The Glucocorticoid PYED-1 Disrupts Mature Biofilms of Candida spp. and Inhibits Hyphal Development in Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
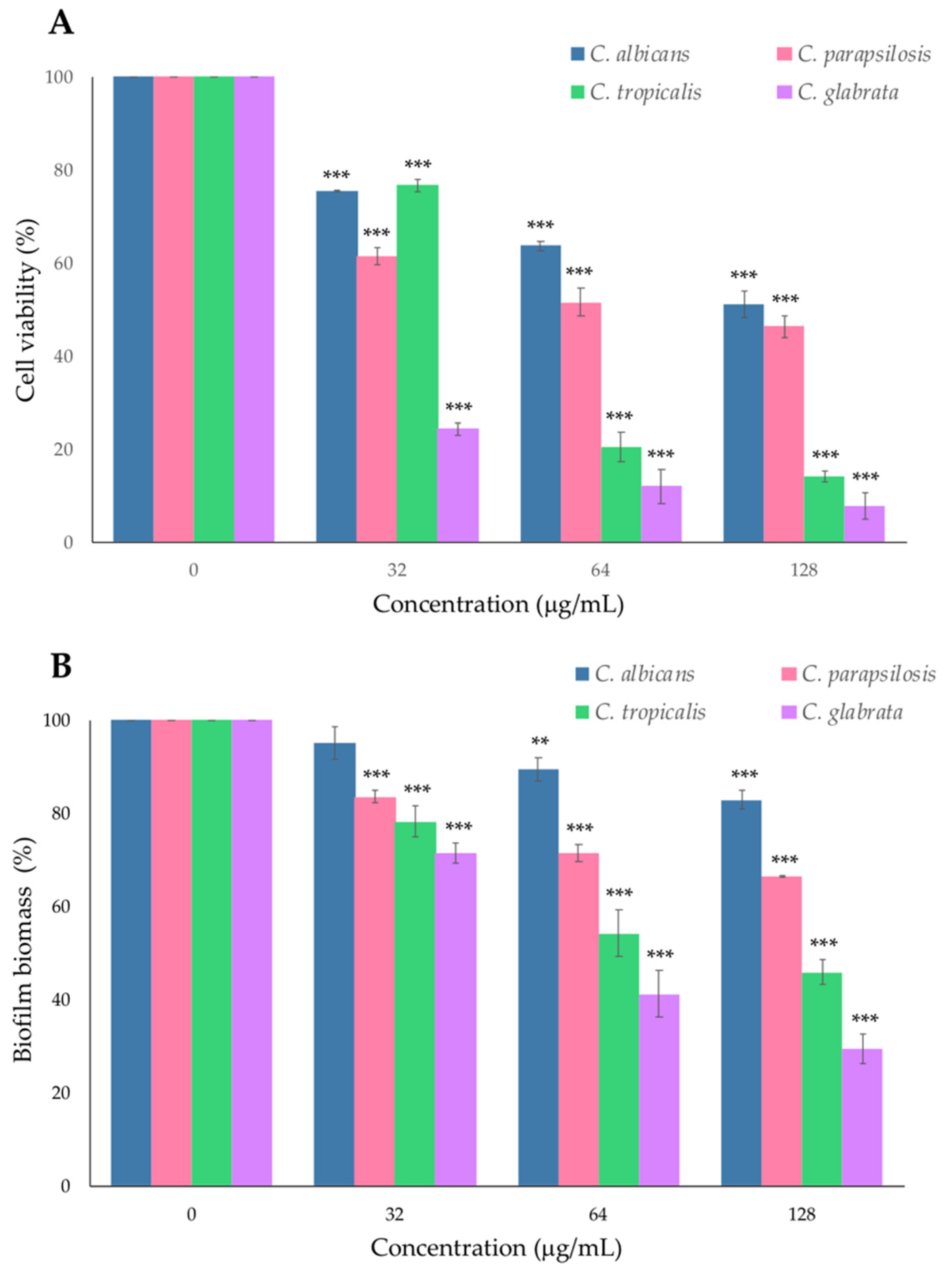
2.1. Antifungal Susceptibility to Glucocorticoid PYED-1 of Candida Species
2.2. Effect of PYED-1 on Preformed Candida spp. Biofilm
2.3. PYED-1 Inhibited the Filamentation and Adhesion of C. albicans
3. Materials and Methods
3.1. Fungal Strain, Culture Condition and Compound Synthesis
3.2. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Fungicide Concentration (MFC)
3.3. Checkboard Microdilution Assay
3.4. Germ Tube Inhibition Assay
3.5. Adhesion Assay
3.6. Anti-Preformed-Biofilm Assay
3.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the SENTRY antifungal surveillance program: Results for Candida species from 1997-2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Chen, Y.C.; Kuo, S.F.; Chen, F.J.; Lee, C.H. The impact of biofilm formation on the persistence of candidemia. Front. Microbiol. 2018, 9, 1196. [Google Scholar] [CrossRef] [Green Version]
- De Fenza, M.; D’Alonzo, D.; Esposito, A.; Munari, S.; Loberto, N.; Santangelo, A.; Lampronti, I.; Tamanini, A.; Rossi, A.; Ranucci, S.; et al. Exploring the effect of chirality on the therapeutic potential of N-alkyl-deoxyiminosugars: Anti-inflammatory response to Pseudomonas aeruginosa infections for application in CF lung disease. Eur. J. Med. Chem. 2019, 175, 63–71. [Google Scholar] [CrossRef]
- De Gregorio, E.; Esposito, A.; Vollaro, A.; De Fenza, M.; D’Alonzo, D.; Migliaccio, A.; Iula, V.D.; Zarrilli, R.; Guaragna, A. N-Nonyloxypentyl-l-Deoxynojirimycin Inhibits Growth, Biofilm Formation and Virulence Factors Expression of Staphylococcus aureus. Antibiotics 2020, 9, 362. [Google Scholar] [CrossRef]
- Esposito, A.; D’Alonzo, D.; De Fenza, M.; Gregorio, E.; Tamanini, A.; Lippi, G.; Dechecchi, M.C.; Guaragna, A. Synthesis and Therapeutic Applications of Iminosugars in Cystic Fibrosis. Int. J. Mol. Sci. 2020, 21, 3353. [Google Scholar] [CrossRef]
- Esposito, A.; D’Alonzo, D.; D’Errico, S.; De Gregorio, E.; Guaragna, A. Toward the Identification of Novel Antimicrobial Agents: One-Pot Synthesis of Lipophilic Conjugates of N-Alkyl d- and l-Iminosugars. Mar. Drugs 2020, 18, 572. [Google Scholar] [CrossRef]
- Esposito, A.; di Giovanni, C.; De Fenza, M.; Talarico, G.; Chino, M.; Palumbo, G.; Guaragna, A.; D’Alonzo, D. A Stereoconvergent Tsuji-Trost Reaction in the Synthesis of Cyclohexenyl Nucleosides. Chemistry 2020, 26, 2597–2601. [Google Scholar] [CrossRef]
- Esposito, A.; De Gregorio, E.; De Fenza, M.; D’Alonzo, D.; Satawani, A.; Guaragna, A. Expeditious synthesis and preliminary antimicrobial activity of deflazacort and its precursors. RSC Adv. 2019, 9, 21519–21524. [Google Scholar] [CrossRef] [Green Version]
- Esposito, A.; Vollaro, A.; Esposito, E.P.; D’Alonzo, D.; Guaragna, A.; Zarrilli, R.; De Gregorio, E. Antibacterial and antivirulence activity of glucocorticoid PYED-1 against Stenotrophomonas maltophilia. Antibiotics 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollaro, A.; Esposito, A.; Antonaki, E.; Iula, V.D.; D’Alonzo, D.; Guaragna, A.; De Gregorio, E. Steroid derivatives as potential antimicrobial agents against Staphylococcus aureus planktonic cells. Microorganisms 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollaro, A.; Esposito, A.; Esposito, E.P.; Zarrilli, R.; Guaragna, A.; De Gregorio, E. PYED-1 inhibits biofilm formation and disrupts the preformed biofilm of Staphylococcus aureus. Antibiotics 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Candida biofilms associated with CVC and medical devices. Mycoses 2012, 55, 46–57. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.M.; Felício, M.R.; Boas, E.V.; Gonçalves, S.; Costa, F.F.; Samy, R.P.; Santos, N.C.; Franco, O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016, 160, 133–144. [Google Scholar] [CrossRef]
- Nobile, C.J.; Mitchell, A.P. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 2005, 15, 1150–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Sardi, J.C.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Robertson, S.N.; Williams, C. Strength in numbers: Antifungal strategies against fungal biofilms. Int. J. Antimicrob. Agents 2013, 43, 114e120. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A. Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. NPJ Biofilms Microbiomes 2019, 21, 94–95. [Google Scholar] [CrossRef] [Green Version]
- de Groot, P.W.J.; Bader, O.; de Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in human fungal pathogens: Glue with plenty of stick. Eukaryot. Cell 2013, 12, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef] [Green Version]
- Gauwerky, K.; Borelli, C.; Korting, H.C. Targeting virulence: A new paradigm for antifungals. Drug Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef]
- EUCAST Definitive Document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [CrossRef]
- Dantas-Medeiros, R.; Zanatta, A.C.; de Souza, L.B.F.C.; Fernandes, J.M.; Amorim-Carmo, B.; Torres-Rêgo, M.; Fernandes-Pedrosa, M.F.; Vilegas, W.; Araújo, T.A.S.; Michel, S.; et al. Antifungal and Antibiofilm Activities of B-Type Oligomeric Procyanidins From Commiphora leptophloeos Used Alone or in Combination with Fluconazole Against Candida spp. Front Microbiol. 2021, 12, 613155. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krom, B.P.; Cohen, J.B.; McElhaney-Feser, G.; Busscher, H.J.; van der Mei, H.C.; Cihlar, R.L. Conditions for optimal Candida biofilm development in microtiter plates. Methods Mol. Biol. 2009, 499, 55–62. [Google Scholar]
- De Gregorio, E.; Esposito, E.P.; Zarrilli, R.; Di Nocera, P.P. Contact-dependent growth inhibition proteins in Acinetobacter baylyi ADP1. Curr. Microbiol. 2018, 75, 1434–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Species | Strain | PYED-1 | Fluconazole | |
|---|---|---|---|---|
| MIC (µg/mL) | MLC (µg/mL) | MIC (g/mL) | ||
| C. albicans | ATCC 10231 | 16 | 64 | 1 |
| 61142 | 16 | 64 | 0.5 | |
| 61280 | 16 | 64 | 0.5 | |
| 61446 | 32 | 64 | 128 | |
| 61540 | 16 | 64 | 0.5 | |
| 61558 | 32 | 128 | 1 | |
| 61691 | 32 | 64 | 64 | |
| 62020 | 16 | 64 | 0.5 | |
| 62033 | 16 | 64 | 1 | |
| C. glabrata | 60932 | 32 | 64 | 0.5 |
| 60940 | 32 | 64 | 0.5 | |
| 61112 | 16 | 128 | 1 | |
| 61115 | 32 | 128 | 4 | |
| 65821 | 64 | >128 | 1 | |
| 67676 | 64 | >128 | 1 | |
| 81263 | 32 | 64 | 0.5 | |
| C. parapsilosis | 60568 | 32 | 128 | 0.5 |
| 61446 | 64 | >128 | 2 | |
| 66627 | 128 | >128 | 0.5 | |
| 80149 | 128 | >128 | 1 | |
| 81208 | 64 | >128 | 0.5 | |
| 81879 | 128 | >128 | 0.5 | |
| 84609 | 128 | >128 | 32 | |
| 84614 | 128 | >128 | 32 | |
| C. tropicalis | 60981 | 16 | 32 | 0.5 |
| 61220 | 64 | >128 | 1 | |
| 81222 | 32 | 64 | 0.5 | |
| 81252 | 32 | 64 | 0.5 | |
| 81419 | 64 | >128 | 0.5 | |
| 82193 | 32 | 128 | 1 | |
| C. krusei | 61159 | 32 | >128 | 32 |
| 67053 | 32 | >128 | 64 | |
| 69788 | 32 | >128 | 32 | |
| 71456 | 32 | >128 | 32 | |
| 81288 | 32 | 128 | 16 | |
| 81667 | 32 | >128 | 32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, A.; Migliaccio, A.; Iula, V.D.; Zarrilli, R.; Guaragna, A.; De Gregorio, E. The Glucocorticoid PYED-1 Disrupts Mature Biofilms of Candida spp. and Inhibits Hyphal Development in Candida albicans. Antibiotics 2021, 10, 1396. https://doi.org/10.3390/antibiotics10111396
Esposito A, Migliaccio A, Iula VD, Zarrilli R, Guaragna A, De Gregorio E. The Glucocorticoid PYED-1 Disrupts Mature Biofilms of Candida spp. and Inhibits Hyphal Development in Candida albicans. Antibiotics. 2021; 10(11):1396. https://doi.org/10.3390/antibiotics10111396
Chicago/Turabian StyleEsposito, Anna, Antonella Migliaccio, Vita Dora Iula, Raffaele Zarrilli, Annalisa Guaragna, and Eliana De Gregorio. 2021. "The Glucocorticoid PYED-1 Disrupts Mature Biofilms of Candida spp. and Inhibits Hyphal Development in Candida albicans" Antibiotics 10, no. 11: 1396. https://doi.org/10.3390/antibiotics10111396
APA StyleEsposito, A., Migliaccio, A., Iula, V. D., Zarrilli, R., Guaragna, A., & De Gregorio, E. (2021). The Glucocorticoid PYED-1 Disrupts Mature Biofilms of Candida spp. and Inhibits Hyphal Development in Candida albicans. Antibiotics, 10(11), 1396. https://doi.org/10.3390/antibiotics10111396










