO3 & chitosan | Plantago lanceolata | Apigenin & gallic acid | B. cereusf K. pneumoniaef P. vulgarisf S. typhif E. colif | [86] |
| Acetate, chitosan, methyl salicylate & methyl jasmonate | Anacardiaceae, Apiaceae, Asteraceae, Brassicaceae, Caryophyllaceae, Cucurbitaceae, Fabaceae, Lamiaceae, Polemoniaceae | n.t | S. aureus subsp. aureus ATCC 6538 E. coli K12 d P. aeruginosa d | [87] |
| Transformation with A. rhizogenes ATCC 15,834 & fungal elicitors. | Sweet basil | Rosmarinic acid | P. aeruginosa PAO1 g P. aeruginosa PA14 g | [88] |
| C. sakazakii bacteria lysate & hydromechanical stress. | D. muscipula J. Ellis | Myricetin, caffeic acid, & ellagic acid | S. aureus ATCC 25923 E. coli ATCC 25922 | [89] |
| Acetyl salicylic acid | Peas | Total phenolics | H. pylori ATCC 43579 | [90] |
| Callus culture | Cotton | Free & bound flavonoids | B. cereus NCIM 2156 h S. aureus NCIM 2654 S. epidermidis NCIM 249 M. smegmatis NCIM 5138 P. aeruginosa NCIM 5032 Proteus vulgaris NCIM 2027 S. typhimurium NCIM 2501 E. coli NCIM 2027 | [91] |
| Callus culture & ex vitro plantlets | Chicory | Total phenolics | S. aureus ATCC 29213 K. pneumoniae ATCC 10,031 B. cereus ATCC 11778 B. subtilis ATCC 6633 P. aeruginosa ATCC 27853 E. coli ATCC 25922 E. aerogenes ATCC 13048 S. epidermis ATCC 12,228 Serratia marcescens ATCC 27117 | [92] |
| Micropropagation | Stevia rebaudiana Bertoni | n.t | E. coli MTCC 41 i B. subtilis MTCC 441 S. mutans MTCC 497 S. aureus MTCC 737 | [93] |
| Micropropagation | Tulbaghia violacea Harv. | Total phenolics, flavonoids & saponins | B. subtilis ATCC 6051 E. coli ATCC 11775 K. pneumoniae ATCC 13,883 S. aureus ATCC 12600. | [94] |
| Micropropagation | Oregano | Carvacrol & thymol | L. monocytogenesj | [47] |
| Micropropagation | Oregano | Total phenolics | H. pylori ATCC 43504 | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopher, A.; Sarkar, D.; Shetty, K. Elicitation of Stress-Induced Phenolic Metabolites for Antimicrobial Applications against Foodborne Human Bacterial Pathogens. Antibiotics 2021, 10, 109. https://doi.org/10.3390/antibiotics10020109
Christopher A, Sarkar D, Shetty K. Elicitation of Stress-Induced Phenolic Metabolites for Antimicrobial Applications against Foodborne Human Bacterial Pathogens. Antibiotics. 2021; 10(2):109. https://doi.org/10.3390/antibiotics10020109
Chicago/Turabian StyleChristopher, Ashish, Dipayan Sarkar, and Kalidas Shetty. 2021. "Elicitation of Stress-Induced Phenolic Metabolites for Antimicrobial Applications against Foodborne Human Bacterial Pathogens" Antibiotics 10, no. 2: 109. https://doi.org/10.3390/antibiotics10020109
APA StyleChristopher, A., Sarkar, D., & Shetty, K. (2021). Elicitation of Stress-Induced Phenolic Metabolites for Antimicrobial Applications against Foodborne Human Bacterial Pathogens. Antibiotics, 10(2), 109. https://doi.org/10.3390/antibiotics10020109







