Abstract
The increasing emergence of antimicrobial resistance in staphylococcal bacteria is a major health threat worldwide due to significant morbidity and mortality resulting from their associated hospital- or community-acquired infections. Dramatic decrease in the discovery of new antibiotics from the pharmaceutical industry coupled with increased use of sanitisers and disinfectants due to the ongoing COVID-19 pandemic can further aggravate the problem of antimicrobial resistance. Staphylococci utilise multiple mechanisms to circumvent the effects of antimicrobials. One of these resistance mechanisms is the export of antimicrobial agents through the activity of membrane-embedded multidrug efflux pump proteins. The use of efflux pump inhibitors in combination with currently approved antimicrobials is a promising strategy to potentiate their clinical efficacy against resistant strains of staphylococci, and simultaneously reduce the selection of resistant mutants. This review presents an overview of the current knowledge of staphylococcal efflux pumps, discusses their clinical impact, and summarises compounds found in the last decade from plant and synthetic origin that have the potential to be used as adjuvants to antibiotic therapy against multidrug resistant staphylococci. Critically, future high-resolution structures of staphylococcal efflux pumps could aid in design and development of safer, more target-specific and highly potent efflux pump inhibitors to progress into clinical use.
1. Introduction
Antimicrobial resistance (AMR) represents a public health concern worldwide and has been described as a “slow-moving tsunami” [1]. Undoubtedly, the selective pressure exerted by the excessive and indiscriminate use of antimicrobial agents such as antibiotics and sanitisers to kill or inhibit various bacterial pathogens has accelerated the emergence and spread of AMR among bacteria [2,3]. Bacteria have developed countermeasures to protect themselves against external stresses such as antimicrobials. In the presence of lethal stress, while the majority of bacterial cells perish, a subpopulation of bacterial cells known as persister cells can survive and resume growth after relief of the imposed lethal stress [4,5]. Staphylococcal bacteria in particular can adopt alternative lifestyles including a phenotype known as “small colony variants” [6] and biofilm formation [7], which can house such persister cells. These specific lifestyles facilitate staphylococci to be more resistant to antimicrobials.
The spread of resistant bacteria from the clinic to the environment (and vice-versa) is increasing [8], causing a growing and worrying threat to public health worldwide. According to the ‘One Health’ concept, the health of humans, animals and the environment is interconnected and the spread of AMR in any of these domains poses a risk to human health. This necessitates global innovative interventions to prevent and manage AMR [9,10]. An alarming situation is when bacteria exhibit non-susceptibility to three or more agents from different antimicrobial classes, which are then defined as multidrug resistant (MDR) bacteria [11]. Infections caused by MDR bacteria are becoming significantly harder to treat causing increases in the length of stay in hospitals, in addition to economic and social costs and mortality [12]. According to the World Health Organisation (WHO) [13], we are heading for a post-antibiotic era, where common infections and small injuries may again be fatal if we fail to act urgently against the rapid spread of AMR. Antimicrobial stewardship (restricting the use) has been a challenge during the ongoing COVID-19 pandemic, with most hospitalised COVID-19 patients given antibiotics as a prevention against secondary bacterial infection [14]. This substantially increased usage, together with increased application of sanitisers and disinfectants globally, creates conditions to further accelerate the development and spread of resistant bacteria and it is therefore anticipated to worsen the AMR threat in the coming years [14,15]. It is worth noting that there is currently no stewardship for biocides [16]. Widespread use of biocides, particularly benzalkonium and chlorhexidine, can contribute to development of biocide tolerance, which has also been found to be correlated with cross-resistance to antibiotics in bacteria including Staphylococcus aureus [17,18].
The identification of new antimicrobial drugs remains a top medical priority given the increasing rate of infections caused by MDR bacteria. This situation has been exacerbated by the lack of interest in the discovery of new antibiotic drugs. Indeed, the new antimicrobial drug discovery pipeline has shrunk since the golden age of antibiotic discovery (1950s–1970s) [19]. Today large pharmaceutical companies seem to have little enthusiasm to develop and deliver a new class of antibiotics to clinic mainly due to the low return of investment in the last few decades. Multiple factors, including short-lived efficacy before the first resistant bacterial strains start to appear (1–4 years) and demanding regulatory requirements, are the main reasons for the lack of investment on the development of novel antimicrobial drugs [20,21]. Therefore, there is a pressing need to identify alternative ways to safeguard the effectiveness of the currently used antimicrobials for the control of bacterial infections [22]. One such orthogonal strategy complimentary to new antimicrobial discovery is the development of nonantimicrobial compounds, termed antibiotic adjuvants, that can diminish the emergence of resistance. These compounds can be used in combination with existing antibiotics to enhance the activities of already approved antibiotics to which resistance has developed [23,24].
Staphylococci are among the most important human pathogens and are well known for their ability to rapidly develop resistance to a broad range of antimicrobial compounds [25]. Additionally, staphylococci possess a remarkable diversity of resistance mechanisms which cause the increasing multidrug resistance seen among these bacteria [26]. Among the resistance mechanisms behind MDR staphylococci, active efflux pumps play a key role in the development of AMR in these bacteria by actively extruding antimicrobials from the cell [27]. The growing threat of efflux pump mediated AMR in staphylococci has driven research into identification of antimicrobial adjuvants. This is required to safeguard the continued utility of anti-staphylococcal drugs and simultaneously decrease the selection of resistant mutants via multidrug efflux. One such promising area of research is the identification and development of efflux pump inhibitors (EPIs). These antimicrobial adjuvants can potentiate a co-administered antibiotic through inhibition of staphylococcal efflux systems [28].
This review aims to present the current state of knowledge surrounding efflux-mediated antimicrobial resistance in staphylococci with their clinical implications and summarise recent updates on EPIs with a view to aiding the development of an effective adjuvant therapy to overcome efflux-related resistance in staphylococci.
2. Staphylococcal Infections as a Global Health Problem Requiring Urgent Attention
Staphylococci are frequent bacterial colonisers of the mucous membranes and skin of humans and other mammals [29]. Staphylococcal species contain two main groups, the coagulase-negative (CNS) and -positive staphylococci, based on their ability to produce the enzyme coagulase [30]. Staphylococcus epidermidis, a CNS, and S. aureus, a coagulase-positive species, are amongst the most frequently isolated nosocomial pathogens [30,31]. S. epidermidis is less virulent than its cousin S. aureus and only rarely causes life-threatening infections, usually in immunocompromised patients [32]. However, S. epidermidis represents the most common opportunistic pathogen involved in hospital-associated infections related to implantable medical devices [29,33]. Such infections have become increasingly concerning in the healthcare field as they can be extremely difficult to treat due to biofilm formation. Biofilms are more resistant than planktonic cells to antibiotics and host immune response [34]. S. epidermidis can also function as a reservoir for virulence and antibiotic resistance genes that can be transferred to S. aureus, augmenting the pathogenicity and antibiotic resistance of this more dangerous pathogen [29]. Being the most virulent staphylococcus species, the well-studied pathogen S. aureus is responsible for a wide spectrum of human infections ranging from minor skin and soft tissue abscesses to major fatal necrotising pneumonia, meningitis, infective endocarditis, sepsis, osteomyelitis, toxic shock syndrome as well as infections related to implanted medical devices [35,36]. Staphylococci are successful pathogens in establishing both acute and chronic infections in different human body sites due to their ability to efficiently regulate a wide arsenal of virulence factors and immune evasion mechanisms [31].
3. Development and Dissemination of Multidrug Resistance in Staphylococci
S. aureus represents the most notorious superbug among Gram-positive bacteria due to its ability to develop resistance to antibiotics [37]. Historically, penicillin was used to treat S. aureus infections. However, by the mid-1940s, penicillin-resistant S. aureus strains began to appear in hospitals only a few years after its introduction into clinical practice. Within a decade, penicillin-resistant strains spread to the community and reached pandemic levels [25,38]. Resistance to penicillin emerged through the acquisition of the blaZ gene. This gene encodes β-lactamase, which hydrolyses the β-lactam ring of penicillin and thus inactivates its antimicrobial activity [39]. To circumvent the problems associated with penicillin resistance, second generation semi-synthetic penicillins, which include methicillin and oxacillin, were introduced in 1959. However, resistance to methicillin was first reported in the UK in 1961, giving rise to methicillin-resistant S. aureus (MRSA) [40,41]. This resistance phenotype was 20 years later identified to be due to the acquisition of a mecA gene that encodes the low affinity penicillin binding protein (PBP) 2a, enabling the bacterium to maintain cell wall synthesis, while other PBPs are inhibited by β-lactam antibiotics [25].
S. aureus is highlighted as a globally pervasive pathogen associated with rising AMR [42,43,44]. Furthermore, this ‘superbug’ has garnered substantial attention from public health researchers and health policy makers due to significant morbidity and mortality caused by MDR S. aureus infections in the hospitals or in the community settings globally [45,46,47]. For example, MRSA strains, which are resistant to all β-lactam antibiotics, were once largely confined to hospitals but recently have been increasingly isolated from community infections [48,49,50]. These community-associated MRSA (CA-MRSA) strains have been rapidly spreading in diverse communities throughout the world since the 1990s with reports of outbreaks in several countries [51,52]. In addition to its dissemination, CA-MRSA can cause complicated infections such as infective endocarditis and sepsis, whose incidence were rare before the emergence of CA-MRSA [47,53].
In addition to β-lactam antibiotics, S. aureus exhibits resistance to numerous other antibiotics such as vancomycin, erythromycin, gentamicin, and oxacillin. This has resulted in increasingly limited antibiotic choices for the treatment of cognate infections [47,53,54]. A notable example of this phenomenon is the development of vancomycin resistance in S. aureus. Vancomycin, a glycopeptide antibiotic that inhibits cell wall biosynthesis, has been used as the drug of choice for the treatment of MRSA infections since the 1980s; however, S. aureus strains exhibiting decreased susceptibility to vancomycin, known as vancomycin-intermediate and vancomycin-resistant MRSA (VISA and VRSA, respectively) have emerged and are now compromising successful therapy [55,56,57,58]. The biochemistry behind the development of resistance in this pathogen is discussed in the following section.
4. Molecular Mechanisms of Antimicrobial Resistance in Staphylococci
Technical advances have gradually unveiled the molecular mechanisms of bacterial AMR. Bacterial resistance can emerge via two major genetic pathways: (i) spontaneous mutations/deletions in the bacterial chromosome which independently result in resistance to some antimicrobials (e.g., S. aureus resistance to quinolones, linezolid and daptomycin) [59,60]; and (ii) acquisition of foreign DNA, referred to as ‘mobile genetic elements’ (MGEs), through either horizontal or vertical gene transfer. MGEs include plasmids, transposons, bacteriophages, insertion sequences, integrons, pathogenicity islands, and chromosomal cassettes that can disseminate a variety of pre-existing antimicrobial resistance determinants across bacterial genomes [61,62,63,64]. While plasmids are mostly extrachromosomal, other MGEs have the ability to integrate into the host DNA [62,65]. MGEs contribute to the high-level emergence of MDR strains [61,66]. For example, S. aureus resistance to methicillin and vancomycin was developed through the acquisition of the Staphylococcal Chromosomal Cassette mec harbouring the mecA gene [67] and the Enterococcal vanA operon residing on a conjugal plasmid [68,69], respectively.
AMR can be mediated by the following biochemical mechanisms: (i) enzymatic modification of the antimicrobial binding site to decrease the affinity for the antimicrobial (e.g., resistance to methicillin by PBP 2a); (ii) enzymatic inactivation/modification of the antimicrobial (e.g., resistance to β-lactam antibiotics by production of β-lactamases); (iii) bypassing the metabolic pathway to avoid antimicrobial action (e.g., resistance to trimethoprim-sulphamethoxazole by production of an insensitive dihydrofolate reductase); (iv) sequestration of the antimicrobial to protect the target (e.g., resistance against host defence antimicrobial peptides such as α-defensins by secretion of staphylokinase); and (v) increased expression of efflux pumps to extrude antimicrobial molecules (e.g., resistance to fluoroquinolones by the NorA efflux pump) [70,71,72,73,74]. Central to the aforementioned resistance mechanisms is the expression of active efflux pumps, which act as the first line of defence against antimicrobials in staphylococci [27], and is discussed in the following sections.
5. Efflux-Mediated Antimicrobial Resistance: Clinical Implications
Efflux pumps are ubiquitous transport proteins which are distributed in the cytoplasmic membrane of bacteria, archaea and eukaryotes [75]. Efflux-mediated drug resistance has increasingly continued to challenge the chemotherapy of bacterial infections and human cancers [76,77]. Several studies have highlighted the contribution of multidrug efflux pumps in the formation of bacterial persisters [78]. Multidrug efflux genes were found to have significantly higher expression levels in persisters compared to efflux knockout strains which showed dramatically reduced expression [79,80]. Efflux pumps enable bacteria to survive for a period of time, increasing the probability of spontaneous mutations which cause high-level resistance to specific antimicrobials to develop. Thus, the activity of efflux pumps can contribute to the establishment of other resistance mechanisms [81,82].
Bacteria essentially utilise efflux pumps as a natural defence mechanism by virtue of their ability to expel a wide variety of toxic compounds in the environment which lowers their concentration inside the cell to sub-toxic levels [41,83]. Drug efflux pumps are considered as an effective resistance mechanism in bacterial pathogens including S. aureus to cope with the diverse range of antimicrobials used clinically to treat infections or as antiseptics and disinfectants to reduce bacterial load [18]. Some bacterial efflux pumps are substrate-specific since they recognise and expel a single substrate or its closely-related derivatives (e.g., tetracycline-specific pumps), whereas MDR efflux pumps recognise and export a broad spectrum of structurally-unrelated substrates [84]. In other words, substrate polyspecificity (or promiscuity toward substrates) is the characteristic feature of MDR efflux proteins [85].
In addition to antimicrobial resistance, efflux pumps in bacteria play numerous vital physiological roles including extrusion of endogenous noxious metabolites, host-derived antimicrobials and virulence factors, suggesting that the synthetic antimicrobials could be “accidental substrates” of these membrane transport proteins [86,87,88]. There is growing evidence from many studies that highlight the contribution of efflux pumps towards bacterial biofilm formation [89], particularly in several important pathogenic species of bacteria such as S. aureus, Acinetobacter baumannii, Escherichia coli, and Pseudomonas aeruginosa. These bacteria can form biofilms on medical devices and cause biofilm infections, which constitute clinical challenges [90,91].
The expression levels of efflux pump genes are generally low under ordinary environmental conditions due to strict control of multiple regulators [92,93,94]. Notably, hospital-associated bacteria, including S. aureus, that use efflux pumps for antimicrobial resistance often express such pumps constituently at higher levels as a result of regulatory mutations, either in the promoter region of the efflux pump or in its regulator gene [83,95,96]. This provides a piece of evidence in favour of the proposition that efflux pumps have primordial physiological functions distinct from antimicrobial resistance but have been fortuitously exploited for antimicrobial resistance roles in bacterial pathogens under intense antimicrobial selective pressures in hospital environments [83].
The use of antimicrobial susceptibility measurements (such as the minimum inhibitory concentration (MIC)) is a common method to reveal differences in antimicrobial efflux activity. Bacteria with higher efflux pump expression are less susceptible to selected antimicrobials compared to those with lower efflux pump expression. However, reflecting the limited sensitivity of this method, other methods have been developed to specifically identify resistance mediated by an efflux pump in bacterial cells [97]. Basically, these methods often utilise a fluorescent molecule known to be a substrate of the efflux pump under investigation. Some directly measure the efflux activity via assessing the amount of a fluorescent substrate that is expelled while others indirectly measure the activity by assessing accumulation of a substrate inside the cell [97,98,99].
6. Major Classes of Bacterial Efflux Pumps
Bacterial drug efflux pumps are currently classified into seven membrane protein families based on a number of properties including protein sequence homology, structural conformation of the protein (the number of transmembrane-spanning (TMS) regions they possess), the energy source used (ATP hydrolysis or the ion electrochemical gradient), and substrate specificity. These families are: (1) the ATP-binding cassette (ABC) family; (2) the major facilitator superfamily (MFS); (3) the small multidrug resistance (SMR) family; (4) the proteobacterial antimicrobial compound efflux (PACE) family; (5) the multidrug and toxic compound extrusion (MATE) family; (6) the resistance-nodulation division (RND) family; and (7) the p-aminobenzoyl-glutamate transporter (AbgT) family [100]. While ABC transporters utilise the energy produced by ATP hydrolysis, other transporters depend on the electrochemical energy stored in the ion gradient, typically the proton motive force (pmf), as the driving force for the extrusion of their substrates. MATE family proteins can also use the sodium membrane gradient as the source of energy [101]. Multidrug efflux pumps identified in S. aureus belong to the ABC, MFS, MATE or SMR superfamilies/families of membrane proteins (Figure 1) and are discussed in the following sections. Interestingly, a new RND type efflux pump, FarE, from S. aureus has been identified to transport antimicrobial fatty acids found on the skin and nasal secretions [102]. Efflux systems that belong to the RND family are usually composed of three proteins. However, here FarE seems to be the only identified component of the efflux system in the staphylococcal cell envelope and may not form a tripartite assembly with partner proteins due to lack of the outer membrane.
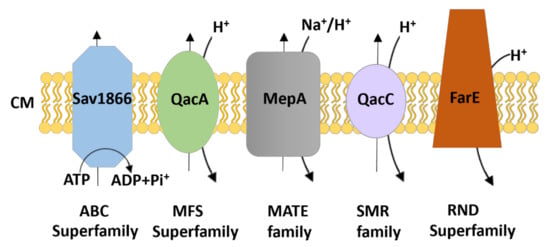
Figure 1.
Schematic representation of the families/superfamilies of multidrug exporters in staphylococci. Each transport system is depicted as a distinct shape and colour along with the energy source for driving substrate export (i.e., ATP hydrolysis for the ABC superfamily and electrochemical energy stored in the ion gradient [H+/Na+] for the others). The transporters classified within the ATP-binding cassette (ABC), major facilitator superfamily (MFS), multidrug and toxic compound extrusion (MATE), small multidrug resistance (SMR), and resistance-nodulation division (RND) family commonly expel their substrates across the cytoplasmic membrane (CM). Examples of S. aureus transporters are included.
7. Prototypical Characterised Efflux Pumps in Staphylococci
Technological advances in genome analysis and bioinformatics have identified a large number of genes encoding putative efflux pumps in bacteria (http://www.membranetransport.org/) (accessed on 26 October 2021); however, the majority of these remain experimentally uncharacterised. More than 15 efflux pumps have been described in S. aureus thus far. These efflux pumps are encoded either on the chromosome or on plasmids [27,103]. In combination, these drug transporters potentiate resistance to a wide spectrum of unrelated antibiotics, such as tetracyclines, macrolides, and quinolones, as well as a vast array of biocides, including quaternary ammonium compounds (QACs), biguanidines and diamidines. Table 1 summarises the information of the prototypical efflux pumps within S. aureus.

Table 1.
Characterised drug efflux pumps in staphylococci.
The SMR transporters, as implied by their name, are small efflux pump proteins approximately 100–130 amino acids in length. They consist of only four TMS and are carried on plasmids. The S. aureus SMR transporter identified thus far is QacC (also known as Smr, QacD or Ebr) [83,136,137]. The QacC multidrug transporter (107 amino acids) is composed of four TMS [145] and predicted to form dimers in the bacterial membrane [136,146]. Similar to the prototypical SMR protein EmrE from E. coli, QacC has been proposed to function as a homo-oligomer [93,147,148]. Mutagenesis and functional assessments have shown that the residue E13 in TMS 1 of QacC is essential for resistance to benzalkonium. This residue, which is highly conserved in SMR members, is part of the both substrate and proton binding site of QacC [149]. In addition, highly conserved residues Y59 and W62 in TMS 3 were found to be functionally important as substitutions resulted in a significant reduction in the ability of QacC to confer resistance to benzalkonium and other compounds [145]. The qacC gene [130,150] can be carried on small rolling-circle replicating plasmids [151,152] and large low-copy-number multiresistance plasmids; its promoter is known to differ between these plasmids systems [130]. QacC expression is not modulated by a transcriptional regulator [141] and currently there is no high-resolution structure available for QacC protein. Similar to QacA, QacC confers resistance to some QACs (benzalkonium and cetrimide) in staphylococcal strains [145,153], but it does not provide resistance against chlorhexidine (CH) [154,155]. It has been shown that S. aureus isolates carrying both qacA and qacC have the potential to survive exposure to a higher dose of QACs compared to those carrying only qacA [156,157]. Such S. aureus strains with elevated resistance to QACs can lead to infections in the hospital necessitating infection control measures to prevent their transmission [157].
Proteins of the MATE family range from 400 to 550 amino acid residues. MATE transporters possess 12 TMS and are chromosomally-encoded [83,158]. The MepA efflux pump is the only MATE transporter found in the staphylococcal chromosome [113,114,159]. MepA (451 amino acids) has 12 TMS and presents 21% amino acid identity to the MATE transporter NorM from Vibrio parahaemolyticus [27,113,160]. MepA is associated with a MDR phenotype in clinical S. aureus isolates, mediating low-level resistance to several monovalent and bivalent biocides and dyes [161], as well as to tigecycline [162], a glycylcycline antibiotic. Like the MFS transporter NorA, MepA is an important fluoroquinolone efflux system in S. aureus that expels hydrophilic fluoroquinolone antibiotics such as norfloxacin and ciprofloxacin. Nonetheless, these antibiotic compounds are weak substrates of MepA but strong substrates for NorA [113,114,160]. In silico modelling of MepA has revealed a probable expansive central binding cavity that represents a substrate translocation pathway similar to other multidrug binding proteins, such as AcrB [160]. Site-directed mutagenesis studies have identified that residues S81, E156, A161, D183, M291, and A302 within the cavity are functionally important [160]. Expression of mepA is regulated by MepR, a member of the MarR family of transcriptional repressors encoded immediately upstream of mepA. MepR dimers bind to inverted repeats contained within operator regions upstream of mepR and mepA. The mepR operator contains one inverted repeat, whereas the mepA operator contains two such MepR binding sites. Thus, MepR reveals higher affinity binding towards the mepA operator site [113,159,163]. MepR is substrate responsive and represses both mepA and its own gene mepR. In the presence of MepA substrates, MepR binding to each of the mepA and mepR operator sites is essentially abrogated in a different manner as a result of a conformational change in MepR, with a markedly reduced binding affinity for the mepA operator site [113,159,163].
Transporters of the ABC family have a structure that consists of four domains; two transmembrane domains and two highly conserved nucleotide-binding domains [164]. The multidrug ABC exporters Sav1866 and AbcA from S. aureus are chromosomally-encoded and contain a transmembrane and nucleotide-binding domain fused together that dimerise to form the complete transporter [107,165]. S. aureus Sav1866 is a homologue of the human ABC transporter P-glycoprotein that causes MDR in cancer cells [108,166]. Functional studies have found that Sav1866 can transport diverse substrates such as ethidium, Hoechst 33,324, tetraphenylphosphonium, verapamil and vinblastine, which are also known P-glycoprotein substrates [108,167]. Sav1866 protein is one of the best-studied bacterial multidrug ABC transporters, whose three-dimensional crystal structure was determined at 3.0 Å resolution [166]. The Sav1866 structure revealed a homodimeric protein of 12 TMS where nucleotide-binding domains exhibited a ‘head-to-tail’ arrangement with a shared interface forming two ATP-binding and hydrolysis sites [107,166]. The plasmid-encoded MsrA efflux pump of S. aureus contains 488 residues with two putative ATP-binding domains but no hydrophobic membrane spanning domains [168]. Similar to MsrA, plasmid-mediated Vga proteins from S. aureus are ABC transporters that have been characterised with no transmembrane domains [112].
The MFS represents the largest and most diverse family of membrane transporters found ubiquitously in all domains of living organisms [89,169]. Proteins of the MFS family range from 350 to 600 amino acid residues and commonly possess 12 or 14 TMS [83,103]. MFS transporters that function as drug and multidrug efflux pumps can be sub-grouped into three well-characterised drug:H+ antiporter (DHA) families, namely DHA1, DHA2 and DHA3 [83,100,170]. DHA1 and DHA3 families have been shown experimentally to have 12 TMS [100,171], whereas DHA2 family transporters such as QacA, QacB, and TetA(K) from S. aureus [93,127], TetA(L) from Bacillus subtilis [172], EmrB from E. coli [173], and LfrA from Mycobacterium smegmatis [174], have been shown to have a 14-TMS topology. Nonetheless, evolutionary studies suggested that DHA2 transporters have evolved from a 12-TMS precursor where the extra two central TMS are localised between TMS 6 and 7 [171,175,176]. The drug resistance phenotypes due to efflux in staphylococci are predominately conferred by MFS efflux pumps [177]. The genes encoding QacA, QacB and TetA(K) are primarily carried on plasmids whereas those encoding other MFS transporters are present in the chromosome (Table 1). The following sections provide an overview of well-characterised S. aureus MFS efflux pumps, focusing on their structural and functional aspects as well as clinical implications.
7.1. NorA, NorB and NorC Efflux Proteins
NorA, together with QacA, represent the best studied MFS multidrug efflux pumps found in S. aureus [103]. NorA was the first endogenous (chromosomally-encoded) efflux pump to be characterised in S. aureus [178,179]. It possesses 12 TMS with 388 amino acids and a molecular weight of 42.2 kDa [103]. In addition to hydrophilic fluoroquinolones antibiotics (e.g., norfloxacin and ciprofloxacin; treatment options against S. aureus infections), NorA confers resistance to a broad range of structurally different compounds including biocides (e.g., cetrimide, benzalkonium), dyes (e.g., ethidium, rhodamines), puromycin and chloramphenicol [180,181,182]. NorA is the predominant efflux pump associated with first-line response to antimicrobials in staphylococci [122,183] and is overexpressed in 43% of S. aureus strains, especially MRSA [184,185] strains. Due to this clinical importance, NorA has been widely studied as a target for the development of EPIs [186,187]. However, due to lack of a high-resolution structure for NorA, chemical approaches and in silico analyses (molecular docking studies) have been implemented to identify EPIs for NorA [181,188,189,190].
NorB and NorC are chromosomally-encoded efflux pumps in S. aureus of 464 and 462 amino acid residues, respectively [103,125,191]. NorA confers resistance to hydrophilic quinolones such as ciprofloxacin and norfloxacin, whereas NorB and NorC also confer resistance to hydrophobic quinolones such as moxifloxacin and sparfloxacin [125,192]. These three pumps, collectively known as ‘Nor efflux pumps’, have broad substrate profiles that include not only quinolones but also other antimicrobials, disinfectants, and dyes [191,193]. The efflux pump NorB has amino acid sequence identity with S. aureus NorA (30%), QacA (39%) and NorC (61%) [27,125]. Expression of the Nor efflux pumps in S. aureus is regulated by the global regulator MgrA (multiple gene regulator). MgrA appears to act as a positive regulator (activator) of norA expression but as a negative regulator (repressor) of norB and norC expression [123,194,195]. Increased expression of norB in a mouse subcutaneous abscess model showed that NorB was important for S. aureus survival in abscesses, suggesting a contribution of NorB in staphylococcal pathogenesis [196,197]. Recently, the X-ray structure of NorC at a resolution of 3.6 Å in complex with a single-domain camelid antibody has been determined [198]. This study demonstrated a proof-of-principle that nanobodies have the capacity to be used as EPIs for NorC and possibly other DHA2 family transporters by locking them in an outward-open conformation.
7.2. TetA(K) and Tet38 Efflux Pumps
In S. aureus, TetA(K) is a key drug efflux pump which confers high levels of resistance to the tetracycline antibiotics [133,199]. TetA(K) has 14 TMS [200,201] and is closely related to chromosome-encoded TetA(L) protein of B. subtilis [202] and Tet38 (46% identity) of S. aureus [192]. The TetA(K) and TetA(L) proteins were found to be multifunctional antiporters because they transport the cations Na+ and K+ across the membrane along with tetracycline and H+. This indicates that these efflux pumps play physiological functions in salt and alkali tolerance in addition to antimicrobial resistance [203,204]. Tet38 has a wider substrate profile mediating resistance to tetracyclines, certain fatty acids [192] and fosfomycin [134,205], a clinically used antibiotic to target the MurA protein involved in bacterial cell wall synthesis [206]. Like NorB, the Tet38 efflux pump contributes to S. aureus colonisation of skin and survival in the environment of an abscess [196,207]. Moreover, it has recently been demonstrated that this protein is involved in the attachment and internalisation of staphylococci into host cells via interaction with host cell receptors CD36 and Toll-Like Receptor 2 (TLR-2) [205,208].
7.3. QacA Multidrug Efflux Protein
QacA comprises 514 amino acids that have been shown to be organised into 14 TMS based on hydropathy studies [209] and membrane topological analyses using alkaline phosphate and β-galactosidase fusions [127]. The QacA efflux pump is the most prevalent plasmid-encoded QAC-resistance mechanism among Gram-positive bacteria. QacA is encoded by the qacA gene which was the first bacterial MDR gene to be reported [93,210,211]. The qacA gene is carried by transmissible plasmids [212] including multiresistance plasmids such as the β-lactamase and heavy-metal resistance plasmid pSK57 [213] as well as pSK1-family plasmids [155,214,215] and pSK105 in S. epidermidis [216]. While qacA is usually found in clinical isolates of S. aureus [127,176,217] and CNS such as S. epidermidis [216], Staphylococcus saprophyticus, and Staphylococcus hominis [156,218,219,220], it has also been identified in Enterococcus faecalis that showed increased CH resistance [221]. This suggests that the plasmid-born qacA gene can spread across bacterial genera [141].
QacA is able to mediate resistance to a diverse range of cationic lipophilic antimicrobial compounds belonging to different chemical classes. The one common feature of these compounds is that they are aromatic and contain a positive charge, being monovalent or bivalent cations. Monovalent cations include QACs (e.g., benzalkonium and cetrimide) and intercalating dyes (e.g., ethidium and acriflavine). Bivalent cations include diamidines (e.g., pentamidine, 4′,6-diamidino-2-phenylindole (DAPI)) and the biguanidines (e.g., CH) [100,128]. It is worth noting that QACs are the most prominent QacA substrates, such as cetrimide, benzalkonium and dequalinium, which are commonly used as antiseptics and disinfectants [128,222].
QacA exhibits significantly higher levels of resistance for a wide range of bivalent cationic substrates such as diamidines and biguanidines as opposed to its close homologue QacB. It has been postulated that QacA evolved from QacB in response to the extensive use of bivalent cations such as chlorhexidine in hospital environments [212]. MIC analysis of diamidine compounds with structural variations indicated that QacA protein interacts with bivalent cationic substrates irrespective of the interamidine linkage, side-chain alterations, or the placement of the amidine group on the aromatic ring [128]. Transport kinetic analyses of monovalent and bivalent fluorescent substrates revealed that QacA has a high affinity binding mechanism for the recognition of bivalent cationic substrates while QacB lacks such characteristics [223]. In addition to cationic antimicrobials, QacA was shown to provide resistance to thrombin-induced platelet microbicidal protein-1 (tPMP-1), a rabbit-derived cationic antimicrobial peptide [224,225]. QacA-dependent tPMP-1 resistant S. aureus strains were found to survive and proliferate within rabbit model of endocarditis. Moreover, tPMP resistance was shown to be an important S. aureus virulence factor correlated with endocarditis in humans [73,225,226]. Of note, the presence of the QacA transporter in the cell membrane of S. aureus strains has been found to be linked to increased membrane fluidity [227,228,229].
The expression of qacA has been shown to be inducible in vivo by the addition of QacA substrates. These substrates directly bind to a regulatory protein known as QacR, a TetR family transcriptional regulator, which represses expression of qacA [230,231]. QacR acts as a repressor of qacA expression by specific binding to the 28-base pair inverted repeat 1 (IR1) operator which overlaps the qacA promoter site, thereby preventing qacA transcription [232]. Induction of qacA expression occurs when QacA substrates bind to QacR, inducing a conformational change in the protein such that QacR releases from the promoter region allowing transcription of qacA to proceed [211,231,233].
In clinical practice, disinfectants (for disinfection of inanimate surfaces) and antiseptics (for disinfection of living tissues such as hand washing and skin decolonisation prior to invasive procedures) are extensively used in hospitals to prevent spread of nosocomial pathogens [234,235]. Studies have investigated the frequency of disinfectant and antiseptic resistance genes carried by S. aureus isolated from clinical settings in different regions of the world. Prevalence of qacA among S. aureus clinical isolates fluctuates depending on the geographic location, varying from 10 to 80% [236]. Carriage of qacA has been reported to be as high as 73.9% in Australia [155], 63% in Europe [237], 8.3–26.3% in the UK [157,238], and 33–61% in Asia [234,239,240,241]. An increasing trend in qacA prevalence has been observed in the U.S. and Asian countries [130], establishing the relevance of this resistance determinant. A recent study on clinical staphylococci isolated from Seattle Children’s Hospital identified a novel qacA nucleotide sequence variant [216] associated with an increased MIC to chlorhexidine (≥4 µg/mL). Varying qacA prevalence across studies is due to different factors, including the number of isolates screened in each study or the type of biocides used regularly, exerting different selective pressures on S. aureus isolates in each region [157]. As evidenced in these studies and others, increasing use of disinfectants and antiseptics has raised concerns about the emergence of resistance to biocides in S. aureus at a quite alarming rate both in medical settings and in the community [219,220].
8. Commonly Used Methodological Approaches for Evaluating Efflux Inhibition in Staphylococci
Multiple biological assays have been applied to assess EPI candidate molecules. The process of evaluating the efficacy of candidate EPIs generally starts with susceptibility assays in bacterial strains using selected antimicrobials in the absence and presence of the EPI, also known as checkerboard assays [78,242]. For these assays in staphylococci, a set of isogenic S. aureus strains are typically used [189,243,244]. Most studies have been performed on NorA where they include the well-established S. aureus SA1199B (NorA overexpressing strain) and its parent wild-type strain SA-1199 as well as SA-K1902 (norA-deleted strain). Test compounds that do not show growth inhibition nor to be a substrate when used alone but interact synergistically with an anti-staphylococcal agent in the efflux pump overexpressing strain compared to the wild-type and pump deleted strains are considered a potential EPI and are taken further for subsequent evaluations.
Measurement of the fluorescence of ethidium bromide (EtBr) is the most widely used method to determine how a designated EPI inhibits the transport of antimicrobial molecules out of the cell. EtBr is often used as a universal fluorescent substrate of efflux pump since active efflux is the only known mechanism of resistance of bacteria against this compound. EtBr emits intense fluorescence only when it intercalates into DNA inside the cell. In the absence of EPI, active efflux reduces the intracellular accumulation of EtBr and thereby causes a reduction of fluorescence in a fluorometric efflux assay whereas in the presence of EPI, increased fluorescence signal intensity is observed which is a positive indicator of EPI efficiency [28,78,245]. The most frequently used antibiotic compound in EPI assessment assays in staphylococci is ciprofloxacin which is a well-known substrate for the NorA efflux pump [243,246,247,248].
The fractional inhibitory concentration index (FICi) value is often used to describe the interaction between EPIs and antimicrobial compounds [249,250] and can be defined by the following formula:
FICi = FICantimicrobial + FICinhibitor
FICi results are interpreted as follows: synergy (FICi ≤ 0.5), additivity or partial synergy (0.5 < FICi ≤ 1), indifference or no interaction ( 1 < FICi ≤ 4), or antagonism (FICi > 4) [251]. The potentiation of antimicrobial activity is often conveniently expressed as the lowest concentration of an EPI that results in n-fold reduction of the MIC of an antimicrobial agent [78].
9. In Quest of Staphylococcal Efflux Pump Inhibitors
As mentioned before, the activity of efflux pumps in extruding various antimicrobials from the cells is recognised as an important mechanism of resistance in clinical strains of staphylococci. Moreover, efflux pumps have also been linked to biofilm formation and virulence of these bacteria. The necessity to tackle the AMR menace has encouraged research into the identification and development of potent EPIs as a promising and valid strategy to block the antimicrobial extrusion, thereby restoring antimicrobial susceptibility and extending the clinical efficacy of existing established antibiotics [252,253]. Moreover, EPIs were shown to be able to significantly decrease biofilm formation and virulence in different bacteria including S. aureus [248,249].
There has been great interest in the search for natural or synthetic compounds that could act as EPIs to potentiate the activity of conventional antimicrobial agents which are substrates of efflux pumps in resistant bacterial strains [254]. Studies show that the combined use of EPIs (as adjuvants) with antimicrobials is expected to: (i) increase the intracellular concentration of the antimicrobials that are extruded by efflux pumps; (ii) decrease the intrinsic resistance of bacteria to antimicrobials; (iii) reverse the acquired resistance related to efflux pump overexpression; and (iv) reduce the frequency of the emergence of resistant mutants [183,255].
The inhibition of efflux pump activity can be achieved by several approaches [28,183,256]. These include: (i) modifications of the chemical structure of existing antibiotics to decrease their affinity for binding sites of the efflux pump transporter; (ii) interference with the regulation of efflux pump gene expression to reduce the production of an active efflux pump in the bacterial cell membrane; (iii) disruption in the energy source required for efflux pump activity; (iv) use of carefully designed compounds that can be inserted inside the efflux pump cavity and act as a molecular plug hindering the transport of antimicrobials; (v) prevention of efflux pump from acquiring its active conformations; (vi) reduction in ability of efflux pump to interact with its substrates (antimicrobials) by using decoy compounds that compete with substrates for the same binding site (competitive inhibition) or cause decrease in the affinity of efflux pump for its substrates (non-competitive inhibition); and (vii) use of membrane permeabilisers that increase the influx of antimicrobial agents with subsequent increase in their intracellular concentration.
For a compound to qualify as a potent EPI it must be able to meet the following criteria [78,185,257]. It must: (i) enhance the efficacy of an antibiotic to which a strain has developed resistance as a result of the drug efflux pump activity; (ii) have no effect on sensitive strains lacking the drug efflux pump; (iii) not potentiate the activity of antibiotics which are not substrates of the efflux pump; (iv) increase the accumulation and reduce the expulsion of antimicrobial compounds which are substrates of the efflux pump; and (e) not affect the proton gradient across the cell membrane.
A diverse array of novel EPI compounds that show promising reversal of the efflux-mediated resistance in staphylococci have been reported to date. Such inhibitors for in vitro use with staphylococcal bacteria have been obtained either from screening of naturally occurring plant metabolites or small synthetic molecules. These compounds are discussed in more detail elsewhere [187,258,259]; this review focuses on summarising the potent staphylococcal EPIs reported over the past decade. Moreover, the selected EPIs were found to potentiate the activity of antimicrobial(s) by inhibiting an efflux pump when tested against an antimicrobial-resistant staphylococcal strain overexpressing the cognate efflux pump. Other EPIs tested against clinical isolates of staphylococci with MDR phenotypes, which are not specified to be due to overexpression of a specific efflux pump, were excluded from this review.
Plants are the natural source of compounds (phytochemicals) with many diverse chemical structures that demonstrate various biological activities. In recent years many studies have focused on the search for effective EPIs of plant origin [184]. This is because the advantages of using plant-derived EPIs are manifold including lowered cost in extraction/production, safe (minimal or almost no toxicity to human cells), high-level activity and environmentally friendly [260,261]. Reserpine, an alkaloid extracted from the roots of Rauwolfia serpentina, was the first identified plant-derived EPI that can reverse NorA-mediated resistance in S. aureus by potentiating the activity of fluoroquinolones [262,263]. In general, reserpine is commonly used as a control reference EPI in experiments assessing inhibitory compounds against NorA and other MDR efflux pumps [264]. However, the clinical application of reserpine to enhance the activities of clinically used fluoroquinolones could not be achieved due to its neurotoxicity at the concentrations required for inhibition of NorA [265]. Table 2 provides a list of new plant-derived EPIs found in the last decade, including their chemical class and efflux pump(s) that they inhibit as well as the antimicrobials to which resistance is reversed in a staphylococcal strain overproducing the relevant efflux pumps. These EPIs are alkaloids, flavonoids, terpenoids and polyphenols, which are the major classes of phytochemicals.

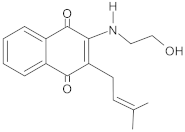
Table 2.
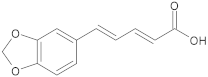
Potent plant-derived EPIs reported over the past decade.
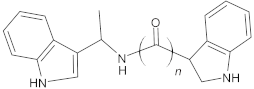
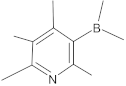
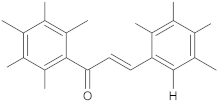
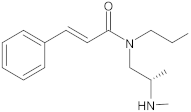
Alternate studies have focused on the search for EPIs of synthetic origin [184]. The most promising synthetic EPIs against staphylococcal efflux pumps reported in the publications over the past decade are listed in Table 3 along with their structures. Synthetic EPIs are mostly based on heterocyclic scaffolds, such as indoles, quinolines, quinolones, boronic acids and flavonoids compounds [78,266]. Especially striking is the existence of structurally complex EPIs for NorA, indicating this efflux transporter is able to bind diverse and bulky molecules [78].

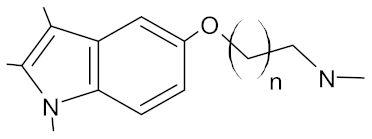
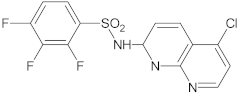
Table 3.
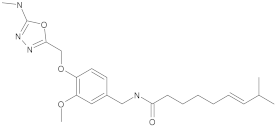
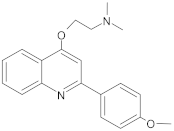
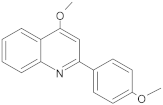
New synthetic EPI compounds reported to have synergism with various antimicrobials in staphylococci.
Despite the effectiveness of several natural and synthetic compounds that can function as an EPI, no discrete EPI compound has yet progressed beyond clinical trials, especially due to their toxicity profiles [249,267]. Therefore, further research is still required to provide a solid basis for subsequent clinical trials by designing and developing analogues of known staphylococcal EPI scaffolds or wholly new EPI compounds with even higher potency and minimal cytotoxicity.
10. Conclusions
Staphylococci are among the leading pathogens of humans, causing infections in hospitals and communities worldwide [5]. Efflux-mediated MDR, particularly in staphylococci, is an urgent clinical problem, rendering many of the current antimicrobials ineffective. To counter this issue, there is an urgent need to find alternative ways to maintain the sensitivity of staphylococci against the currently available antimicrobials by decreasing their MIC. Targeting of bacterial efflux pumps via EPIs holds great promise as an approach to enhance the potency of existing antibiotic treatment regimens when co-administered. The results of the studies summarised in this review suggest that various new EPIs have been found in the last decade to have a potential to be utilised as adjuvants to antibiotic therapy against MDR staphylococci. However, to date, there are still no EPIs on the market that can be used in combination with an antibiotic for treatment of infections caused by bacteria including staphylococci, mainly because of toxicity problems and/or poor pharmacokinetic properties [78]. Recent significant advancement in protein structural determination [268] gives a new hope that in the near future, high-resolution structures of staphylococcal MDR efflux pump bound to their substrate/inhibitor will likely be resolved. This foundational knowledge will be beneficial in promoting the design and development of further EPIs exhibiting more target-specific inhibition, being effective at a low dose and safer (non-toxic), which will hopefully progress into clinical use.
Author Contributions
Writing—Original Draft Preparation, A.D.-R.; Drafted and Edited the Manuscript, Writing—Review and Editing, A.D.-R. and M.H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded from a grant from Flinders University to M.H.B., A.D-R. was supported by an Australian Government Research Training Program Scholarship (AGRTP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reinl, J. UN declaration on antimicrobial resistance lacks targets. Lancet 2016, 388, 1365. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Loss, G.; Simões, P.M.; Valour, F.; Cortês, M.F.; Gonzaga, L.; Bergot, M.; Trouillet-Assant, S.; Josse, J.; Diot, A.; Ricci, E. Staphylococcus aureus small colony variants (SCVs): News from a chronic prosthetic joint infection. Front. Cell. Infect. Microbiol. 2019, 9, 363. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Trinh, P.; Zaneveld, J.R.; Safranek, S.; Rabinowitz, P.M. One health relationships between human, animal, and environmental microbiomes: A mini-review. Front. Public Health 2018, 6, 235. [Google Scholar] [CrossRef]
- Collignon, P.J.; McEwen, S.A. One health—Its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Cay, R.; Fehlberg, L.C.; Carvalhaes, C.G.; Nicoletti, A.G.; Gales, A.C. Molecular diagnosis contributing for multi-drug resistant infection control. Curr. Treat. Options Infect. Dis. 2014, 6, 17–39. [Google Scholar] [CrossRef]
- Barrasa-Villar, J.I.; Aibar-Remón, C.; Prieto-Andrés, P.; Mareca-Doñate, R.; Moliner-Lahoz, J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin. Infect. Dis. 2017, 65, 644–652. [Google Scholar] [CrossRef] [PubMed]
- WHO: World Health Organization. Antibiotic Resistance Fact Sheet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 31 July 2020).
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Buehrle, D.J.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob. Resist. 2020, 2, dlaa049. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Challenging biocide tolerance with antiseptic stewardship. J. Hosp. Infect. 2018, 100, e37–e39. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Chakraborty, R.; Mandal, S.M. Biocides and health-care agents are more than just antibiotics: Inducing cross to co-resistance in microbes. Ecotoxicol. Environ. Saf. 2019, 174, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.S. Antibacterial discovery: 21st century challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Venter, H. Reversing resistance to counter antimicrobial resistance in the World Health Organisation’s critical priority of most dangerous pathogens. Biosci. Rep. 2019, 39, BSR20180474. [Google Scholar] [CrossRef]
- Leitão, J.H. New insights into antibacterial compounds: From synthesis and discovery to molecular mechanisms of action. Antibiotics 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jiang, G.; Gao, R.; Chen, G.; Ren, Y.; Liu, J.; van der Mei, H.C.; Busscher, H.J. Circumventing antimicrobial-resistance and preventing its development in novel, bacterial infection-control strategies. Expert Opin. Drug Deliv. 2020, 17, 1151–1164. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotic adjuvants: Rescuing antibiotics from resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug efflux pumps in Staphylococcus aureus: An update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef]
- Mikulášová, M.; Chovanová, R.; Vaverková, Š. Synergism between antibiotics and plant extracts or essential oils with efflux pump inhibitory activity in coping with multidrug-resistant staphylococci. Phytochem. Rev. 2016, 15, 651–662. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- França, A.; Gaio, V.; Lopes, N.; Melo, L.D. Virulence factors in coagulase-negative Staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci—An overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef]
- Widerström, M.; Wiström, J.; Sjöstedt, A.; Monsen, T. Coagulase-negative staphylococci: Update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.Y.; Dykes, G.A.; Choo, W.S. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit. Rev. Microbiol. 2019, 45, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Dam, Q.; Tran, T.; Nguyen, A.; Adler-Shohet, F.; Kim, S.; Schmidt, K.; Lieberman, J.; Bradley, J. Epidemiology and hospital readmission associated with complications of Staphylococcus aureus bacteremia in pediatrics over a 25-year period. Epidemiol. Infect. 2017, 145, 2631–2639. [Google Scholar] [CrossRef]
- Banerjee, S.; Sionov, R.V.; Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Anandamide alters the membrane properties, halts the cell division and prevents drug efflux in multidrug resistant Staphylococcus aureus. Sci. Rep. 2021, 11, 1–22. [Google Scholar]
- Jiang, J.-H.; Bhuiyan, M.S.; Shen, H.-H.; Cameron, D.R.; Rupasinghe, T.W.; Wu, C.-M.; Le Brun, A.P.; Kostoulias, X.; Domene, C.; Fulcher, A.J. Antibiotic resistance and host immune evasion in Staphylococcus aureus mediated by a metabolic adaptation. Proc. Natl. Acad. Sci. USA 2019, 116, 3722–3727. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Bui, C.M. Pandemics, public health emergencies and antimicrobial resistance-putting the threat in an epidemiologic and risk analysis context. Arch. Public Health 2017, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miragaia, M. Factors contributing to the evolution of mecA-mediated beta-lactam resistance in Staphylococci: Update and new insights from whole genome sequencing (WGS). Front. Microbiol. 2018, 9, 2723. [Google Scholar] [CrossRef]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 2014, 66, 572–577. [Google Scholar] [CrossRef]
- Venter, H.; Henningsen, M.L.; Begg, S.L. Antimicrobial resistance in healthcare, agriculture and the environment: The biochemistry behind the headlines. Essays Biochem. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Wernli, D.; Jorgensen, P.S.; Morel, C.M.; Carroll, S.; Harbarth, S.; Levrat, N.; Pittet, D. Mapping global policy discourse on antimicrobial resistance. BMJ Glob. Health 2017, 2, e000378. [Google Scholar] [CrossRef] [PubMed]
- Sirijatuphat, R.; Sripanidkulchai, K.; Boonyasiri, A.; Rattanaumpawan, P.; Supapueng, O.; Kiratisin, P.; Thamlikitkul, V. Implementation of global antimicrobial resistance surveillance system (GLASS) in patients with bacteremia. PLoS ONE 2018, 13, e0190132. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Senn, L.; Clerc, O.; Zanetti, G.; Basset, P.; Prod’hom, G.; Gordon, N.C.; Sheppard, A.E.; Crook, D.W.; James, R.; Thorpe, H.A.; et al. The stealthy superbug: The role of asymptomatic enteric carriage in maintaining a long-term hospital outbreak of ST228 methicillin-resistant Staphylococcus aureus. MBio 2016, 7, e02039-15. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.C.; Dallas, S.D.; Wang, Y.; Olsen, R.J.; Lawson, K.A.; Wilson, J.; Frei, C.R. Emerging multidrug resistance in community-associated Staphylococcus aureus involved in skin and soft tissue infections and nasal colonization. J. Antimicrob. Chemother. 2017, 72, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- Agostino, J.W.; Ferguson, J.K.; Eastwood, K.; Kirk, M.D. The increasing importance of community-acquired methicillin-resistant Staphylococcus aureus infections. Med. J. Aust. 2017, 207, 388–393. [Google Scholar] [CrossRef]
- Choo, E.J. Community-associated methicillin-resistant Staphylococcus aureus in nosocomial infections. Infect. Chemother. 2017, 49, 158–159. [Google Scholar] [CrossRef]
- Kong, E.F.; Johnson, J.K.; Jabra-Rizk, M.A. Community-associated methicillin-resistant Staphylococcus aureus: An enemy amidst us. PLoS Pathog. 2016, 12, e1005837. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Sasaki, T.; Baba, T.; Lu, Y.; Imajo, E.; Sato, Y.; Tanno, S.; Furuichi, M.; Kawada, M.; Hiramatsu, K. Regional outbreak of community-associated methicillin-resistant Staphylococcus aureus ST834 in Japanese children. BMC Infect. Dis. 2019, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- McDougal, L.K.; Fosheim, G.E.; Nicholson, A.; Bulens, S.N.; Limbago, B.M.; Shearer, J.E.; Summers, A.O.; Patel, J.B. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob. Agents Chemother. 2010, 54, 3804–3811. [Google Scholar] [CrossRef] [PubMed]
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-resistant Staphylococcus aureus (MRSA): Prevalence and antimicrobial sensitivity pattern among patients—A multicenter study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8321834. [Google Scholar] [CrossRef]
- Choo, E.J.; Chambers, H.F. Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect. Chemother. 2016, 48, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Yarlagadda, V.; Ghosh, C.; Haldar, J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. MedChemComm 2017, 8, 516–533. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar] [PubMed]
- Wang, F.; Zhou, H.; Olademehin, O.P.; Kim, S.J.; Tao, P. Insights into key interactions between vancomycin and bacterial cell wall structures. ACS Omega 2018, 3, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Touati, A.; Bellil, Z.; Barache, D.; Mairi, A. Fitness cost of antibiotic resistance in Staphylococcus aureus: A systematic review. Microb. Drug Resist. 2021, 9, 1218–1231. [Google Scholar] [CrossRef]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell. Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Penades, J.R.; Ingmer, H. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol. 2017, 25, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, M.; Piwowarczyk, R.; Madry, A.; Zagorski-Przybylo, R.; Hydzik, M.; Wladyka, B. Prevalence of antibiotic and heavy metal resistance determinants and virulence-related genetic elements in plasmids of Staphylococcus aureus. Front. Microbiol. 2019, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Sargison, F.A.; Fitzgerald, J.R. Advances in transposon mutagenesis of Staphylococcus aureus: Insights into pathogenesis and antimicrobial resistance. Trends Microbiol. 2020, 29, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Jamrozy, D.; Coll, F.; Mather, A.E.; Harris, S.R.; Harrison, E.M.; MacGowan, A.; Karas, A.; Elston, T.; Estee Torok, M.; Parkhill, J.; et al. Evolution of mobile genetic element composition in an epidemic methicillin-resistant Staphylococcus aureus: Temporal changes correlated with frequent loss and gain events. BMC Genomics 2017, 18, 684. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. The science of antibiotic discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Earls, M.R.; Kinnevey, P.M.; Brennan, G.I.; Lazaris, A.; Skally, M.; O’Connell, B.; Humphreys, H.; Shore, A.C.; Coleman, D.C. The recent emergence in hospitals of multidrug-resistant community-associated sequence type 1 and spa type t127 methicillin-resistant Staphylococcus aureus investigated by whole-genome sequencing: Implications for screening. PLoS ONE 2017, 12, e0175542. [Google Scholar] [CrossRef]
- Weigel, L.M.; Clewell, D.B.; Gill, S.R.; Clark, N.C.; McDougal, L.K.; Flannagan, S.E.; Kolonay, J.F.; Shetty, J.; Killgore, G.E.; Tenover, F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003, 302, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.K.; Yin, S.; Boyle-Vavra, S. The role of the Staphylococcal VraTSR regulatory system on vancomycin resistance and vanA operon expression in vancomycin-resistant Staphylococcus aureus. PLoS ONE 2014, 9, e85873. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Gao, J.; Tang, W. Nosocomial infection and its molecular mechanisms of antibiotic resistance. Biosci. Trends 2016, 10, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 2. [Google Scholar] [CrossRef]
- Nawrocki, K.L.; Crispell, E.K.; McBride, S.M. Antimicrobial peptide resistance mechanisms of Gram-positive bacteria. Antibiotics 2014, 3, 461–492. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hipolito, C.J.; Maturana, A.D.; Ito, K.; Kuroda, T.; Higuchi, T.; Katoh, T.; Kato, H.E.; Hattori, M.; Kumazaki, K.; et al. Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature 2013, 496, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Andersson, D.I. Evolutionary consequences of drug resistance: Shared principles across diverse targets and organisms. Nat. Rev. Genet. 2015, 16, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Melander, C. The challenge of overcoming antibiotic resistance: An adjuvant approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef]
- Lamut, A.; Peterlin Mašič, L.; Kikelj, D.; Tomašič, T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 2019, 39, 2460–2504. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Z.; Li, Y.; Zou, J.; Ma, Q.; Zhao, Y.; Ke, Y.; Zhu, Y.; Chen, H.; Baker, M.A. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol. Cell 2016, 62, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ke, Y.; Bai, F. Active efflux in dormant bacterial cells—New insights into antibiotic persistence. Drug Resist. Updates 2017, 30, 7–14. [Google Scholar] [CrossRef]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Ebbensgaard, A.E.; Løbner-Olesen, A.; Frimodt-Møller, J. The role of efflux pumps in the transition from low-level to clinical sntibiotic resistance. Antibiotics 2020, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.J.; Maher, C.; Elbourne, L.D.; Eijkelkamp, B.A.; Paulsen, I.T.; Hassan, K.A. Physiological Functions of Bacterial “Multidrug” Efflux Pumps. Chem. Rev. 2021, 121, 5417–5478. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 55s–64s. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.K.; Vargiu, A.V.; Malloci, G.; Dreier, J.; Ruggerone, P. Molecular rationale behind the differential substrate specificity of bacterial RND multi-drug transporters. Sci. Rep. 2017, 7, 8075. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. Multidrug-resistance efflux pumps—Not just for resistance. Nat. Rev. Microbiol. 2006, 4, 629–636. [Google Scholar] [CrossRef]
- Hassan, K.A.; Naidu, V.; Edgerton, J.R.; Mettrick, K.A.; Liu, Q.; Fahmy, L.; Li, L.; Jackson, S.M.; Ahmad, I.; Sharples, D. Short-chain diamines are the physiological substrates of PACE family efflux pumps. Proc. Natl. Acad. Sci. USA 2019, 116, 18015–18020. [Google Scholar] [CrossRef]
- Short, F.L.; Liu, Q.; Ashwood, H.E.; Naidu, V.; Li, L.; Mabbutt, B.C.; Hassan, K.A.; Paulsen, I.T. Spermidine and spermine are the natural substrates of the Acinetobacter baumannii AmvA multidrug efflux pump. Commun. Biol. 2021, 4, 1114. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef]
- Grkovic, S.; Brown, M.H.; Skurray, R.A. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 2002, 66, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Brown, M.H.; Skurray, R.A. Proton-dependent multidrug efflux systems. Microbiol. Rev. 1996, 60, 575–608. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef]
- Webber, M.A.; Piddock, L.J. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef]
- Blair, J.M.; Piddock, L.J. How to measure export via bacterial multidrug resistance efflux pumps. mBio 2016, 7, e00840-16. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; McCusker, M.P.; Viveiros, M.; Couto, I.; Fanning, S.; Pages, J.M.; Amaral, L. A simple method for assessment of MDR bacteria for over-expressed efflux pumps. Open Microbiol. J. 2013, 7, 72–82. [Google Scholar] [CrossRef]
- Whittle, E.E.; Legood, S.W.; Alav, I.; Dulyayangkul, P.; Overton, T.W.; Blair, J. Flow cytometric analysis of efflux by dye accumulation. Front. Microbiol. 2019, 10, 2319. [Google Scholar] [CrossRef]
- Chitsaz, M.; Brown, M.H. The role played by drug efflux pumps in bacterial multidrug resistance. Essays Biochem. 2017, 61, 127–139. [Google Scholar] [PubMed]
- Kumar, A.; Schweizer, H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005, 57, 1486–1513. [Google Scholar] [CrossRef] [PubMed]
- Alnaseri, H.; Arsic, B.; Schneider, J.E.; Kaiser, J.C.; Scinocca, Z.C.; Heinrichs, D.E.; McGavin, M.J. Inducible expression of a resistance-nodulation-division-type efflux pump in Staphylococcus aureus provides resistance to linoleic and arachidonic acids. J. Bacteriol. 2015, 197, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, M.; Ammini, P.; Jones Adjei, L.M.S.; Shrestha, U.; Kumar, S.; Varela, M.F. Modulation of antimicrobial efflux pumps of the major facilitator superfamily in Staphylococcus aureus. AIMS Microbiol. 2018, 4, 1. [Google Scholar] [CrossRef]
- Littlejohn, T.G.; DiBerardino, D.; Messerotti, L.J.; Spiers, S.J.; Skurray, R.A. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene 1991, 101, 59–66. [Google Scholar] [CrossRef]
- Sasatsu, M.; Shima, K.; Shibata, Y.; Kono, M. Nucleotide sequence of a gene that encodes resistance to ethidium bromide from a transferable plasmid in Staphylococcus aureus. Nucleic Acids Res. 1989, 17, 10103. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Brown, M.H.; Dunstan, S.J.; Skurray, R.A. Molecular characterization of the staphylococcal multidrug resistance export protein QacC. J. Bacteriol. 1995, 177, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Poget, S.F.; Harris, R.; Cahill, S.M.; Girvin, M.E. 1H, 13C, 15N backbone NMR assignments of the Staphylococcus aureus small multidrug-resistance pump (Smr) in a functionally active conformation. Biomol. NMR Assign. 2010, 4, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Putman, M.; van Veen, H.W.; Konings, W.N. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 672–693. [Google Scholar] [CrossRef]
- Bay, D.C.; Turner, R.J. Small multidrug resistance efflux pumps. In Efflux-Mediated Antimicrobial Resistance in Bacteria; Li, X.Z., Elkins, C.A., Zgurskaya, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 45–71. [Google Scholar]
- Grinius, L.L.; Goldberg, E.B. Bacterial multidrug resistance is due to a single membrane protein which functions as a drug pump. J. Biol. Chem. 1994, 269, 29998–30004. [Google Scholar] [CrossRef]
- Bay, D.C.; Rommens, K.L.; Turner, R.J. Small multidrug resistance proteins: A multidrug transporter family that continues to grow. Biochim. Biophys. Acta 2008, 1778, 1814–1838. [Google Scholar] [CrossRef] [PubMed]
- LaBreck, P.T.; Bochi-Layec, A.C.; Stanbro, J.; Dabbah-Krancher, G.; Simons, M.P.; Merrell, D.S. Systematic analysis of efflux pump-mediated antiseptic resistance in Staphylococcus aureus suggests a need for greater antiseptic stewardship. mSphere 2020, 5, e00959-19. [Google Scholar] [CrossRef] [PubMed]
- Leelaporn, A.; Firth, N.; Paulsen, I.T.; Hettiaratchi, A.; Skurray, R.A. Multidrug resistance plasmid pSK108 from coagulase-negative staphylococci; Relationships to Staphylococcus aureus qacC plasmids. Plasmid 1995, 34, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Ussery, D.W.; Ingmer, H. The qacC gene has recently spread between rolling circle plasmids of Staphylococcus, indicative of a novel gene transfer mechanism. Front. Microbiol. 2016, 7, 1528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wassenaar, T.M.; Ussery, D.; Nielsen, L.N.; Ingmer, H. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur. J. Microbiol. Immunol. 2015, 5, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, T.G.; Paulsen, I.T.; Gillespie, M.T.; Tennent, J.M.; Midgley, M.; Jones, I.G.; Purewal, A.S.; Skurray, R.A. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 1992, 74, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Horner, C.; Mawer, D.; Wilcox, M. Reduced susceptibility to chlorhexidine in Staphylococci: Is it increasing and does it matter? J. Antimicrob. Chemother. 2012, 67, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Baines, S.L.; Jensen, S.O.; Firth, N.; da Silva, A.G.; Seemann, T.; Carter, G.P.; Williamson, D.A.; Howden, B.P.; Stinear, T.P. Remodeling of pSK1 family plasmids and enhanced chlorhexidine tolerance in a dominant hospital lineage of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2019, 63, e02356-18. [Google Scholar] [CrossRef] [PubMed]
- Leelaporn, A.; Paulsen, I.T.; Tennent, J.M.; Littlejohn, T.G.; Skurray, R.A. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J. Med. Microbiol. 1994, 40, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Gemmell, C.G.; Hunter, I.S. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J. Antimicrob. Chemother. 2008, 61, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Villet, R.A.; Truong-Bolduc, Q.C.; Wang, Y.; Estabrooks, Z.; Medeiros, H.; Hooper, D.C. Regulation of expression of abcA and its response to environmental conditions. J. Bacteriol. 2014, 196, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Le Bouter, A.; Leclercq, R.; Cattoir, V. Molecular basis of resistance to macrolides, lincosamides and streptogramins in Staphylococcus saprophyticus clinical isolates. Int. J. Antimicrob. Agents 2011, 37, 118–123. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Jiao, H.; Meng, J.; Qiao, M.; Du, H.; He, M.; Ming, K.; Liu, J.; Wang, D.; Wu, Y. Baicalin inhibits biofilm formation and the quorum-sensing system by regulating the MsrA drug efflux pump in Staphylococcus saprophyticus. Front. Microbiol. 2019, 10, 2800. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.J.; Locher, K.P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007, 581, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Velamakanni, S.; Yao, Y.; Gutmann, D.A.; van Veen, H.W. Multidrug transport by the ABC transporter Sav1866 from Staphylococcus aureus. Biochemistry 2008, 47, 9300–9308. [Google Scholar] [CrossRef] [PubMed]
- Allignet, J.; Loncle, V.; el Sohl, N. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene 1992, 117, 45–51. [Google Scholar] [CrossRef]
- Vimberg, V.; Cavanagh, J.P.; Novotna, M.; Lenart, J.; Ngoc, B.N.T.; Vesela, J.; Pain, M.; Koberska, M.; Novotna, G.B. Ribosome-mediated attenuation of vga (A) expression is shaped by the antibiotic resistance specificity of Vga (A) protein variants. Antimicrob. Agents Chemother. 2020, 64, e00666-20. [Google Scholar] [CrossRef] [PubMed]
- Allignet, J.; El Solh, N. Characterization of a new staphylococcal gene, vgaB, encoding a putative ABC transporter conferring resistance to streptogramin A and related compounds. Gene 1997, 202, 133–138. [Google Scholar] [CrossRef]
- Chesneau, O.; Ligeret, H.; Hosan-Aghaie, N.; Morvan, A.; Dassa, E. Molecular analysis of resistance to streptogramin A compounds conferred by the Vga proteins of staphylococci. Antimicrob. Agents Chemother. 2005, 49, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, G.W.; McAleese, F.; Seo, S.M. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 2005, 49, 1857–1864. [Google Scholar] [CrossRef]
- McAleese, F.; Petersen, P.; Ruzin, A.; Dunman, P.M.; Murphy, E.; Projan, S.J.; Bradford, P.A. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob. Agents Chemother. 2005, 49, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- De Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Muniz, D.F.; dos Santos Barbosa, C.R.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C. Chemical synthesis, molecular docking and MepA efflux pump inhibitory effect by 1, 8-naphthyridines sulfonamides. Eur. J. Pharm. Sci. 2021, 160, 105753. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, C.; Schwarz, S. Florfenicol-chloramphenicol exporter gene fexA is part of the novel transposon Tn558. Antimicrob. Agents Chemother. 2005, 49, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.L.; Smith, K.P.; Kumar, S.H.; Floyd, J.T.; Varela, M.F. LmrS is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 5406–5412. [Google Scholar] [CrossRef] [PubMed]
- Nava, A.R.; Mauricio, N.; Sanca, A.J.; Dominguez, D.C. Evidence of calcium signaling and modulation of the LmrS multidrug resistant efflux pump activity by Ca2+ ions in S. aureus. Front. Microbiol. 2020, 11, 573388. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; O’Toole, P.W.; Shen, W.; Amrine-Madsen, H.; Jiang, X.; Lobo, N.; Palmer, L.M.; Voelker, L.; Fan, F.; Gwynn, M.N.; et al. Novel chromosomally encoded multidrug efflux transporter MdeA in Staphylococcus aureus. Antimicrob. Agents Chemother. 2004, 48, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Shiota, S.; Mizushima, T.; Kuroda, T.; Tsuchiya, T. Functional gene cloning and characterization of MdeA, a multidrug efflux pump from Staphylococcus aureus. Biol. Pharm. Bull. 2006, 29, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.L.; Grinius, L.; Hooper, D.C. NorA functions as a multidrug efflux protein in both cytoplasmic membrane vesicles and reconstituted proteoliposomes. J. Bacteriol. 2002, 184, 1370–1377. [Google Scholar] [CrossRef]
- Costa, S.S.; Sobkowiak, B.; Parreira, R.; Edgeworth, J.D.; Viveiros, M.; Clark, T.G.; Couto, I. Genetic diversity of norA, coding for a main efflux pump of Staphylococcus aureus. Front. Genet. 2019, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.C.; Hooper, D.C. Phosphorylation of MgrA and its effect on expression of the NorA and NorB efflux pumps of Staphylococcus aureus. J. Bacteriol. 2010, 192, 2525–2534. [Google Scholar] [CrossRef]
- Rajabi, S.; Shivaee, A.; Khosravi, M.A.; Eshaghi, M.; Shahbazi, S.; Hosseini, F. Evaluation of multidrug efflux pump expression in clinical isolates of Staphylococcus aureus. Gene Rep. 2020, 18, 100537. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Strahilevitz, J.; Hooper, D.C. NorC, a new efflux pump regulated by MgrA of Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 1104–1107. [Google Scholar] [CrossRef]
- Kumar, S.; Mahendran, I.; Athreya, A.; Ranjan, R.; Penmatsa, A. Isolation and structural characterization of a Zn2+-bound single-domain antibody against NorC, a putative multidrug efflux transporter in bacteria. J. Biol. Chem. 2020, 295, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Brown, M.H.; Littlejohn, T.G.; Mitchell, B.A.; Skurray, R.A. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: Membrane topology and identification of residues involved in substrate specificity. Proc. Natl. Acad. Sci. USA 1996, 93, 3630–3635. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.A.; Brown, M.H.; Skurray, R.A. QacA multidrug efflux pump from Staphylococcus aureus: Comparative analysis of resistance to diamidines, biguanidines, and guanylhydrazones. Antimicrob. Agents Chemother. 1998, 42, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Khare, S.; Athreya, A.; Hussain, N.; Gulati, A.; Penmatsa, A. Dissection of protonation sites for antibacterial recognition and transport in QacA, a multi-drug efflux transporter. J. Mol. Biol. 2019, 431, 2163–2179. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Pourmand, M.R.; Mashhadi, R.; Ghazvini, K. Epidemiology of efflux pumps genes mediating resistance among Staphylococcus aureus; A systematic review. Microb. Pathog. 2020, 139, 103850. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hideka, K.; Shiota, S.; Kuroda, T.; Tsuchiya, T. Gene cloning and characterization of SdrM, a chromosomally-encoded multidrug efflux pump, from Staphylococcus aureus. Biol. Pharm. Bull. 2006, 29, 554–556. [Google Scholar] [CrossRef]
- Ginn, S.L.; Brown, M.H.; Skurray, R.A. The TetA (K) tetracycline/H+ antiporter from Staphylococcus aureus: Mutagenesis and functional analysis of motif C. J. Bacteriol. 2000, 182, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.; Wang, Y.; Hooper, D. Tet38 efflux pump contributes to fosfomycin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2018, 62, e00927-18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-T.; Saising, J.; Tribelli, P.M.; Nega, M.; Diene, S.M.; François, P.; Schrenzel, J.; Spröer, C.; Bunk, B.; Ebner, P. Inactivation of farR causes high rhodomyrtone resistance and increased pathogenicity in Staphylococcus aureus. Front. Microbiol. 2019, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Scherf, J.R.; dos Santos, C.R.B.; de Freitas, T.S.; Rocha, J.E.; Macêdo, N.S.; Lima, J.N.M.; Coutinho, H.D.M.; da Cunha, F.A.B. Effect of terpinolene against the resistant Staphylococcus aureus strain, carrier of the efflux pump QacC and β-lactamase gene, and its toxicity in the Drosophila melanogaster model. Microb. Pathog. 2020, 149, 104528. [Google Scholar] [CrossRef] [PubMed]
- Bjorland, J.; Steinum, T.; Sunde, M.; Waage, S.; Heir, E. Novel plasmid-borne gene qacJ mediates resistance to quaternary ammonium compounds in equine Staphylococcus aureus, Staphylococcus simulans, and Staphylococcus intermedius. Antimicrob. Agents Chemother. 2003, 47, 3046–3052. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Sundheim, G.; Holck, A. The qacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry. J. Appl. Microbiol. 1999, 86, 378–388. [Google Scholar] [CrossRef]
- Heir, E.; Sundheim, G.; Holck, A.L. The Staphylococcus qacH gene product: A new member of the SMR family encoding multidrug resistance. FEMS Microbiol. Lett. 1998, 163, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Narui, K.; Noguchi, N.; Wakasugi, K.; Sasatsu, M. Cloning and characterization of a novel chromosomal drug efflux gene in Staphylococcus aureus. Biol. Pharm. Bull. 2002, 25, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Schindler, B.D.; Kaatz, G.W. Multidrug efflux pumps of Gram-positive bacteria. Drug Resist. Updates 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Omote, H.; Hiasa, M.; Matsumoto, T.; Otsuka, M.; Moriyama, Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 2006, 27, 587–593. [Google Scholar] [CrossRef]
- Kaatz, G.W.; DeMarco, C.E.; Seo, S.M. MepR, a repressor of the Staphylococcus aureus MATE family multidrug efflux pump MepA, is a substrate-responsive regulatory protein. Antimicrob. Agents Chemother. 2006, 50, 1276–1281. [Google Scholar] [CrossRef]
- Schindler, B.D.; Patel, D.; Seo, S.M.; Kaatz, G.W. Mutagenesis and modeling to predict structural and functional characteristics of the Staphylococcus aureus MepA multidrug efflux pump. J. Bacteriol. 2013, 195, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.A.; Raygada, J.L.; Mendiratta, K.; Seo, S.M.; Kaatz, G.W. Multidrug efflux pump overexpression in Staphylococcus aureus after single and multiple in vitro exposures to biocides and dyes. Microbiology 2008, 154, 3144–3153. [Google Scholar] [CrossRef]
- Fang, R.; Sun, Y.; Dai, W.; Zheng, X.; Tian, X.; Zhang, X.; Wang, C.; Cao, J.; Zhou, T. Mutations in the MepRAB efflux system contribute to the in vitro development of tigecycline resistance in Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2020, 22, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Birukou, I.; Seo, S.M.; Schindler, B.D.; Kaatz, G.W.; Brennan, R.G. Structural mechanism of transcription regulation of the Staphylococcus aureus multidrug efflux operon mepRA by the MarR family repressor MepR. Nucleic Acids Res. 2014, 42, 2774–2788. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Schrader-Fischer, G.; Berger-Bachi, B. The AbcA transporter of Staphylococcus aureus affects cell autolysis. Antimicrob. Agents Chemother. 2001, 45, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.J.; Locher, K.P. Structure of a bacterial multidrug ABC transporter. Nature 2006, 443, 180–185. [Google Scholar] [CrossRef]
- Stockner, T.; de Vries, S.J.; Bonvin, A.M.; Ecker, G.F.; Chiba, P. Data-driven homology modelling of P-glycoprotein in the ATP-bound state indicates flexibility of the transmembrane domains. FEBS J. 2009, 276, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.; Ross, J.I.; Cove, J.H. Msr(A) and related macrolide/streptogramin resistance determinants: Incomplete transporters? Int. J. Antimicrob. Agents 2003, 22, 228–236. [Google Scholar] [CrossRef]
- Quistgaard, E.M.; Löw, C.; Guettou, F.; Nordlund, P. Understanding transport by the major facilitator superfamily (MFS): Structures pave the way. Nat. Rev. Mol. Cell Biol. 2016, 17, 123. [Google Scholar] [CrossRef]
- Saier, M.H., Jr.; Reddy, V.S.; Tamang, D.G.; Vastermark, A. The transporter classification database. Nucleic Acids Res. 2014, 42, D251–D258. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H., Jr. The major facilitator superfamily (MFS) revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef]
- Jin, J.; Guffanti, A.A.; Beck, C.; Krulwich, T.A. Twelve-transmembrane-segment (TMS) version (DeltaTMS VII-VIII) of the 14-TMS Tet(L) antibiotic resistance protein retains monovalent cation transport modes but lacks tetracycline efflux capacity. J. Bacteriol. 2001, 183, 2667–2671. [Google Scholar] [CrossRef][Green Version]
- Tanabe, M.; Szakonyi, G.; Brown, K.A.; Henderson, P.J.; Nield, J.; Byrne, B. The multidrug resistance efflux complex, EmrAB from Escherichia coli forms a dimer in vitro. Biochem. Biophys. Res. Commun. 2009, 380, 338–342. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhang, L.; Nikaido, H. Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 2004, 48, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H., Jr. Tracing pathways of transport protein evolution. Mol. Microbiol. 2003, 48, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.A.; Skurray, R.A.; Brown, M.H. Active export proteins mediating drug resistance in staphylococci. J. Mol. Microbiol. Biotechnol. 2007, 12, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Christena, L.R.; Subbarao, H.M.V.; Venkatasubramanian, U.; Thiagarajan, R.; Sivaramakrishnan, V.; Kasilingam, K.; Saisubramanian, N.; Ganesan, S.S. Identification of benzochromene derivatives as a highly specific NorA efflux pump inhibitor to mitigate the drug resistant strains of S. aureus. RSC Adv. 2016, 6, 30258–30267. [Google Scholar] [CrossRef]
- Yoshida, H.; Bogaki, M.; Nakamura, S.; Ubukata, K.; Konno, M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 1990, 172, 6942–6949. [Google Scholar] [CrossRef]
- Palazzotti, D.; Bissaro, M.; Bolcato, G.; Astolfi, A.; Felicetti, T.; Sabatini, S.; Sturlese, M.; Cecchetti, V.; Barreca, M.L.; Moro, S. Deciphering the molecular recognition mechanism of multidrug resistance Staphylococcus aureus NorA efflux pump using a supervised molecular dynamics approach. Int. J. Mol. Sci. 2019, 20, 4041. [Google Scholar] [CrossRef] [PubMed]
- Neyfakh, A.A.; Borsch, C.; Kaatz, G. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 1993, 37, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, F.; Lepri, S.; Goracci, L.; Schindler, B.D.; Seo, S.M.; Kaatz, G.W.; Cruciani, G. Improved potency of indole-based NorA efflux pump inhibitors: From serendipity toward rational design and development. J. Med. Chem. 2017, 60, 517–523. [Google Scholar] [CrossRef]
- Zimmermann, S.; Tuchscherr, L.; Rodel, J.; Loffler, B.; Bohnert, J.A. Optimized efflux assay for the NorA multidrug efflux pump in Staphylococcus aureus. J. Microbiol. Methods 2017, 142, 39–40. [Google Scholar] [CrossRef]
- Handzlik, J.; Matys, A.; Kieć-Kononowicz, K. Recent advances in multi-drug resistance (MDR) efflux pump inhibitors of Gram-positive bacteria S. aureus. Antibiotics 2013, 2, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Urzúa, A.; Sanhueza, L.; Walter, M.; Fincheira, P.; Muñoz, P.; Mendoza, L.; Wilkens, M. Essential oil, extracts, and sesquiterpenes obtained from the heartwood of pilgerodendron uviferum act as potential inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. Front. Microbiol. 2019, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- AlMatar, M.; Albarri, O.; Makky, E.A.; Köksal, F. Efflux pump inhibitors: New updates. Pharmacol. Rep. 2021, 73, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, B.V.; Babu, T.M.C.; Reddy, N.V.; Rajendra, W. Homology modeling, molecular dynamics, and virtual screening of NorA efflux pump inhibitors of Staphylococcus aureus. Drug Des. Devel. Ther. 2016, 10, 3237. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Uniyal, A.; Tiwari, V. A comprehensive review on pharmacology of efflux pumps and their inhibitors in antibiotic resistance. Eur. J. Pharmacol. 2021, 903, 174151. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.M.; de Macedo, E.V.; Oliveira, F.; Ferreira, J.H.L.; Gutierrez, S.J.C.; Pelaez, W.J.; Lima, F.C.; de Siqueira Junior, J.P.; Coutinho, H.D.M.; Kaatz, G.W.; et al. Inhibition of the NorA efflux pump of Staphylococcus aureus by synthetic riparins. J. Appl. Microbiol. 2016, 121, 1312–1322. [Google Scholar] [CrossRef]
- Felicetti, T.; Cannalire, R.; Burali, M.S.; Massari, S.; Manfroni, G.; Barreca, M.L.; Tabarrini, O.; Schindler, B.D.; Sabatini, S.; Kaatz, G.W.; et al. Searching for novel inhibitors of the S. aureus NorA efflux pump: Synthesis and biological evaluation of the 3-Phenyl-1,4-benzothiazine Analogues. ChemMedChem 2017, 12, 1293–1302. [Google Scholar] [CrossRef]
- Singh, S.; Kalia, N.P.; Joshi, P.; Kumar, A.; Sharma, P.R.; Kumar, A.; Bharate, S.B.; Khan, I.A. Boeravinone B, a novel dual inhibitor of NorA bacterial efflux pump of Staphylococcus aureus and human P-glycoprotein, reduces the biofilm formation and intracellular invasion of bacteria. Front. Microbiol. 2017, 8, 1868. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Onodera, Y.; Lee, J.C.; Hooper, D.C. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J. Bacteriol. 2008, 190, 7123–7129. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.; Dunman, P.; Strahilevitz, J.; Projan, S.; Hooper, D. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 2005, 187, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Hsing, L.C.; Villet, R.; Bolduc, G.R.; Estabrooks, Z.; Taguezem, G.F.; Hooper, D.C. Reduced aeration affects the expression of the NorB efflux pump of Staphylococcus aureus by posttranslational modification of MgrA. J. Bacteriol. 2012, 194, 1823–1834. [Google Scholar] [CrossRef]
- Briaud, P.; Camus, L.; Bastien, S.; Doléans-Jordheim, A.; Vandenesch, F.; Moreau, K. Coexistence with Pseudomonas aeruginosa alters Staphylococcus aureus transcriptome, antibiotic resistance and internalization into epithelial cells. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Villet, R.A.; Estabrooks, Z.A.; Hooper, D.C. Native efflux pumps contribute resistance to antimicrobials of skin and the ability of Staphylococcus aureus to colonize skin. J. Infect. Dis. 2014, 209, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, M.; Grossi, M.; Zennaro, A.; Fanelli, G.; Micheli, G.; Barras, F.; Colonna, B.; Prosseda, G. The varied role of efflux pumps of the MFS family in the interplay of bacteria with animal and plant cells. Microorganisms 2019, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Athreya, A.; Gulati, A.; Nair, R.M.; Mahendran, I.; Ranjan, R.; Penmatsa, A. Structural basis of inhibition of a transporter from Staphylococcus aureus, NorC, through a single-domain camelid antibody. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guay, G.G.; Rothstein, D.M. Expression of the tetK gene from Staphylococcus aureus in Escherichia coli: Comparison of substrate specificities of TetA (B), TetA (C), and TetK efflux proteins. Antimicrob. Agents Chemother. 1993, 37, 191–198. [Google Scholar] [CrossRef]
- Guay, G.G.; Khan, S.A.; Rothstein, D.M. The tet (K) gene of plasmid pT181 of Staphylococcus aureus encodes an efflux protein that contains 14 transmembrane helices. Plasmid 1993, 30, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Brown, M.H.; Skurray, R.A. Membrane topology of the metal-tetracycline/H+ antiporter TetA (K) from Staphylococcus aureus. J. Bacteriol. 1997, 179, 3786–3789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, J.; Guffanti, A.A.; Bechhofer, D.H.; Krulwich, T.A. Tet (L) and tet (K) tetracycline-divalent metal/H+ antiporters: Characterization of multiple catalytic modes and a mutagenesis approach to differences in their efflux substrate and coupling ion preferences. J. Bacteriol. 2002, 184, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guffanti, A.A.; Wei, Y.; Ito, M.; Krulwich, T.A. Two types of Bacillus subtilis tetA (L) deletion strains reveal the physiological importance of TetA (L) in K+ acquisition as well as in Na+, alkali, and tetracycline resistance. J. Bacteriol. 2000, 182, 2088–2095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krulwich, T.A.; Jin, J.; Guffanti, A.A.; Bechhofer, D.H. Functions of tetracycline efflux proteins that do not involve tetracycline. J. Mol. Microbiol. Biotechnol. 2001, 3, 237–246. [Google Scholar] [PubMed]
- Truong-Bolduc, Q.; Wang, Y.; Hooper, D. Tet38 of Staphylococcus aureus binds to host cell receptor complex CD36-Toll-like receptor 2 and protects from teichoic acid synthesis inhibitors tunicamycin and Congo red. Infect. Immun. 2019, 87, e00194-19. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ma, Y.; Chen, C.; Guo, Y.; Hu, F.; Liu, Y.; Xu, X.; Wang, M. Prevalence of fosfomycin resistance and mutations in murA, glpT, and uhpT in methicillin-resistant Staphylococcus aureus strains isolated from blood and cerebrospinal fluid samples. Front. Microbiol. 2016, 6, 1544. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.; Bolduc, G.; Medeiros, H.; Vyas, J.; Wang, Y.; Hooper, D. Role of the Tet38 efflux pump in Staphylococcus aureus internalization and survival in epithelial cells. Infect. Immun. 2015, 83, 4362–4372. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.; Wang, Y.; Hooper, D. Staphylococcus aureus Tet38 efflux pump structural modeling and roles of essential residues in drugs efflux and host cell internalization. Infect. Immun. 2021, 89, e00811-20. [Google Scholar] [CrossRef]
- Rouch, D.A.; Cram, D.S.; DiBerardino, D.; Littlejohn, T.G.; Skurray, R.A. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: Common ancestry with tetracycline- and sugar-transport proteins. Mol. Microbiol. 1990, 4, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Tennent, J.M.; Lyon, B.R.; Gillespie, M.T.; May, J.W.; Skurray, R.A. Cloning and expression of Staphylococcus aureus plasmid-mediated quaternary ammonium resistance in Escherichia coli. Antimicrob. Agents Chemother. 1985, 27, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Skurray, R.A. Staphylococcal multidrug efflux protein QacA. J. Mol. Microbiol. Biotechnol. 2001, 3, 163–170. [Google Scholar] [PubMed]
- Paulsen, I.T.; Brown, M.H.; Skurray, R.A. Characterization of the earliest known Staphylococcus aureus plasmid encoding a multidrug efflux system. J. Bacteriol. 1998, 180, 3477–3479. [Google Scholar] [CrossRef]
- Gillespie, M.T.; May, J.W.; Skurray, R.A. Plasmid-encoded resistance to acriflavine and quaternary ammonium compounds in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 1986, 34, 47–51. [Google Scholar] [CrossRef]
- Lyon, B.R.; Skurray, R. Antimicrobial resistance of Staphylococcus aureus: Genetic basis. Microbiol. Rev. 1987, 51, 88–134. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.Y.; Firth, N.; Davis, A.M.; Kwong, S.M.; Krysiak, M.; Lee, Y.T.; O’Brien, F.G.; Grubb, W.B.; Coombs, G.W.; Bond, C.S. Evolution of a 72-kilobase cointegrant, conjugative multiresistance plasmid in community-associated methicillin-resistant Staphylococcus aureus isolates from the early 1990s. Antimicrob. Agents Chemother. 2019, 63, e01560-19. [Google Scholar] [CrossRef]
- Addetia, A.; Greninger, A.L.; Adler, A.; Yuan, S.; Makhsous, N.; Qin, X.; Zerr, D.M. A novel, widespread qacA allele results in reduced chlorhexidine susceptibility in Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2019, 63, e02607-18. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.A.; Xu, Z.; Watkins, R.E.; Brennan, R.G.; Skurray, R.A.; Brown, M.H. Optimized production and analysis of the staphylococcal multidrug efflux protein QacA. Protein Expr. Purif. 2009, 64, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.; Pereira, T.B.; Miyazaki, N.H.; Villas Boas, M.H. Widespread distribution of qacA/B gene among coagulase-negative Staphylococcus spp. in Rio de Janeiro, Brazil. J. Hosp. Infect. 2010, 75, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; O’Donoghue, M.M.; Ito, T.; Hiramatsu, K.; Boost, M.V. Prevalence of antiseptic-resistance genes in Staphylococcus aureus and coagulase-negative staphylococci colonising nurses and the general population in Hong Kong. J. Hosp. Infect. 2011, 78, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Minbiole, K.P.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Bauer, J.; Preikschat, P.; Schwaiger, K.; Molle, G.; Holzel, C. First detection of the antiseptic resistance gene qacA/B in Enterococcus faecalis. Microb. Drug Resist. 2012, 18, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; O’Rourke, B.A.; Skurray, R.A.; Brown, M.H. Role of transmembrane segment 10 in efflux mediated by the staphylococcal multidrug transport protein QacA. J. Biol. Chem. 2006, 281, 792–799. [Google Scholar] [CrossRef]
- Mitchell, B.A.; Paulsen, I.T.; Brown, M.H.; Skurray, R.A. Bioenergetics of the staphylococcal multidrug export protein QacA. Identification of distinct binding sites for monovalent and divalent cations. J. Biol. Chem. 1999, 274, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Tang, Y.Q.; Shen, A.J.; Bayer, A.S.; Selsted, M.E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 1997, 65, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Kupferwasser, L.I.; Skurray, R.A.; Brown, M.H.; Firth, N.; Yeaman, M.R.; Bayer, A.S. Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in staphylococci: Role of the qacA locus. Antimicrob. Agents Chemother. 1999, 43, 2395–2399. [Google Scholar] [CrossRef]
- Bayer, A.S.; Cheng, D.; Yeaman, M.R.; Corey, G.R.; McClelland, R.S.; Harrel, L.J.; Fowler, V.G., Jr. In vitro resistance to thrombin-induced platelet microbicidal protein among clinical bacteremic isolates of Staphylococcus aureus correlates with an endovascular infectious source. Antimicrob. Agents Chemother. 1998, 42, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Prasad, R.; Chandra, J.; Koul, A.; Smriti, M.; Varma, A.; Skurray, R.A.; Firth, N.; Brown, M.H.; Koo, S.-P. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 2000, 68, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Kupferwasser, L.; Brown, M.H.; Skurray, R.A.; Grkovic, S.; Jones, T.; Mukhopadhay, K.; Yeaman, M. Low-level resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein 1 in vitro associated with qacA gene carriage is independent of multidrug efflux pump activity. Antimicrob. Agents Chemother. 2006, 50, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; Proctor, R.A.; Bayer, A.S.; Yeaman, M.R.; Lalk, M.; Engelmann, S.; Mishra, N.N. Proteomic and membrane lipid correlates of re-duced host defense peptide susceptibility in a snoD mutant of Staphylococcus aureus. Antibiotics 2019, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural mechanisms of QacR induction and multidrug recognition. Science 2001, 294, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, S.; Brown, M.H.; Roberts, N.J.; Paulsen, I.T.; Skurray, R.A. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J. Biol. Chem. 1998, 273, 18665–18673. [Google Scholar] [CrossRef]
- Grkovic, S.; Brown, M.H.; Schumacher, M.A.; Brennan, R.G.; Skurray, R.A. The staphylococcal QacR multidrug regulator binds a correctly spaced operator as a pair of dimers. J. Bacteriol. 2001, 183, 7102–7109. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO J. 2002, 21, 1210–1218. [Google Scholar] [CrossRef]
- Wang, C.; Cai, P.; Zhan, Q.; Mi, Z.; Huang, Z.; Chen, G. Distribution of antiseptic-resistance genes qacA/B in clinical isolates of meticillin-resistant Staphylococcus aureus in China. J. Hosp. Infect. 2008, 69, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Gebel, J.; Exner, M.; French, G.; Chartier, Y.; Christiansen, B.; Gemein, S.; Goroncy-Bermes, P.; Hartemann, P.; Heudorf, U.; Kramer, A.; et al. The role of surface disinfection in infection prevention. GMS Hyg. Infect. Control 2013, 8, Doc10. [Google Scholar]
- Zaki, M.E.S.; Bastawy, S.; Montasser, K. Molecular study of resistance of Staphylococcus aureus to antiseptic quaternary ammonium compounds. J. Glob. Antimicrob. Resist. 2019, 17, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Boos, M.; Beyer, A.; Fluit, A.C.; Schmitz, F.J. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and -susceptible European isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 47, 896–897. [Google Scholar] [CrossRef]
- Vali, L.; Davies, S.E.; Lai, L.L.; Dave, J.; Amyes, S.G. Frequency of biocide resistance genes, antibiotic resistance and the effect of chlorhexidine exposure on clinical methicillin-resistant Staphylococcus aureus isolates. J. Antimicrob. Chemother. 2008, 61, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Sheng, W.H.; Wang, J.L.; Chen, D.; Chen, M.L.; Chen, Y.C.; Chang, S.C. Longitudinal analysis of chlorhexidine susceptibilities of nosocomial methicillin-resistant Staphylococcus aureus isolates at a teaching hospital in Taiwan. J. Antimicrob. Chemother. 2008, 62, 514–517. [Google Scholar] [CrossRef]
- Sheng, W.H.; Wang, J.T.; Lauderdale, T.L.; Weng, C.M.; Chen, D.; Chang, S.C. Epidemiology and susceptibilities of methicillin-resistant Staphylococcus aureus in Taiwan: Emphasis on chlorhexidine susceptibility. Diagn. Microbiol. Infect. Dis. 2009, 63, 309–313. [Google Scholar] [CrossRef]
- Htun, H.L.; Hon, P.Y.; Holden, M.T.; Ang, B.; Chow, A. Chlorhexidine and octenidine use, carriage of qac genes, and reduced antiseptic susceptibility in methicillin-resistant Staphylococcus aureus isolates from a healthcare network. Clin. Microbiol. Infect. 2019, 25, 1154.e1–1154.e7. [Google Scholar] [CrossRef] [PubMed]
- Schindler, B.D.; Jacinto, P.; Kaatz, G.W. Inhibition of drug efflux pumps in Staphylococcus aureus: Current status of potentiating existing antibiotics. Future Microbiol. 2013, 8, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, T.; Cannalire, R.; Pietrella, D.; Latacz, G.; Lubelska, A.; Manfroni, G.; Barreca, M.L.; Massari, S.; Tabarrini, O.; Kieć-Kononowicz, K. 2-Phenylquinoline S. aureus NorA efflux pump inhibitors: Evaluation of the importance of methoxy group introduction. J. Med. Chem. 2018, 61, 7827–7848. [Google Scholar] [CrossRef] [PubMed]
- Cannalire, R.; Mangiaterra, G.; Felicetti, T.; Astolfi, A.; Cedraro, N.; Massari, S.; Manfroni, G.; Tabarrini, O.; Vaiasicca, S.; Barreca, M.L. Structural Modifications of the Quinolin-4-yloxy Core to Obtain New Staphylococcus aureus NorA Inhibitors. Int. J. Mol. Sci. 2020, 21, 7037. [Google Scholar] [CrossRef]
- Dreier, J.; Ruggerone, P. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 660. [Google Scholar] [CrossRef]
- Sabatini, S.; Gosetto, F.; Iraci, N.; Barreca, M.L.; Massari, S.; Sancineto, L.; Manfroni, G.; Tabarrini, O.; Dimovska, M.; Kaatz, G.W. Re-evolution of the 2-phenylquinolines: Ligand-based design, synthesis, and biological evaluation of a potent new class of Staphylococcus aureus NorA efflux pump inhibitors to combat antimicrobial resistance. J. Med. Chem. 2013, 56, 4975–4989. [Google Scholar] [CrossRef]
- Carotti, A.; Ianni, F.; Sabatini, S.; di Michele, A.; Sardella, R.; Kaatz, G.W.; Lindner, W.; Cecchetti, V.; Natalini, B. The “racemic approach” in the evaluation of the enantiomeric NorA efflux pump inhibition activity of 2-phenylquinoline derivatives. J. Pharm. Biomed. Anal. 2016, 129, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Piccioni, M.; Felicetti, T.; de Marco, S.; Manfroni, G.; Pagiotti, R.; Nocchetti, M.; Cecchetti, V.; Pietrella, D. Investigation on the effect of known potent S. aureus NorA efflux pump inhibitors on the staphylococcal biofilm formation. RSC Adv. 2017, 7, 37007–37014. [Google Scholar] [CrossRef]
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically approved drugs inhibit the Staphylococcus aureus multidrug NorA efflux pump and reduce biofilm formation. Front. Microbiol. 2019, 10, 2762. [Google Scholar] [CrossRef]
- Oo, T.; Saiboonjan, B.; Srijampa, S.; Srisrattakarn, A.; Sutthanut, K.; Tavichakorntrakool, R.; Chanawong, A.; Lulitanond, A.; Tippayawat, P. Inhibition of Bacterial Efflux Pumps by Crude Extracts and Essential Oil from Myristica fragrans Houtt. (Nutmeg) Seeds against Methicillin-Resistant Staphylococcus aureus. Molecules 2021, 26, 4662. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.Y.; Jamshidi, S.; Mark Sutton, J.; Rahman, K.M. Current advances in developing inhibitors of bacterial multidrug efflux pumps. Curr. Med. Chem. 2016, 23, 1062–1081. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Shahrour, H.; Pitts, B.; Stewart, P.S.; Sánchez-Gómez, S.; Martínez-de-Tejada, G. A permeability-increasing drug synergizes with bacterial efflux pump inhibitors and restores susceptibility to antibiotics in multi-drug resistant Pseudomonas aeruginosa strains. Sci. Rep. 2019, 9, 3452. [Google Scholar] [CrossRef]
- Chovanová, R.; Mezovská, J.; Vaverková, Š.; Mikulášová, M. The inhibition the Tet (K) efflux pump of tetracycline resistant Staphylococcus epidermidis by essential oils from three Salvia species. Lett. Appl. Microbiol. 2015, 61, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, K.L.; de Aquino, T.M.; Mendonça Junior, F.J.B. An update on Staphylococcus aureus NorA efflux pump inhibitors. Curr. Top. Med. Chem. 2020, 20, 2168–2185. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 2001, 45, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Tian, X.; Koseki, S.; Chen, S.; Ye, X.; Ding, T. Novel antibacterial modalities against methicillin resistant Staphylococcus aureus derived from plants. Crit. Rev. Food Sci. Nutr. 2019, 59, S153–S161. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Ali, A.; Bonomo, R.A.; Khan, A.U. Efflux pumps as interventions to control infection caused by drug-resistance bacteria. Drug Discov. Today 2020, 25, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 2020, 10, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.-J.; Fluit, A.; Lückefahr, M.; Engler, B.; Hofmann, B.; Verhoef, J.; Heinz, H.; Hadding, U.; Jones, M. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 42, 807–810. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Kumar, S.; Lekshmi, M.; Parvathi, A.; Ojha, M.; Wenzel, N.; Varela, M.F. Functional and structural roles of the major facilitator superfamily bacterial multidrug efflux pumps. Microorganisms 2020, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Godavari, A.G.; Tambat, R.; Kumar, S.; Nandanwar, H.; Sobhia, M.E.; Jachak, S.M. Synthesis, biological evaluation and computational studies of acrylohydrazide derivatives as potential Staphylococcus aureus NorA efflux pump inhibitors. Bioorg. Chem. 2020, 104, 104225. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.-S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. First identification of boronic species as novel potential inhibitors of the Staphylococcus aureus NorA efflux pump. J. Med. Chem. 2014, 57, 2536–2548. [Google Scholar] [CrossRef] [PubMed]
- Vermote, A.; van Calenbergh, S. Small-molecule potentiators for conventional antibiotics against Staphylococcus aureus. ACS Infect. Dis. 2017, 3, 780–796. [Google Scholar] [CrossRef]
- Klenotic, P.A.; Morgan, C.E.; Edward, W.Y. Cryo-EM as a tool to study bacterial efflux systems and the membrane proteome. Faculty Rev. 2021, 10, 24. [Google Scholar] [CrossRef]
- Pereira da Cruz, R.; Sampaio de Freitas, T.; do Socorro Costa, M.; Lucas dos Santos, A.T.; Ferreira Campina, F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; de Souza Araújo, A.A.; de Siqueira Júnior, J.P. Effect of α-bisabolol and its β-cyclodextrin complex as TetK and NorA efflux pump inhibitors in Staphylococcus aureus strains. Antibiotics 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Limaverde, P.W.; Campina, F.F.; da Cunha, F.A.; Crispim, F.D.; Figueredo, F.G.; Lima, L.F.; Oliveira-Tintino, C.D.D.M.; de Matos, Y.M.; Morais-Braga, M.F.B.; Menezes, I.R. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017, 109, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Cabral, V.; Luo, X.; Junqueira, E.; Costa, S.S.; Mulhovo, S.; Duarte, A.; Couto, I.; Viveiros, M.; Ferreira, M.-J.U. Enhancing activity of antibiotics against Staphylococcus aureus: Zanthoxylum capense constituents and derivatives. Phytomedicine 2015, 22, 469–476. [Google Scholar] [CrossRef]
- Rezende-Júnior, L.M.; Andrade, L.M.D.S.; Leal, A.L.A.B.; Mesquita, A.B.D.S.; Santos, A.L.P.D.A.D.; Neto, J.D.S.L.; Siqueira-Junior, J.P.; Nogueira, C.E.S.; Kaatz, G.W.; Coutinho, H.D.M. Chalcones isolated from Arrabidaea brachypoda flowers as inhibitors of NorA and MepA multidrug efflux pumps of Staphylococcus aureus. Antibiotics 2020, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.F.; Tintino, S.R.; de Freitas, T.S.; Campina, F.F.; Irwin, R.D.A.; Siqueira-Júnior, J.P.; Coutinho, H.D.; Cunha, F.A. In vitro e in silico evaluation of the inhibition of Staphylococcus aureus efflux pumps by caffeic and gallic acid. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Kalia, N.P.; Mahajan, P.; Mehra, R.; Nargotra, A.; Sharma, J.P.; Koul, S.; Khan, I.A. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J. Antimicrob. Chemother. 2012, 67, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Kumari, N.; Pahwa, S.; Agrahari, U.C.; Bhutani, K.K.; Jachak, S.M.; Nandanwar, H. NorA efflux pump inhibitory activity of coumarins from Mesua ferrea. Fitoterapia 2013, 90, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Singh, S.; Wani, A.; Sharma, S.; Jain, S.K.; Singh, B.; Gupta, B.D.; Satti, N.K.; Koul, S.; Khan, I.A. Osthol and curcumin as inhibitors of human Pgp and multidrug efflux pumps of Staphylococcus aureus: Reversing the resistance against frontline antibacterial drugs. MedChemComm 2014, 5, 1540–1547. [Google Scholar] [CrossRef]
- Coêlho, M.L.; Ferreira, J.H.L.; de Siqueira Júnior, J.P.; Kaatz, G.W.; Barreto, H.M.; Cavalcante, A.A.D.C.M. Inhibition of the NorA multi-drug transporter by oxygenated monoterpenes. Microb. Pathog. 2016, 99, 173–177. [Google Scholar] [CrossRef]
- Wang, S.Y.; Sun, Z.L.; Liu, T.; Gibbons, S.; Zhang, W.J.; Qing, M. Flavonoids from Sophora moorcroftiana and their synergistic antibacterial effects on MRSA. Phytother. Res. 2014, 28, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, K.; Ramasamy, M.; Savarimuthu, I.; Paulraj, M.G. Indirubin potentiates ciprofloxacin activity in the NorA efflux pump of Staphylococcus aureus. Scand. J. Infect. Dis. 2010, 42, 500–505. [Google Scholar] [CrossRef]
- Holler, J.G.; Christensen, S.B.; Slotved, H.-C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.-E.; Petersen, B.; Mølgaard, P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef]
- Freitas, P.R.; de Araújo, A.C.J.; dos Santos Barbosa, C.R.; Muniz, D.F.; de Almeida, R.S.; de Menezes, I.R.A.; da Costa, J.G.M.; Rodrigues, F.F.G.; Rocha, J.E.; Pereira-Junior, F.N. Inhibition of the MepA efflux pump by limonene demonstrated by in vitro and in silico methods. Folia Microbiologica 2021, 1–6. [Google Scholar] [CrossRef]
- Shiu, W.K.; Malkinson, J.P.; Rahman, M.M.; Curry, J.; Stapleton, P.; Gunaratnam, M.; Neidle, S.; Mushtaq, S.; Warner, M.; Livermore, D.M. A new plant-derived antibacterial is an inhibitor of efflux pumps in Staphylococcus aureus. Int. J. Antimicrob. Agents 2013, 42, 513–518. [Google Scholar] [CrossRef]
- Ribeiro, A.M.B.; de Sousa, J.N.; Costa, L.M.; de Alcântara Oliveira, F.A.; dos Santos, R.C.; Nunes, A.S.S.; da Silva, W.O.; Cordeiro, P.J.M.; Neto, J.D.S.L.; de Siqueira-Júnior, J.P. Antimicrobial activity of Phyllanthus amarus Schumach & Thonn and inhibition of the NorA efflux pump of Staphylococcus aureus by Phyllanthin. Microb. Pathog. 2019, 130, 242–246. [Google Scholar]
- Mirza, Z.M.; Kumar, A.; Kalia, N.P.; Zargar, A.; Khan, I.A. Piperine as an inhibitor of the MdeA efflux pump of Staphylococcus aureus. J. Med. Microbiol. 2011, 60, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Mirza, Z.M.; Kumar, A.; Verma, V.; Qazi, G.N. Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 810–812. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.; Campina, F.F.; Silva, R.L.; Costa, M.D.S.; Menezes, I.R.; Calixto-Júnior, J.T.; Siqueira-Junior, J.P.; Coutinho, H.D.; Leal-Balbino, T.C. Evaluation of the tannic acid inhibitory effect against the NorA efflux pump of Staphylococcus aureus. Microb. Pathog. 2016, 97, 9–13. [Google Scholar] [CrossRef]
- Rath, S.K.; Singh, S.; Kumar, S.; Wani, N.A.; Rai, R.; Koul, S.; Khan, I.A.; Sangwan, P.L. Synthesis of amides from (E)-3-(1-chloro-3, 4-dihydronaphthalen-2-yl) acrylic acid and substituted amino acid esters as NorA efflux pump inhibitors of Staphylococcus aureus. Bioorg. Med. Chem. 2019, 27, 343–353. [Google Scholar] [CrossRef]
- Diniz-Silva, H.T.; Magnani, M.; de Siqueira, S.; de Souza, E.L.; de Siqueira-Júnior, J.P. Fruit flavonoids as modulators of norfloxacin resistance in Staphylococcus aureus that overexpresses norA. LWT-Food Sci. Technol. 2017, 85, 324–326. [Google Scholar] [CrossRef]
- Siqueira, M.M.R.; Freire, P.D.T.C.; Cruz, B.G.; de Freitas, T.S.; Bandeira, P.N.; dos Santos, H.S.; Nogueira, C.E.S.; Teixeira, A.M.R.; Pereira, R.L.S.; da Cunha Xavier, J. Aminophenyl chalcones potentiating antibiotic activity and inhibiting bacterial efflux pump. Eur. J. Pharm. Sci. 2021, 158, 105695. [Google Scholar] [CrossRef]
- Doléans-Jordheim, A.; Veron, J.B.; Fendrich, O.; Bergeron, E.; Montagut-Romans, A.; Wong, Y.S.; Furdui, B.; Freney, J.; Dumontet, C.; Boumendjel, A. 3-Aryl-4-methyl-2-quinolones targeting multiresistant Staphylococcus aureus bacteria. ChemMedChem 2013, 8, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Gosetto, F.; Serritella, S.; Manfroni, G.; Tabarrini, O.; Iraci, N.; Brincat, J.P.; Carosati, E.; Villarini, M.; Kaatz, G.W. Pyrazolo [4, 3-c][1, 2] benzothiazines 5, 5-dioxide: A promising new class of Staphylococcus aureus NorA efflux pump inhibitors. J. Med. Chem. 2012, 55, 3568–3572. [Google Scholar] [CrossRef]
- Caspar, Y.; Jeanty, M.; Blu, J.; Burchak, O.; le Pihive, E.; Maigre, L.; Schneider, D.; Jolivalt, C.; Paris, J.-M.; Hequet, A. Novel synthetic bis-indolic derivatives with antistaphylococcal activity, including against MRSA and VISA strains. J. Antimicrob. Chemother. 2015, 70, 1727–1737. [Google Scholar] [CrossRef]
- Holler, J.G.; Slotved, H.-C.; Mølgaard, P.; Olsen, C.E.; Christensen, S.B. Chalcone inhibitors of the NorA efflux pump in Staphylococcus aureus whole cells and enriched everted membrane vesicles. Bioorg. Med. Chem. 2012, 20, 4514–4521. [Google Scholar] [CrossRef]
- Radix, S.; Jordheim, A.D.; Rocheblave, L.; N’Digo, S.; Prignon, A.-L.; Commun, C.; Michalet, S.; Dijoux-Franca, M.-G.; Mularoni, A.; Walchshofer, N. N, N′-disubstituted cinnamamide derivatives potentiate ciprofloxacin activity against overexpressing NorA efflux pump Staphylococcus aureus 1199B strains. Eur. J. Med. Chem. 2018, 150, 900–907. [Google Scholar] [CrossRef]
- Lowrence, R.C.; Raman, T.; Makala, H.V.; Ulaganathan, V.; Subramaniapillai, S.G.; Kuppuswamy, A.A.; Mani, A.; Neelakantan, S.C.; Nagarajan, S. Dithiazole thione derivative as competitive NorA efflux pump inhibitor to curtail multi drug resistant clinical isolate of MRSA in a zebrafish infection model. Appl. Microbiol. Biotechnol. 2016, 100, 9265–9281. [Google Scholar] [CrossRef] [PubMed]
- Hequet, A.; Burchak, O.N.; Jeanty, M.; Guinchard, X.; le Pihive, E.; Maigre, L.; Bouhours, P.; Schneider, D.; Maurin, M.; Paris, J.-M. 1-(1H-Indol-3-yl) ethanamine derivatives as potent Staphylococcus aureus NorA efflux pump inhibitors. ChemMedChem 2014, 9, 1534–1545. [Google Scholar] [CrossRef]
- Wani, N.A.; Singh, S.; Farooq, S.; Shankar, S.; Koul, S.; Khan, I.A.; Rai, R. Amino acid amides of piperic acid (PA) and 4-ethylpiperic acid (EPA) as NorA efflux pump inhibitors of Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2016, 26, 4174–4178. [Google Scholar] [CrossRef]
- Muniz, D.F.; dos Santos Barbosa, C.R.; de Menezes, I.R.A.; de Sousa, E.O.; Pereira, R.L.S.; Júnior, J.T.C.; Pereira, P.S.; de Matos, Y.M.; da Costa, R.H.; de Morais Oliveira-Tintino, C.D. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef]
- Figueredo, F.G.; Parente, R.E.L.; Cavalcante-Figueredo, M.R.; Figueiredo, J.G.; da Silva, R.L.P.; Matias, E.F.F.; Silva, T.M.S.; Camara, C.A.; de Morais Oliveira-Tintino, C.D.; Tintino, S.R. Inhibition of Staphylococcus aureus TetK and MsrA efflux pumps by hydroxyamines derived from lapachol and norlachol. J. Bioenerg. Biomembr. 2021, 53, 149–156. [Google Scholar] [CrossRef]
- Corona-Castañeda, B.; Chérigo, L.; Fragoso-Serrano, M.; Gibbons, S.; Pereda-Miranda, R. Modulators of antibiotic activity from Ipomoea murucoides. Phytochemistry 2013, 95, 277–283. [Google Scholar] [CrossRef]
- De Morais Oliveira-Tintino, C.D.; Muniz, D.F.; dos Santos Barbosa, C.R.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; Krautler, M.I.L. The 1, 8-naphthyridines sulfonamides are NorA efflux pump inhibitors. J. Glob. Antimicrob. Resist. 2021, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Khan, A.; Kumari, A.; Ali, I.; Ahmad, F.; Lone, B.A.; Ahmad, N.; Khan, I.A.; Rajput, V.S.; Grover, A. 1, 3, 4-oxadiazole conjugates of capsaicin as potent NorA efflux pump inhibitors of Staphylococcus aureus. Bioorg. Chem. 2021, 113, 105031. [Google Scholar] [CrossRef]
- Sabatini, S.; Gosetto, F.; Manfroni, G.; Tabarrini, O.; Kaatz, G.W.; Patel, D.; Cecchetti, V. Evolution from a natural flavones nucleus to obtain 2-(4-Propoxyphenyl) quinoline derivatives as potent inhibitors of the S. aureus NorA efflux pump. J. Med. Chem. 2011, 54, 5722–5736. [Google Scholar] [CrossRef] [PubMed]
- Thota, N.; Reddy, M.V.; Kumar, A.; Khan, I.A.; Sangwan, P.L.; Kalia, N.P.; Koul, J.L.; Koul, S. Substituted dihydronaphthalenes as efflux pump inhibitors of Staphylococcus aureus. Eur. J. Med. Chem. 2010, 45, 3607–3616. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).