Abstract
We describe the genomic and phenotypic characteristics of a novel member of Streptococcus with multidrug resistance (MDR) isolated from hospital samples. Strains SP218 and SP219 were identified as a novel Streptococcus, S. sputorum, using whole-genome sequencing and biochemical tests. Average nucleotide identity values of strains SP218 and SP219 with S. pseudopneumoniae IS7493 and S. pneumoniae ST556 were 94.3% and 93.3%, respectively. Genome-to-genome distance values of strains SP218 and SP219 with S. pseudopneumoniae IS7493 and S. pneumoniae ST556 were 56.70% (54–59.5%) and 56.40% (52.8–59.9%), respectively. The biochemical test results distinguished these strains from S. pseudopneumoniae and S. pneumoniae, particularly hydrolysis of equine urate and utilization of ribose to produce acid. These isolates were resistant to six major classes of antibiotics, which correlated with horizontal gene transfer and mutation. Notably, strain SP219 exhibited cytotoxicity against human lung epithelial cell line A549. Our results indicate the pathogenic potential of S. sputorum, and provide valuable insights into mitis group of streptococci.
1. Introduction
The mitis group of streptococci (MGS) consists of 20 currently described Streptococcus species that cause a wide range of infections in humans, including pneumonia, infective endocarditis, and septicemia [1,2,3]. Some MGS strains are highly virulent with various pathologies, such as Streptococcus pneumoniae, whereas others are low-pathogenicity commensal species.
Discrimination among MGS bacteria is important for obtaining accurate epidemiological information about MGS and their clinical significance, as their pathogenic potentials and sensitivities to various drugs differ considerably [4,5]. With the decreasing cost of genome sequencing, MGS can be classified more precisely through whole-genome sequencing [3,6]. Additionally, the identification of novel numbers, such as Streptococcus pseudopneumoniae [7], Streptococcus tigurinus [8], and Streptococcus dentisani [9], supplemented the understanding of MGS and avoided the misdiagnosis in clinical settings. In this study, we performed whole-genome phylogenetic analysis and identified a novel species of MGS which were collected from a hospital in Beijing in 2015. According to the phenotypic and phylogenetic data, SP218 and SP219 represent a novel species in the genus Streptococcus, for which the name Streptococcus sputorum sp. nov. is proposed. Remarkably, it was multidrug resistance and exhibited cytotoxicity against the human lung epithelial cell line A549. Our data demonstrated that the whole-genome analysis is an effective way to accurately identify the novel species in MGS, and the characterization of those novel species enabled a more in-depth acknowledgement of MGS diversity. Further analysis of the whole genome sequencing result found that this MDR species contains a lot antibiotic resistant genes and resistance mutations. It should be a part of the antibiotic resistance network of MGS.
2. Results and Discussion
2.1. Phylogenetic Analysis of the Isolates
We collected 18 strains of Streptococcus sp. that were isolated from sputum samples of emergency ward patients in a hospital in Beijing in 2015 (7 females and 11 males). The 18 strains were initially identified as S. pneumoniae using the VITEK-2 system (BioMérieux, Marcy l’Etoile, France). To clarify their phylogenetic relationships, Illumina sequencing was performed and a maximum-likelihood phylogenetic tree of the 18 strains was constructed based on the concatenated sequences of 687 core genes, along with genome sequences for 19 representative species [3] of MGS downloaded from RefSeq (Figure 1). Sixteen strains clustered with S. pneumoniae, while the other two strains (SP218 and SP219), which were isolated from different patients, constituted a distinct lineage from S. pneumoniae and S. pseudopneumoniae. Average nucleotide identity (ANI) values between strain SP218 and SP219 and between S. pseudopneumoniae IS7493 and S. pneumoniae ST556 were 94.3% and 93.3%, respectively. Genome-to-genome distance (GGD) values between strain SP218 and SP219 and between S. pseudopneumoniae IS7493 and S. pneumoniae ST556 were 56.70% (54–59.5%) and 56.40% (52.8–59.9%), respectively. The threshold values of ANI and GGD for species discrimination are 95–96% and 70%, respectively. The SP218 and SP219 genomes contain 2,267,731 and 2,267,305 nucleotides, respectively, and both have GC contents of 39.74 mol%, within the range of values for Streptococcus species (33.8–43.4 mol%) [10]. The isolates SP218 and SP219 showed identical ANI and GGD values for species discrimination, confirming that these isolates represent the same species. These results suggest that strain SP218 and SP219 represent a novel species belonging to genus Streptococcus.
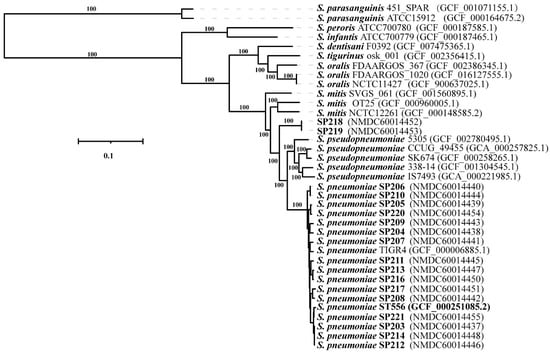
Figure 1.
Whole-genome maximum-likelihood phylogenetic tree constructed using 687 core genome sequences from 18 clinical isolates and 19 genomes obtained from Refseq belonging to MGS. The sequences of S. parasanguinis ISU2812 and S. parasanguinis NCTC10234 were used as the root. Numbers at major branches represent bootstrap values based on 100 replications. The bar indicates changes per nucleotide position.
2.2. Morphological and Physiological Characterization of the New Species
Colonies of strains SP218 and SP219 on sheep blood agar were alpha-hemolytic, smooth, and white to grayish with a diameter of 0.5–1 mm after incubation at 37 °C with CO2 for 24 h (Figure S1A,D). The cells formed chains in broth culture (Figure S1B,E). As demonstrated using transmission electron microscopy, the strains do not have capsules (Figure S1C,F), as is the case for S. pseudopneumoniae [7]. However, in contrast to S. pseudopneumoniae, SP218 and SP219 were susceptible to optochin. The inhibition zones was 18 mm (>14 mm) when incubated under an atmosphere of elevated CO2 or ambient conditions. SP218 and SP219 can dissolve the bile, showed a OD-value 0.2, which differed from S. pseudopneumoniae (showed a median OD-value 1.8 [7]).
To classify the new species through physiological characterization, we tested the strains using the Rapid ID 32 Strep system, which can be used to differentiate among most Streptococcus species for which phenotypic characteristics have been described. The biochemical characteristics of the two isolates were represented by Rapid ID 32 Strep profiles of 4–2–1–7–2–4–1–1–1–0–0 and 0–2–1–0–2–4–1–1–1–0–0, respectively. These results do not align with any species that the Rapid ID 32 Strep system can classify. Some traits clearly distinguished these strains from their nearest phylogenetic relatives (S. pseudopneumoniae and S. pneumoniae), as shown in Table S1. Strains SP218 and SP219 hydrolyzed equine urate and utilized ribose to produce acid, whereas S. pseudopneumoniae and S. pneumoniae did not. SP219 exhibited β-galactosidase activity (Table S1). Given the phenotypic and phylogenetic data described above, we propose that SP218 and SP219 belong to a novel species of Streptococcus: Streptococcus sputorum sp. nov.
2.3. Antibiotic Resistance via HGT
Antibiotic susceptibility profiles were determined for all 18 clinical isolates (Table 1). Strains SP218 and SP219 were MDR, as they were not susceptible to six major classes of antibiotics: β-lactams, erythromycin, fluoroquinolone, tetracycline, trimethoprim, and sulfamethoxazole. They were, however, susceptible to clindamycin. The strains showed broader resistance than 10 of the tested S. pneumoniae isolates, and had higher resistance level to fluoroquinolone than other S. pneumoniae isolates in this study.

Table 1.
Antibiotic susceptibility profiles of the clinical isolates in this study *.
To clarify the mechanisms through which this new species acquired resistance, we analyzed genes associated with antibiotic resistance. The S. pneumoniae genome encodes six penicillin-binding proteins (PBPs), among which PBP2x and PBP1a contribute to cephalosporin resistance [11]. A maximum-likelihood tree was constructed from serial amino acid sequences of PBP1a and PBP2x from tested clinical isolates to identify variation in these proteins. As shown in Figure 2A, the amino acid sequences of PBP1a and PBP2x from the 18 clinical isolates in this study clustered into two highly supported monophyletic clades (with 100% bootstrap confidence). All isolates in the clade containing SP218 and SP219 were insensitive to cephalosporins (cefuroxime and ceftriaxone), while the other clade exhibited cephalosporin sensitivity. SP218 and SP219 clustered with non-susceptible β-lactam isolates; this clustering did not match the phylogenetic relationships observed for the core genome, indicating the occurrence of interspecies recombination events. Further evidence of gene transfer comes from GenBank, as the pbp1a sequence obtained here was identical to that of S. pneumoniae SMRU689 (CQZX01000009) (Figure 2B). Moreover, pbp2x was highly homologous (99.07%) to the corresponding gene of S. pneumoniae GCF_900170105.1 (NZ_FWWA01000144). A fragment with greater than 90% nucleotide similarity to S. pneumoniae GPSC7 (CAANHW010000003) was found in the region approximately 20 kb upstream of pbp2x, which contained a sulA gene with mutations conferring resistance to sulfonamides. These data strongly suggest that the new species, S. sputorum, obtained gene fragments from S. pneumoniae and recombined them into its genome. It consistent with previous reports, gene exchanges were occur frequently among this group [12]. Recombination among S. pneumoniae, Streptococcus mitis, and Streptococcus oralis contribute to the development and dissemination of resistance to β-lactam antibiotics [13,14].
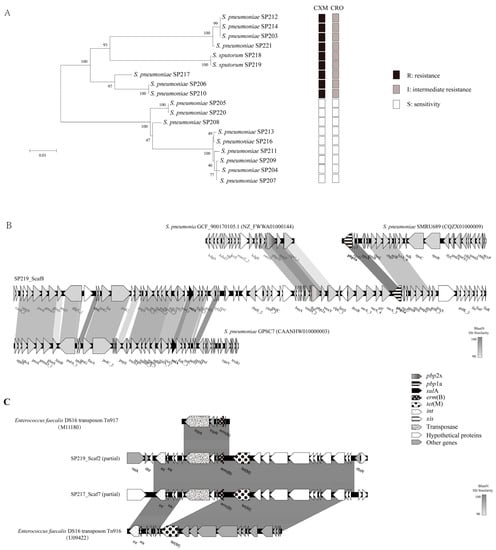
Figure 2.
(A) Maximum-likelihood tree generated using the MEGA software based on the serial amino acid sequences of PBP1a and PBP2x from tested clinical isolates, and their cephalosporin resistance profiles. Bootstrap values of 1000 replications are shown. The bar indicates changes per amino acid position. Black squares: resistance; gray squares: intermediate resistance; white squares: sensitivity. (B) Genomic organization of pbp1a-, pbp2x-, and sulA-carrying scaffolds showing highest homology with S. pneumoniae and comparison with related sequences available in GenBank. Genes are labeled and are textured based on functional classification, as shown in the key. Gray shading represents similarity at the nucleotide level. (C) Genomic organization of Tn3872 of strain S219 and S. pneumoniae SP217, and comparison with related sequences available in GenBank. Genes are labeled and are textured based on functional classification, as shown in the key. Gray shading represents similarity at the nucleotide level.
SP218 and SP219 acquired erythromycin and tetracycline resistance through integration of Tn3872 belonging to the Tn916 family [15], which is widely distributed in S. pneumoniae. Tn916 family genes were found in all 18 isolates analyzed in this study. SP218 and SP219 contained Tn3872 sequences identical to that of the isolate S. pneumoniae SP217 (Figure 2C). These results suggest that horizontal gene exchange has been involved in the acquisition of resistance by these clones. Thus, this study provides evidence of interspecies genetic transfer between the new species and S. pneumoniae.
All S. pneumoniae in this study were sensitive to fluoroquinolone except SP217. Relative to S. pneumoniae SP217, the strains SP218 and SP219 displayed higher resistance level to fluoroquinolone. Mutations in GyrA (S81Y and S114G) and ParC (S52G, D56E, S79I, and N91D) confer resistance to fluoroquinolone in S. pneumoniae [16]. These mutations were also found in GyrA and ParC of SP218 and SP219. Furthermore, GyrA and ParC of SP218 and SP219 shared 97.89% and 97.04% idenity with the proteins in S. streptococcus SP217 and there could be unknown mutation involved in fluoroquinolone resistance in strains of the novel species.
2.4. Virulence Genes and Cytotoxicity of the New Species
To gain insights into the genetic features that promote adhesion, virulence, and colonization in these strains, we searched the Virulence Factor Database [17] for orthologs of virulence genes. SP218 and SP219 harbored 21 virulence genes in common with the reference S. pneumoniae genome (Table S2), including genes associated with adherence, enzymes, iron uptake, manganese uptake, and pneumolysin toxins, which have hemolytic activity. These findings indicate the pathogenic potential of these microorganisms. However, strains SP218 and SP219 did not harbor the capsule gene cluster, suggesting that they do not generate capsules. This is consistent with our transmission electron microscopy results, described above (Figure S1).
Interactions between pathogens and host mucosal epithelial cells are prerequisites for pneumococcal disease development. Therefore, we investigated the ability of strain SP219 to adhere to and invade A549 cells. As shown in Figure 3, it adhered to and invaded A549 cells. However, the efficiencies of adhesion and invasion were significantly lower for un-encapsulated strain SP219 than encapsulated S. pneumoniae ST556. We also examined the cytotoxicity of SP219 to A549 cells based on lactate dehydrogenase (LDH) release. SP219 infection caused significantly higher levels of cell necrosis and LDH release than S. pneumoniae ST556 infection in A549 cells. This suggests that SP219 could more effectively breach the first line of host defenses and damage human lung epithelial cells.
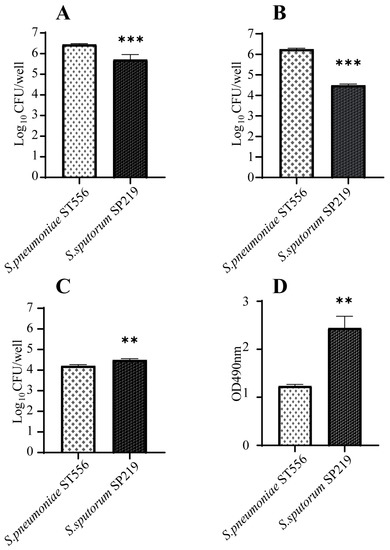
Figure 3.
Interactions between A549 cells and S. sputorum strains. The bacterial suspensions were inoculated onto washed A549 cell monolayers and incubated for 2 h at 37 °C under 5% CO2 for adhesion assays (A). Bacterial invasion was also monitored after incubation for 2 h and no bacterial invasion was observed. The bacterial suspensions were inoculated onto washed A549 cell monolayers and incubated for 4 h at 37 °C under 5% CO2 for invasion (B) and cytotoxicity assays (C,D). For the cytotoxicity assays, (C) number of adherable living A549 cells after invasion assays, (D) LDH release test. The multiplicity of infection was approximately 50:1. p-values <0.001 (***), p-values <0.01 (**).
3. Conclusions
S. pneumoniae is a clinically important pathogen of the respiratory tract that causes various invasive infections. The closely related species S. pseudopneumoniae was first described in 2004 [7], and an increasing number of reports indicate that S. pseudopneumoniae is an emerging pathogen [2,18,19]. Therefore, the need to distinguish among Streptococcus species in clinical isolates is growing. Whole-genome analysis is a useful strategy for improving the accuracy of Streptococcus identification and classification. Our phenotypic and phylogenetic data clearly differentiate the two novel strains from S. pneumoniae. The presence of virulence genes and cytotoxicity factors suggests that S. sputorum could be pathogenic. Multiple HGT events resulted in the MDR phenotype of this new species. SP218 and SP219 were isolated from different patients at the same hospital, indicating the potential infectivity and transmissibility of S. sputorum. Thus, our study provides important insights into MGS.
4. Materials and Methods
4.1. Bacterial Strains, MIC Determination, and Morphological and Physiological Characterization of the Novel Strain
The 18 clinical strains were isolated for sputum samples by performing dilution plates. The purified strains were stored at −80 °C, and grown in brain heart infusion broth (BHI, BD, USA) or modified trypticase soy agar (TSA, BD, USA) containing 5% defibrinated sheep blood under a 5% CO2 atmosphere at 37 °C for 18–24 h prior to use. The 18 strains were initially identified as S. pneumoniae using the VITEK-2 system (BioMérieux, Marcy l’Etoile, France). The minimum inhibitory concentrations (MICs) of antibiotics were determined using the broth microdilution method according to Clinical and Laboratory Standards Institute guidelines (http://www.clsi.org/; accessed on 10 May 2021). A JEM-1400 transmission electron microscope (Jeol, Minato-Ku, Japan) and an SU1080 scanning electron microscope (Hitachi, Tokyo, Japan) were used to observe cell morphology, size and capsule growth state. For bile solubility testing, an inoculum was prepared from colonies taken from blood agar plates incubated overnight (37 °C) using a tube containing 2 mL saline with cell density adjusted to that of the McFarland 4 standard (approximately 1.5 × 109 cells/mL). Then the suspension was divided equally into two tubes, with each containing each 1 mL. Then, 200 µL 10% sodium deoxycholate was added to the test tube and 200 µL saline was added to the control tube. Both tubes were incubated for 10 min at 37 °C, and then their absorbances were measured. The difference in absorbance between the test tube and control tube was calculated as an OD value. A negative difference between the test tube and the blank control was set to 0.0 OD value [20]. API Rapid ID 32 strips (BioMerieux, Marcy I’Etiole, France) were used for biochemical testing of the novel and reference strains, following the manufacturer’s instructions.
4.2. Whole-Genome Sequencing and Genome Analysis
Total DNA was purified using the TIANamp Genomic DNA Kit (Tiangen, Beijing, China) following the manufacturer’s instructions. Genome sequencing of all isolates in this study was performed using the Illumina HiSeq 2000 system (Illumina, San Diego, CA, USA). Coverage was 200-fold and the scaffold N50 value was approximately 200 kb. Assembly was performed using Spades software [21]. Predicted protein-coding sequences were detected using the NCBI Prokaryotic Genomes Automatic Annotation Pipeline (prokka 1.14.6). Antibiotic resistance genes were named in accordance with The Comprehensive Antibiotic Resistance Database and confirmed manually through Basic Local Alignment Search Tool (BLAST) and literature searches. Virulence genes were identified in accordance with the Virulence Factor Database [22] for orthologs of virulence genes. To clarify their phylogenetic relationships, a maximum-likelihood (ML) phylogenetic tree was constructed using RAxML version 8.2.12 [23]. The ML tree was constructed based on the concatenated sequences of 211 core genes, along with reference Streptococcus genome sequences downloaded from GenBank. Linear genetic diagrams were constructed using Easyfig version 2.1 [24]. Sequence comparisons were generated using mafft v7.487. Pairwise ANI values were estimated by calculating the average identity value of all BLAST results between each pair of genomes [25]. GGD was assessed using the web service of the Genome-to-genome Distance Calculator 2.1 [26]. Phylogenetic trees of the serial sequences PBP1a and PBP2x were constructed and genetic distances were determined using MEGA X [27].
4.3. Cell Adhesion and Invasion Assays
Cell adhesion and invasion assays had been modified from previously described [28]. For the cell adhesion assay, the strains were inoculated into BHI culture medium and grown at 37 °C to the middle logarithmic phase (optical density at 600 nm, OD600 = 0.5). Then, 5 × 106 colony-forming units (CFUs) bacteria were added to 1 × 105 cultured cells in each well (multiplicity of infection, approximately 50:1). Bacteria and cells were incubated for 120 min at 37 °C. At the end of incubation, the cells were extensively rinsed with phosphate-buffered saline five times and then incubated with 0.25% trypsin and 400 μL 0.025% Triton-X100 for 5 min. The cells were removed from the well. The suspension was serially diluted and plated onto TSA plates containing 5% sheep blood, which were incubated at 37 °C under 5% CO2 for 17–18 h for CFU determination. Adherent cells were counted at the end of each experiment. For each experiment, six replicates were assessed at least three times. Representative experiments are presented in the figures. For the cell invasion assay, all of the initial steps were the same as those used in the adhesion assay, except that bacteria and cells were incubated together for 4 h and penicillin (100 μg/mL) and gentamicin (50 μg/mL) were added to the culture medium for 60 min at the end of incubation to kill the bacteria outside of the cell. The MIC of SP218 and 219 to penicillin and gentamycin were 1 μg/mL.
4.4. LDH Release Test
The fresh strains were inoculated into BHI culture medium and cultured at 37 °C to the middle logarithmic phase (OD600 = 0.5). Then, 5 × 106 CFUs bacteria were added to 1 × 105 cultured cells in each well (multiplicity of infection, approximately 50:1). Bacteria and cells were incubated for 4 h at 37 °C. After incubation, the supernatant was centrifuged at 400× g for 5 min. The LDH Cytotoxicity Assay Kit (Beyotime, Shenzhen, China) was used for cytotoxicity testing of the novel and reference strains, following the manufacturer’s instructions. Then the absorbance was measured at 490 nm. Dual-wavelength determination was performed using any wavelength of 600 nm or greater as the reference wavelength.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10121532/s1, Table S1: Characteristics used to distinguish strains SP218 and SP219 from S. pneumoniae and S. pseudopneumoniae. Table S2: Virulence factor genes in the genomes of SP218, SP219, and the reference genome of S. pneumoniae annotated using the Virulence Factor Database (http://www.mgc.ac.cn/VFs/; accessed on 19 March 2021). Figure S1: (A,D) Images of the colony morphology of SP218 and SP219. Bar = 2 mm; (B,E) microscopy images of SP218 and SP219. Bar = 10 µm; (C,F) transmission-electron-microscope images of SP218 and SP219. Bar = 1 µm. SP218 and SP219 grown at 37 °C on TSA medium containing 5% defibrinated sheep blood in a 5% CO2 atmosphere. Reference [29] is cited in the supplementary materials.
Author Contributions
Conceptualization, C.W. and J.F.; methodology, C.W, M.W. and Y.L.; validation, Y.L. and J.F.; formal analysis, C.W. and Y.Z.; investigation, L.C., G.Z., and C.W.; resources, Y.L.; data curation, Y.Z.; writing—original draft preparation, C.W.; writing—review and editing, J.F.; visualization, Y.S.; supervision, J.F.; project administration, J.F.; funding acquisition, C.W. and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number: 31872632, 31861133001 and 31870134) and the Joint Funds of the International Development Research Center of Canada (Grant number: 109282-001).
Data Availability Statement
All datasets were provided by requests to the author for correspondence.
Conflicts of Interest
The authors declare no conflict of interest.
Nucleotide Sequence Accession Numbers
The draft genome sequence of isolates have been deposited in National Microbiology Data Center under the accession number NMDC60014437 to NMDC60014448, NMDC60014450 to NMDC60014455.
References
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Fuursted, K.; Littauer, P.J.; Greve, T.; Scholz, C.F. Septicemia with Streptococcus pseudopneumoniae: Report of three cases with an apparent hepatic or bile duct association. Infect. Dis. 2016, 48, 636–639. [Google Scholar] [CrossRef]
- Jensen, A.; Scholz, C.F.P.; Kilian, M. Re-evaluation of the taxonomy of the Mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis subsp. dentisani comb. nov., Streptococcus tigurinus as Streptococcus oralis subsp. tigurinus comb. nov., and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int. J. Syst. Evol. Microbiol. 2016, 66, 4803–4820. [Google Scholar] [CrossRef]
- Kitten, T.; Munro, C.L.; Zollar, N.Q.; Lee, S.P.; Patel, R.D. Oral streptococcal bacteremia in hospitalized patients: Taxonomic identification and clinical characterization. J. Clin. Microbiol. 2012, 50, 1039–1042. [Google Scholar] [CrossRef][Green Version]
- Shelburne, S.A.; Sahasrabhojane, P.; Saldana, M.; Yao, H.; Su, X.; Horstmann, N.; Thompson, E.; Flores, A.R. Streptococcus mitis strains causing severe clinical disease in cancer patients. Emerg. Infect. Dis. 2014, 20, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nemoto, R.; Kodana, M.; Tarumoto, N.; Sakai, J.; Kawamura, T.; Ikebuchi, K.; Mitsutake, K.; Murakami, T.; Maesaki, S.; et al. Rapid and Accurate Species Identification of Mitis Group Streptococci Using the MinION Nanopore Sequencer. Front. Cell Infect. Microbiol. 2020, 10, 11. [Google Scholar] [CrossRef]
- Arbique, J.C.; Poyart, C.; Trieu-Cuot, P.; Quesne, G.; Carvalho Mda, G.; Steigerwalt, A.G.; Morey, R.E.; Jackson, D.; Davidson, R.J.; Facklam, R.R. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J. Clin. Microbiol. 2004, 42, 4686–4696. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, A.; Mueller, N.J.; Tarr, P.E.; Eich, G.; Schulthess, B.; Bahlmann, A.S.; Keller, P.M.; Bloemberg, G.V. Streptococcus tigurinus, a novel member of the Streptococcus mitis group, causes invasive infections. J. Clin. Microbiol. 2012, 50, 2969–2973. [Google Scholar] [CrossRef]
- Camelo-Castillo, A.; Benítez-Páez, A.; Belda-Ferre, P.; Cabrera-Rubio, R.; Mira, A. Streptococcus dentisani sp. nov., a novel member of the mitis group. Int. J. Syst. Evol. Microbiol. 2014, 64, 60–65. [Google Scholar] [CrossRef]
- Gao, X.Y.; Zhi, X.Y.; Li, H.W.; Klenk, H.P.; Li, W.J. Comparative genomics of the bacterial genus Streptococcus illuminates evolutionary implications of species groups. PLoS ONE 2014, 9, e101229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ju, Y.; Tang, N.; Li, Y.; Zhang, G.; Song, Y.; Fang, H.; Yang, L.; Feng, J. Systematic analysis of supervised machine learning as an effective approach to predicate β-lactam resistance phenotype in Streptococcus pneumoniae. Brief. Bioinform. 2020, 21, 1347–1355. [Google Scholar] [CrossRef]
- Kilian, M.; Riley, D.R.; Jensen, A.; Brüggemann, H.; Tettelin, H. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. mBio 2014, 5, e01490-14. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Nolte, O.; Bergmann, C.; Ip, M.; Hakenbeck, R. Crossing the barrier: Evolution and spread of a major class of mosaic pbp2x in Streptococcus pneumoniae, S. mitis and S. oralis. Int. J. Med. Microbiol. 2007, 297, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, J.; Maurer, P.; Rieger, M.; Hakenbeck, R. Streptococcus pneumoniae R6 interspecies transformation: Genetic analysis of penicillin resistance determinants and genome-wide recombination events. Mol. Microbiol. 2012, 86, 692–706. [Google Scholar] [CrossRef]
- Brenciani, A.; Bacciaglia, A.; Vecchi, M.; Vitali, L.A.; Varaldo, P.E.; Giovanetti, E. Genetic elements carrying erm(B) in Streptococcus pyogenes and association with tet(M) tetracycline resistance gene. Antimicrob. Agents Chemother. 2007, 51, 1209–1216. [Google Scholar] [CrossRef]
- Brueggemann, A.B.; Coffman, S.L.; Rhomberg, P.; Huynh, H.; Almer, L.; Nilius, A.; Flamm, R.; Doern, G.V. Fluoroquinolone resistance in Streptococcus pneumoniae in United States since 1994–1995. Antimicrob. Agents Chemother. 2002, 46, 680–688. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Keith, E.R.; Podmore, R.G.; Anderson, T.P.; Murdoch, D.R. Characteristics of Streptococcus pseudopneumoniae isolated from purulent sputum samples. J. Clin. Microbiol. 2006, 44, 923–927. [Google Scholar] [CrossRef]
- Kilian, M.; Tettelin, H. Identification of Virulence-Associated Properties by Comparative Genome Analysis of Streptococcus pneumoniae, S. pseudopneumoniae, S. mitis, Three, S. oralis Subspecies, and S. infantis. mBio 2019, 10, e01985-19. [Google Scholar] [CrossRef] [PubMed]
- Slotved, H.C.; Facklam, R.R.; Fuursted, K. Assessment of a novel bile solubility test and MALDI-TOF for the differentiation of Streptococcus pneumoniae from other mitis group streptococci. Sci. Rep. 2017, 7, 7167. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Lee, I.; Kim, Y.O.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Meier-Kolthof, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Novick, S.; Shagan, M.; Blau, K.; Lifshitz, S.; Givon-Lavi, N.; Grossman, N.; Bodner, L.; Dagan, R.; Mizrachi Nebenzahl, Y. Adhesion and invasion of Streptococcus pneumoniae to primary and secondary respiratory epithelial cells. Mol. Med. Rep. 2017, 15, 65–74. [Google Scholar] [CrossRef]
- Lim, Y.K.; Park, S.N.; Shin, J.H.; Chang, Y.H.; Shin, Y.; Paek, J.; Kim, H.; Kook, J.K. Streptococcus chosunense sp. nov., Isolated from Human Postoperative Maxillary Cyst. Curr. Microbiol. 2019, 76, 1193–1198. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).